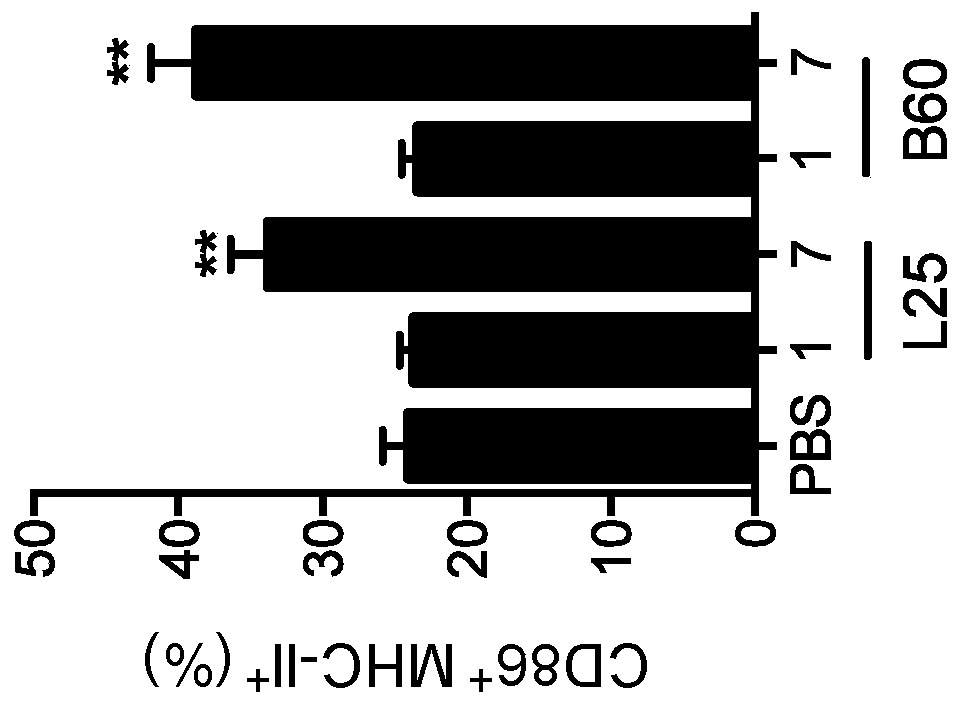
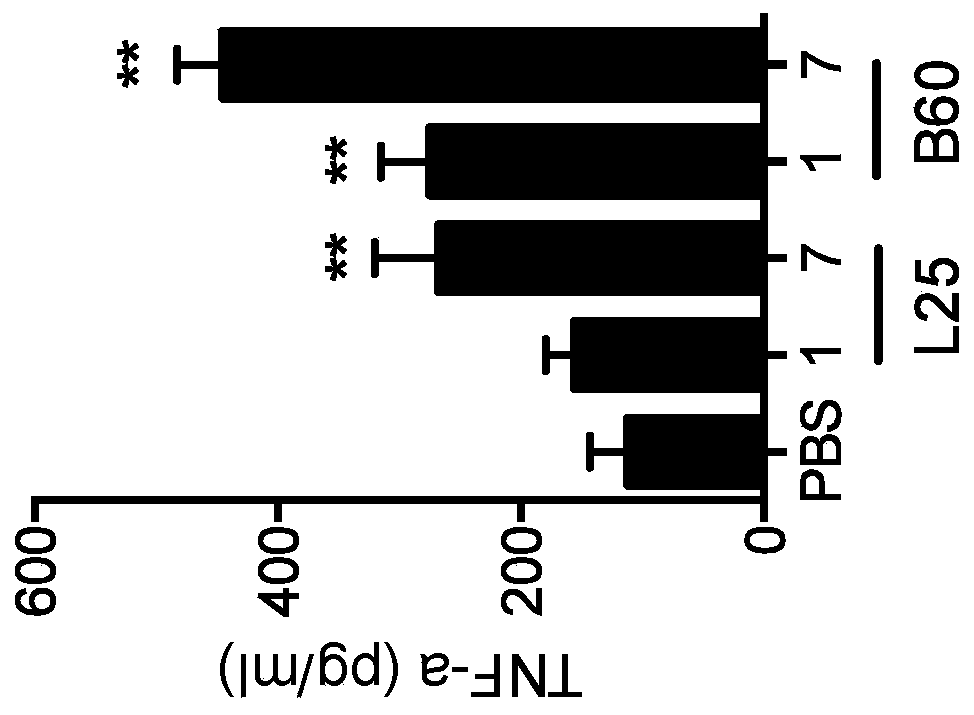
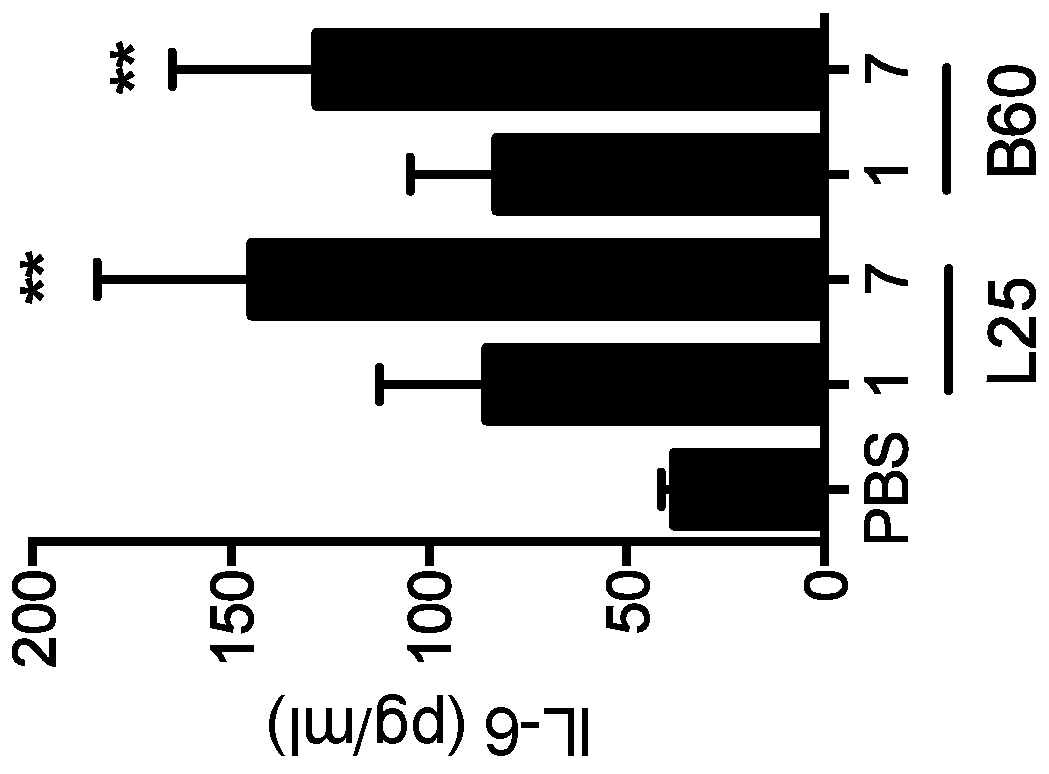
Biomaterials for modulating immune responses
A technology of immune cells and compositions, applied in vaccines, drug combinations, drug delivery, etc., can solve the problems of scarce dendritic cells and slow research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0624] Example 1: Mesoporous silica (MPS) vaccine for enhancing anti-tumor immunity
[0625]Biomaterials have shown great potential to synergistically integrate with existing cancer vaccine strategies and enhance their effectiveness. We have recently developed injectable biomaterial vaccines through the spontaneous assembly of mesoporous silica (MPS) microparticles into 3D scaffolds in vivo. When formulated with GM-CSF and CpG, MPS vaccines modulate host dendritic cell (DC) activation and trafficking. Here we demonstrate that a single injection of MPS vaccine induces persistent germinal center activity over 30 days, for example in draining lymph nodes. Thus, when immunized with a small linear Her2 / neu peptide within the binding domain of trastuzumab, the MPS vaccine elicited more than 2 orders of magnitude higher IgG1 and IgG2a antibody titers than the bolus vaccine, and the antibodies were Native Her2 is structurally shown to be responsive to breast cancer cells. To furt...
Embodiment 2
[0626] Example 2: D,L-lactide and glycolide (PLG) scaffolds comprising PEI
[0627] Coating of PLG scaffolds with polyethyleneimine (PEI) enhanced dendritic cell (DC) activation. Application of PEI to the PLG system prior to antigen adsorption enhanced antitumor responses in cancer vaccine models.
[0628] Compared with the control, the scaffold loaded with PEI promoted TLR5 activity to increase 3-4 times in vitro ( Figure 19A ). Furthermore, murine DCs seeded on PEI-PLG scaffolds produced more than 3-fold more IL-12 and approximately 30-fold more IFN-α than cells seeded on scaffolds without PEI ( Figure 19B ). These results suggest that PEI-modified PLG can locally activate DCs and other antigen-presenting cells, APCs, possibly through the TLR5 pathway.
[0629] Antigens from B16-F10 melanoma tumor lysates were adsorbed onto the PEI-PLG system to generate cancer vaccines. Implantation of PEI-antigen-coated vaccines into mice induced local production of in situ immuno...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com