Nanometer assembly based on immune checkpoint inhibitor and preparation method and application of nanometer assembly
A nano-assembly and methyl technology, applied in the field of medicine, can solve the problems of low treatment response rate, achieve good biocompatibility, enhance immune response, and relieve tumor immunosuppressive environment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: PLL-1-mt molecular synthesis.
[0056] 1) Boc protection of 1-methyl-D-tryptophan amino group (Boc-1-mt): use an analytical balance to accurately weigh 1-methyl-D-tryptophan, NaHCO 3 and di(tert-butyl)dicarbonate were dissolved in a mixed solution of water and tetrahydrofuran at a volume ratio of 1:1, and the mixture was stirred at 0°C for 10 minutes, and then kept at room temperature for 24 hours. After the reaction, the THF was spin-dried, and the aqueous layer was acidified to pH 1.0 with 1M HCl, and then extracted with ethyl acetate. Evaporation of ethyl acetate gave a milky white solid which was the target product Boc-1-mt.
[0057] (2) Synthesis of Boc-1-mt active intermediate ester (Boc-1-mt-NHS ester1): a certain amount of N-hydroxysuccinimide and 1-ethyl-(3-dimethylaminopropyl) carbon Diimine hydrochloride was dissolved in anhydrous DMF, and Boc-1-mt was added. The reaction mixture was stirred at room temperature for 4 hours and used without furth...
Embodiment 2
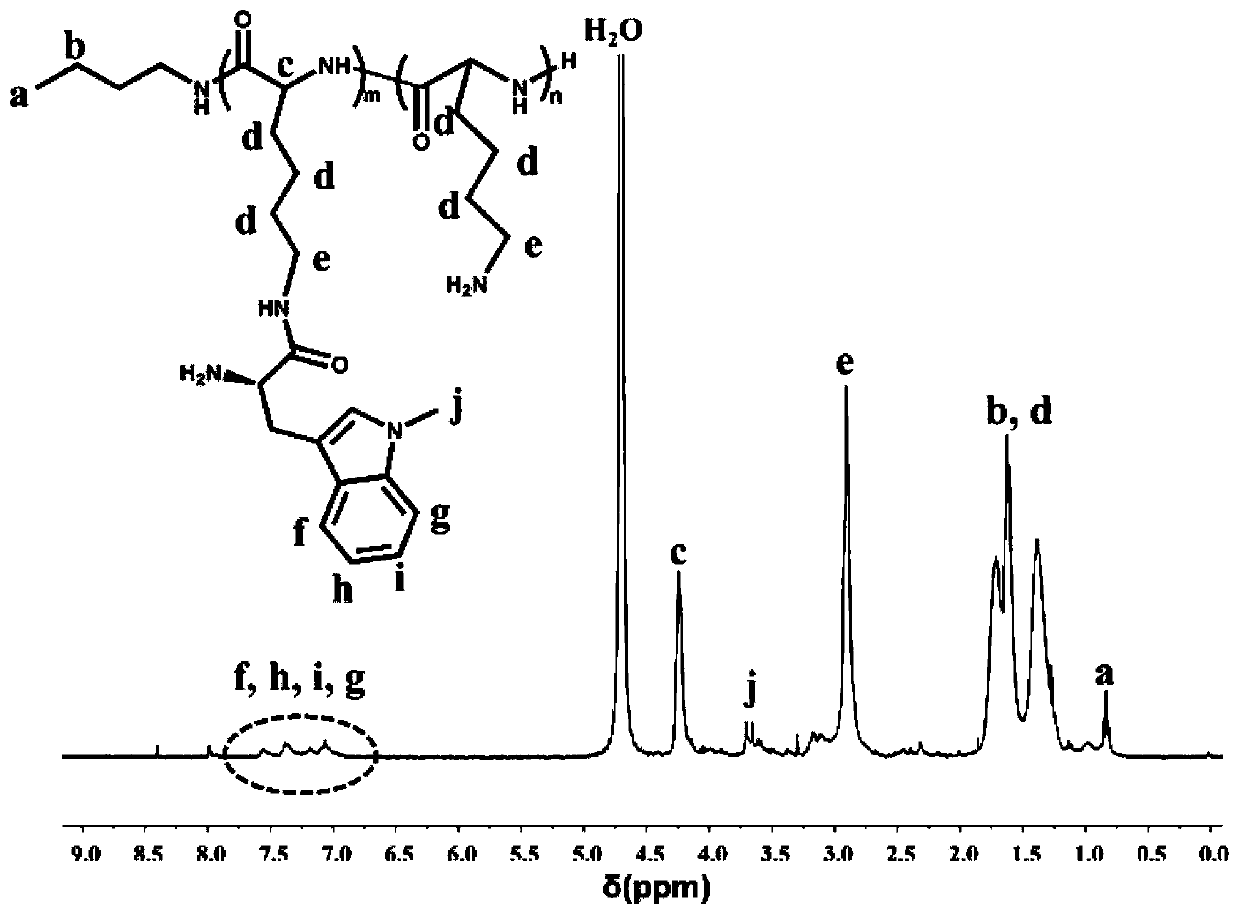
[0060] Embodiment 2: proton nuclear magnetic resonance spectrum ( 1 H-NMR) to identify the molecular chemical structure of PLL-1-mt.
