Preparation and application of myoglobin monoclonal antibody
A myoglobin and single-chain antibody technology, which is applied in the field of eukaryotic expression vectors expressing myoglobin monoclonal antibodies and early diagnosis of acute myocardial infarction. Large discrepancies, poor detection accuracy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
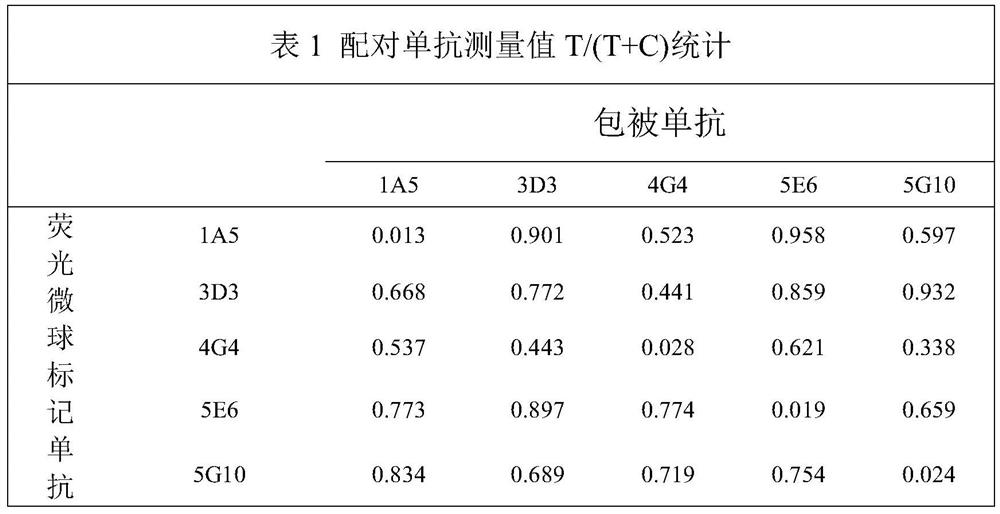
Image
Examples
Embodiment 1
[0007] Example 1: Myoglobin (Myo) dominant antigen epitope selection
[0008] Taking myoglobin (Myo) as the target antigen, the hydrophilicity and antigenicity of its epitope sequence were analyzed by using the biological software DNAssist2.0, and the A-dominant epitope and the B-dominant epitope were selected. At the same time, the results of sequence comparison showed that the selected two dominant epitope sequences of A and B have a broad spectrum and are the common epitopes of all myoglobin (Myo); and the A and B epitopes have no obvious identity with other protein sequences. Origin, only exists in the myoglobin (Myo) sequence.
Embodiment 2
[0009] Example 2: Concatenation of myoglobin (Myo) dominant antigenic epitopes
[0010] In order to enhance the stimulation of the selected antigenic epitope to the immune system of mice so as to facilitate subsequent experiments, the two dominant antigenic epitope sequences of A and B of myoglobin (Myo) were respectively repeated and then passed through the flexible fragment (four consecutive Glycine) connection to obtain the amino acid sequence of the recombinant protein.
Embodiment 3
[0011] Embodiment 3: optimize the nucleotide sequence of encoding recombinant Myo protein
[0012] In order to increase the expression of recombinant protein in Escherichia coli, under the premise that the amino acid sequence of the recombinant protein remains unchanged, the amino acid sequence encoding the recombinant protein is converted into the corresponding nucleotide sequence according to the preferred codons of Escherichia coli, and the upstream and downstream After adding the nucleotide sequences corresponding to the restriction sites BamHI and EcoRI respectively, they were synthesized by General Biosystems Anhui Co., Ltd. The synthesized target gene was cloned into the pMD25-T vector (Bao Bioengineering Dalian Co., Ltd.).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com