Nanobody against SARS-COV-2 virus S protein RBD structure domain and use thereof
A SARS-COV-2, nanobody technology, applied in the direction of antiviral immunoglobulins, antiviral agents, antibodies, etc., can solve the problem of inconspicuousness in the initial stage of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] The acquisition of embodiment 1 Nanobody
[0099] 1. Construction of Nanobody Library
[0100] Take a 15ml centrifuge tube, first add the same amount of separation liquid as the blood sample, carefully draw the blood sample and add it on the liquid surface of the separation liquid, 450-650g, centrifuge for 20-30min; It is divided into four layers from bottom to bottom, followed by plasma layer, ring-shaped milky white lymphocyte layer, transparent separation liquid layer and red blood cell layer; use a straw to carefully draw the second layer of ring-shaped milky white lymphocyte layer into another 15ml centrifuge tube; Add 10ml of cleaning solution to the centrifuge tube, mix the cells, and centrifuge at 250g for 10 minutes; after centrifugation, check whether the supernatant is clear, if it is not clear enough, increase the centrifugation time; wash the cells twice with 1xPBS, add trizol to lyse the cells and store at -80°C.
[0101] Take the peripheral lymphocyte ly...
Embodiment 2
[0120] Example 2 Binding of nanobody to RBD protein
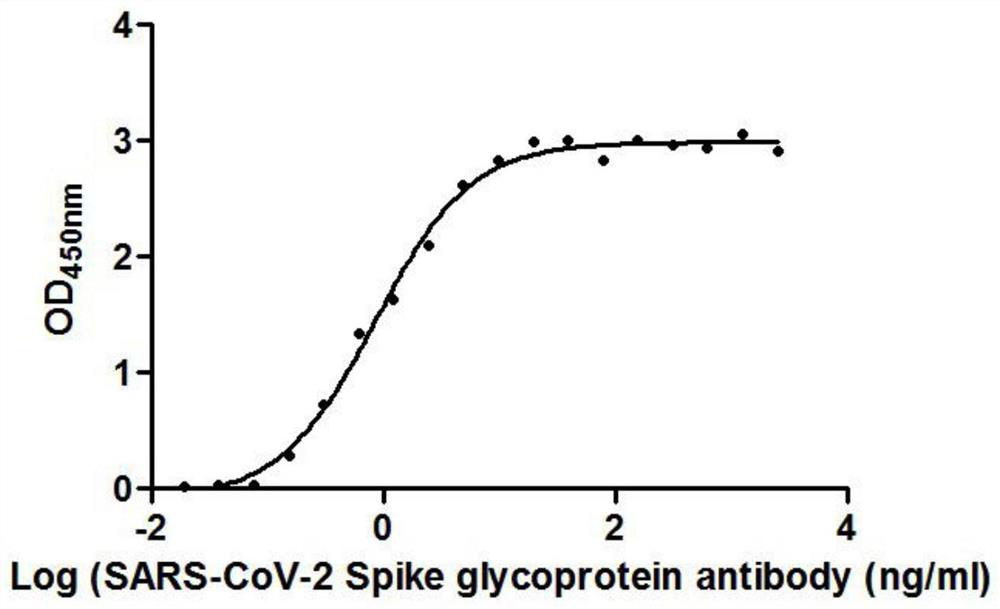
[0121] The 19 different nanobodies screened in Example 1 were combined with RBD respectively, and the EC50 value of antibody binding to RBD was detected. The RBD protein was coated on a microwell plate at 2 μg / ml. The EC50 values of the 19 nanobodies are shown in Table 3. Show.
[0122] table 3
[0123] Nanobodies EC50(ng / ml) Nanobodies EC50(ng / ml) B4 91.69 G5 14.20 B6 325.24 A1 51.79 B9 153.27 F1 0.42 E3 254.45 G3 135.22 E6 5.54 C7 150.26 E7 3.26 D4 254.45 F4 50.14 H3 25.90 F5 25.33 H4 36.21 F8 76.25 H6 50.14 G2 52.19
[0124] It can be seen from the data in Table 1 that the EC50 of the 19 strains of antibodies binding to RBD ranges from 0.42ng / ml to 325.24ng / ml.
[0125] figure 1 is the binding curve of F1 antibody and RBD protein, by figure 1 It can be seen that the RBD protein is coated on the microwell plate at 2 μg / ml, an...
Embodiment 3
[0126] Example 3 Inhibition of Nanobody Binding to ACE2 Protein and RBD Protein
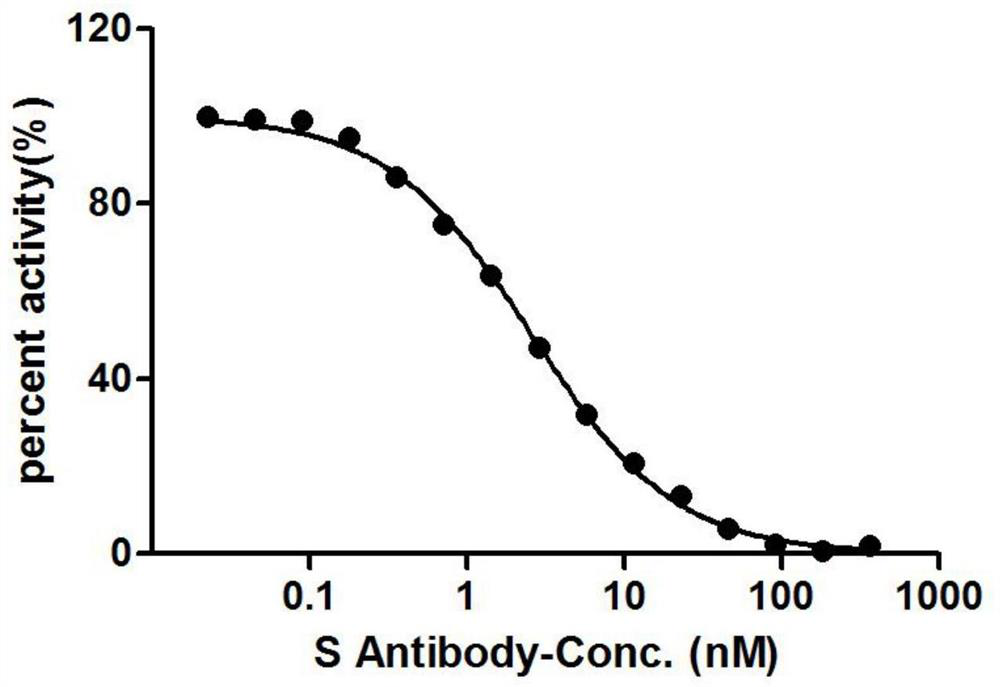
[0127] The RBD protein was coated on a microwell plate at 2 μg / ml, and the 19 different strains of nanobodies screened in Example 1 were mixed with 1 ug / ml of HRP-coupled ACE2 protein, and the OD450nm value was measured. The anti-IC50 values adopted by the 19 strains of Nanobodies are shown in Table 4.
[0128] Table 4
[0129]
[0130]
[0131] Note: - indicates that the antibody has not been tested
[0132] It can be known from the data in Table 2 that all the 19 antibody strains compete with the ACE2 protein, and the IC50 ranges from 2.433nM to 100.02nM.
[0133] figure 2 It is the inhibition curve of the binding of F1 nanobody to ACE2 protein and RBD protein; the OD450nm value decreases with the increase of the concentration of F1 nanobody, indicating that there is a competitive relationship between the nanobody and ACE2 protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com