[0061] Weigh about 5 mg of PLL-1-mt prodrug and heavy water (D 2 O) dissolved and placed in a nuclear magnetic tube, using 400MHz proton nuclear magnetic resonance spectrum to measure its hydrogen nuclear magnetic resonance spectrum, using tetramethylsilane as an internal standard, and recording the chemical shift value (ppm) of the compound. The result is as figure 1 As shown, the NMR results can confirm that the characteristic peaks of PLL and 1-mt appear simultaneously in the newly synthesized molecule. pass 1 H-NMR spectrum can confirm the successful synthesis of PLL-1-mt molecule. Through the nuclear magnetic analysis of the PLL, it is obtained that m+n is 44, and through the ultraviolet spectrum analysis, it is obtained that m is 4 and n is 40.
Embodiment 3
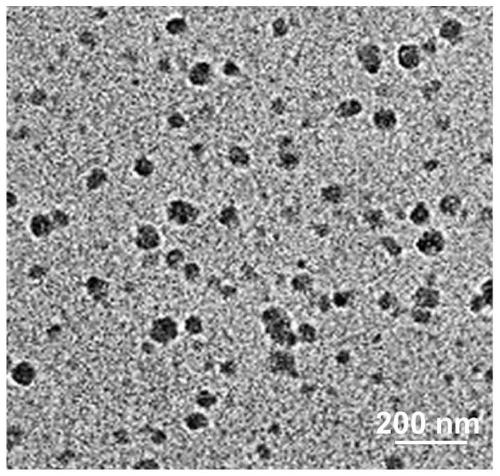
[0062] Example 3: Preparation of aPD-L1@HC / PM nano vein preparation.
[0063] Accurately weigh 2 mg of HA-Ce6 molecule, dissolve it in 1.5 mL of water, and precisely weigh 0.5 mg of PLL-1-mt molecule, and dissolve it in 0.5 mL of water. Under ultrasonic conditions, the PLL-1-mt aqueous solution was added dropwise to the HA-Ce6 aqueous solution, and after reacting for 30 minutes, the nanocomposite (HC / PM ), and then post-adsorption of aPD-L1 monoclonal antibody. Disperse aPD-L1 in 10 μL PBS, then slowly drop it into the HC / PM nanocomposite solution using a stirrer under ice bath, and let stand for 10 minutes to obtain aPD-L1@HC / PM nanocomposite. Among them, HA-Ce6 references W.J.Li, C.F.Zheng, Z.Y.Pan, C.Chen, D.H.Hu, G.H.Gao, S.D.Kang, H.D.Cui, P.Gong, L.T.Cai, Smarthyaluronidase-activedtheranostic micelles for dual-modal imaging guided photodynamic therapy .Biomaterials 2016,10,10-19. Preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com