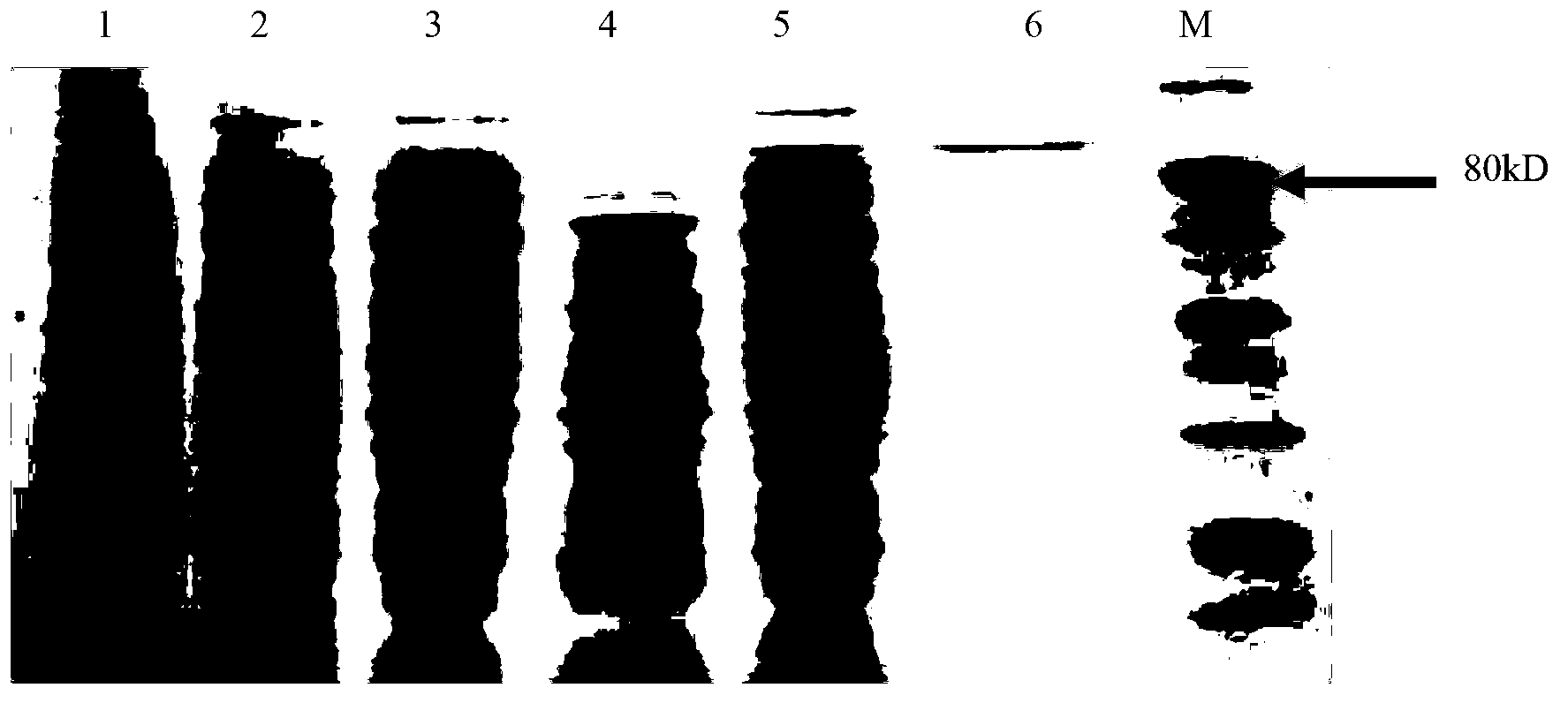Neutral alpha-galactosidase M-GALC, and coding gene and application thereof
A technology of galactosidase and gene, applied in the field of neutral α-galactosidase M-GALC and its coding gene and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, the acquisition of target protein M-GALC and its coding gene
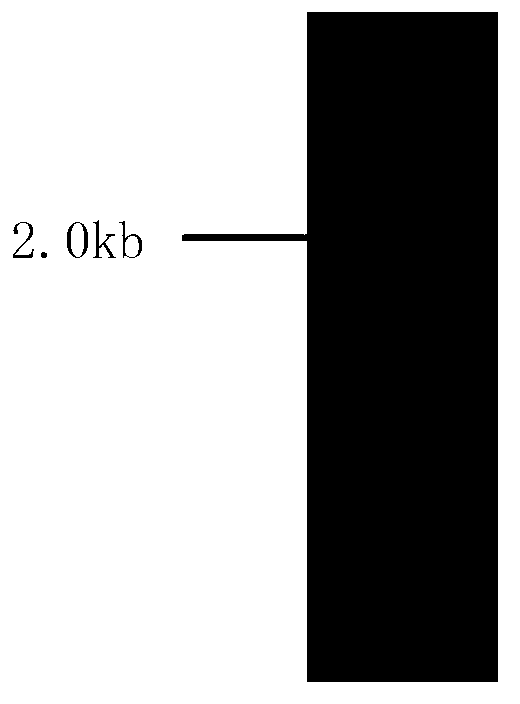
[0040] A chaetomium globosom strain MK was screened from soil samples collected from a soybean product processing factory. Using the genomic DNA of the strain as a template, PCR amplification was performed with a pair of degenerate primers designed by ourselves, and a length of 0.8 The bp DNA fragment was used as a template, and Tail-PCR was performed with self-designed nested primers and degenerate primers to obtain a 3800bp gene fragment, the nucleotide sequence of which was shown in sequence 3 of the sequence listing. The sequence was analyzed to find an open reading frame of 2244bp, and its nucleotide sequence is shown in sequence 2 of the sequence listing.
[0041] The total RNA of the above-mentioned chaetomium globosom strain MK was extracted, and cDNA was obtained by reverse transcription; using the cDNA as a template, the following primers were used for PCR amplification:
[0042] QF:...
Embodiment 2
[0045] Embodiment 2, the preparation of target protein M-GALC
[0046] 1. Construction of recombinant expression vector
[0047] The total RNA of the above-mentioned chaetomium globosom strain MK was extracted, and cDNA was obtained by reverse transcription; using the cDNA as a template, the following primers were used for PCR amplification:
[0048] ZYF: 5'- GAATTC ATGAGTTTCGCTTCAATGTC-3' (ECOR I recognition sequence is underlined);
[0049] ZYR: 5'- GCGGCCGC CTACTGCCTCTCGAGCAGGA-3' (not I recognition sequence is underlined);
[0050]The PCR product was recovered and sequenced to obtain a DNA fragment connected to the 5' end of sequence listing sequence 2 with the ECOR I restriction recognition sequence and the 3' end with the Not I restriction recognition sequence. After the DNA fragment was double-digested with EcoR I and Not I, it was connected to the vector backbone fragment of the prokaryotic expression vector pET-30a(+) (purchased from Novegon) that had been cut w...
Embodiment 3
[0083] Example 3, Characterization of target protein M-GALC as α-galactosidase
[0084] In this example, the eluate obtained from the recombinant bacterium X in step 3 of Example 2 was used as the detection object.
