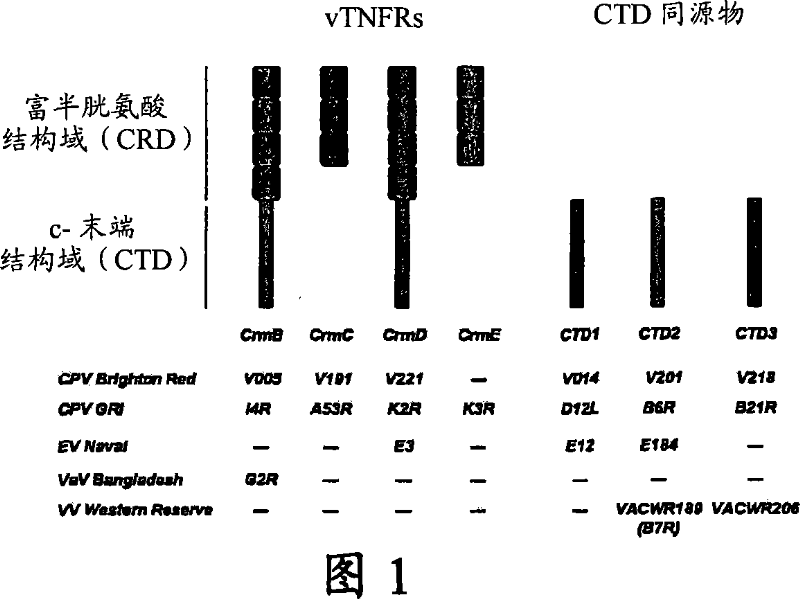
Chemokine binding activity of viral TNF receptors and related proteins
A chemokine and virus technology, which is applied in the field of chemokine binding activity of viral TNF receptors and related proteins, can solve the problems that are not clear, TNF binding is not an essential function, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Materials and methods
[0051] 1.1-pox virus
[0052] CPV, EV and VV proliferate in vitro by infecting confluent monolayers of Bsc-I cells.
[0053] 1.2-Cloning of Camelpox Virus CrmB and Production of VaV CrmB
[0054] Using oligonucleotides CMLV 264 Eco (5'-GCGGAATTCATGAAGTCCGTATTATACTCG) and CMLV 264 Xho (5'-GCGCTCGAGTAAAAAGTGGGTGGGTTTGG) and purified CMLVDNA as a template to amplify the ORF 264 (corresponding to the CrmB gene) of the camelpox virus strain CMS by PCR . The PCR product was cloned into EcoRI / XhoI digested pBacl (Novagen) to generate plasmid pRA1. The lack of mutations in the amplified gene was confirmed by DNA sequencing. According to the manufacturer's instructions, the "QuikChange Multi Site-Directed Mutagenesis Kit (Strategene)" was used to obtain DNA corresponding to VaV (strain Bangladesh 1975; ORF 188) through multiple site-directed mutagenesis of plasmid pRA1. The introduced mutations are shown in Figure 6. After several consecutive round...
Embodiment 2
[0071] Example 2: Results
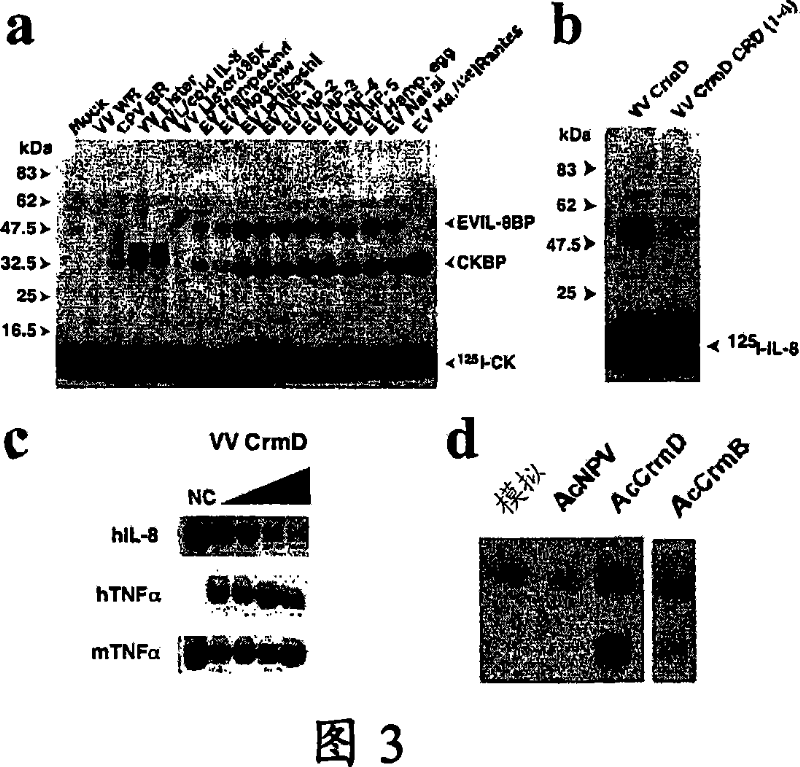
[0072] 2.1-vTNFR CrmD-binding chemokine encoded by EV and CPV
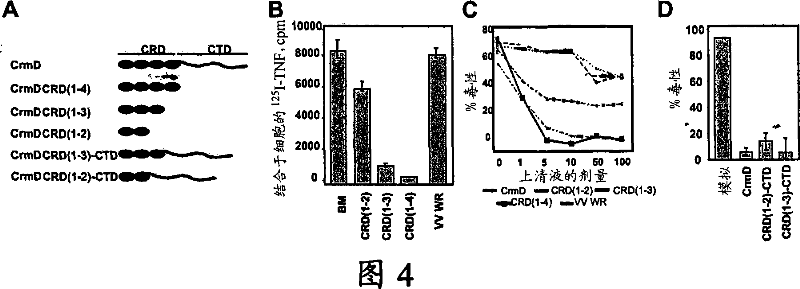
[0073] In order to find a new virus secreted protein that binds to chemokines, we performed cross-linking experiments with 1251-CXCL8 and identified a new secreted vCKBP encoded by poxvirus EV (Figure 3a). This activity is lacking in VV samples, and has a larger molecular size than the 35kDa vCKBP encoded by VV and other poxviruses. vCKPB is a kind of cross-linking CXCL8 but does not block its organism due to its low affinity for CXCL8. Active protein (Alcami et al., 1998, Graham et al., 1997, Lalani et al., 1998). Unexpectedly, we found that the vTNFR CrmD encoded by EV (known to bind TNF) has additional CXCL8 binding properties (Figure 3b). Using the truncated CrmD protein expressed in the VV expression system, we have confirmed that the three N-terminal CRDs of CrmD are necessary to block TNF activity (Figures 4a, 4b, and 4c), while CTD is not for TNF binding. It is necessary, but CTD gi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com