[0085] 1. Optimum temperature and temperature stability
[0086] 1. Optimum temperature
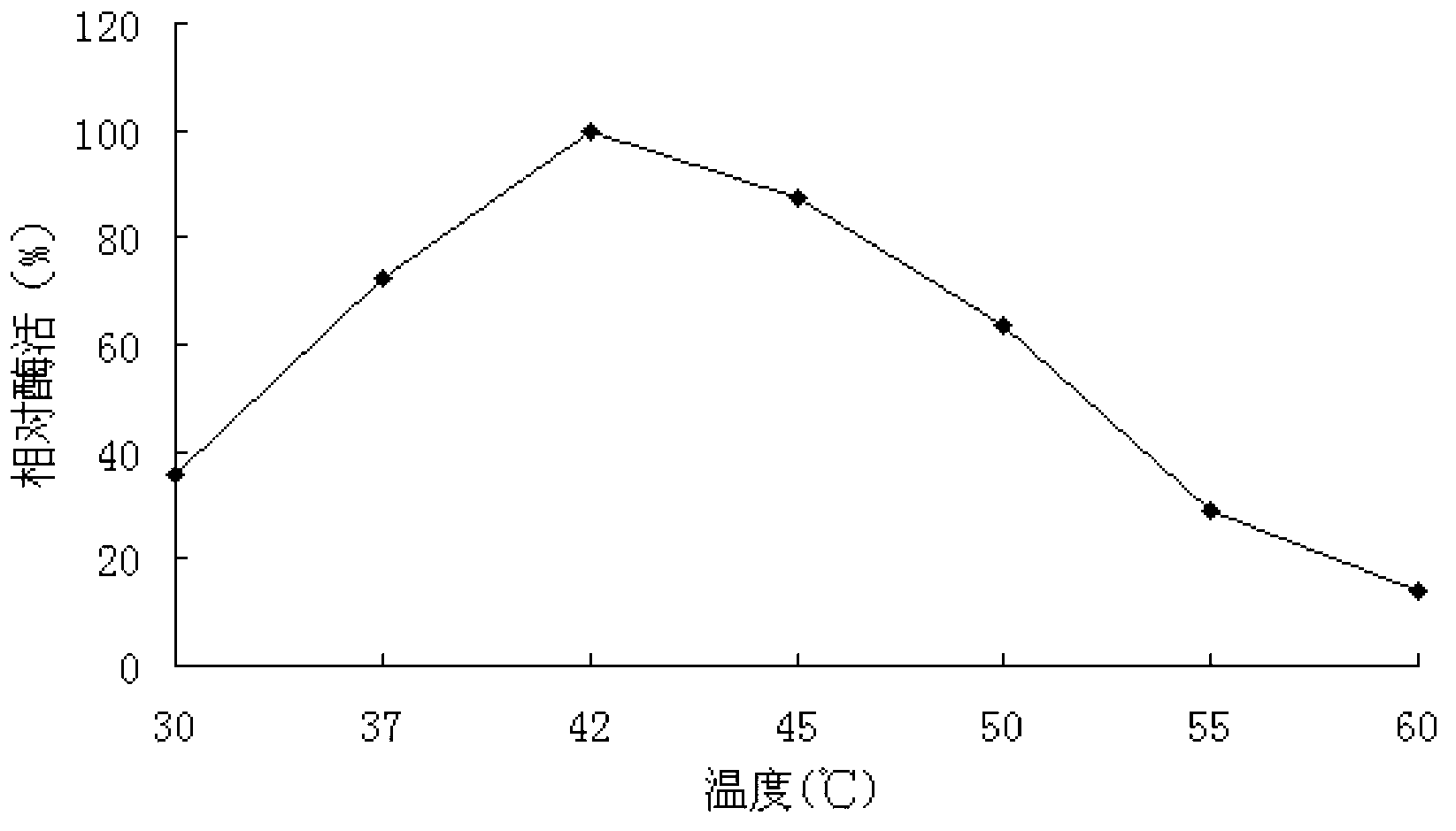
[0087] Carry out according to the method in step 6 of Example 2, the difference lies in: the α-galactosidase enzyme activity is measured under the condition of temperature of 30, 37, 42, 45, 50, 55, 60 or 65°C.
[0088] When the temperature is 42°C, the measured enzyme activity is 0.35U / ml, which is recorded as 100%, and the percentage of the measured enzyme activity relative to 0.35U / ml under different temperature conditions is calculated, that is, the relative enzyme activity (%) . The experiment was repeated 3 times, and the results were averaged, such as image 3 shown.
[0089] image 3 Among them, when the reaction temperatures were 30, 37, 42, 45, 50, 55, and 60°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com