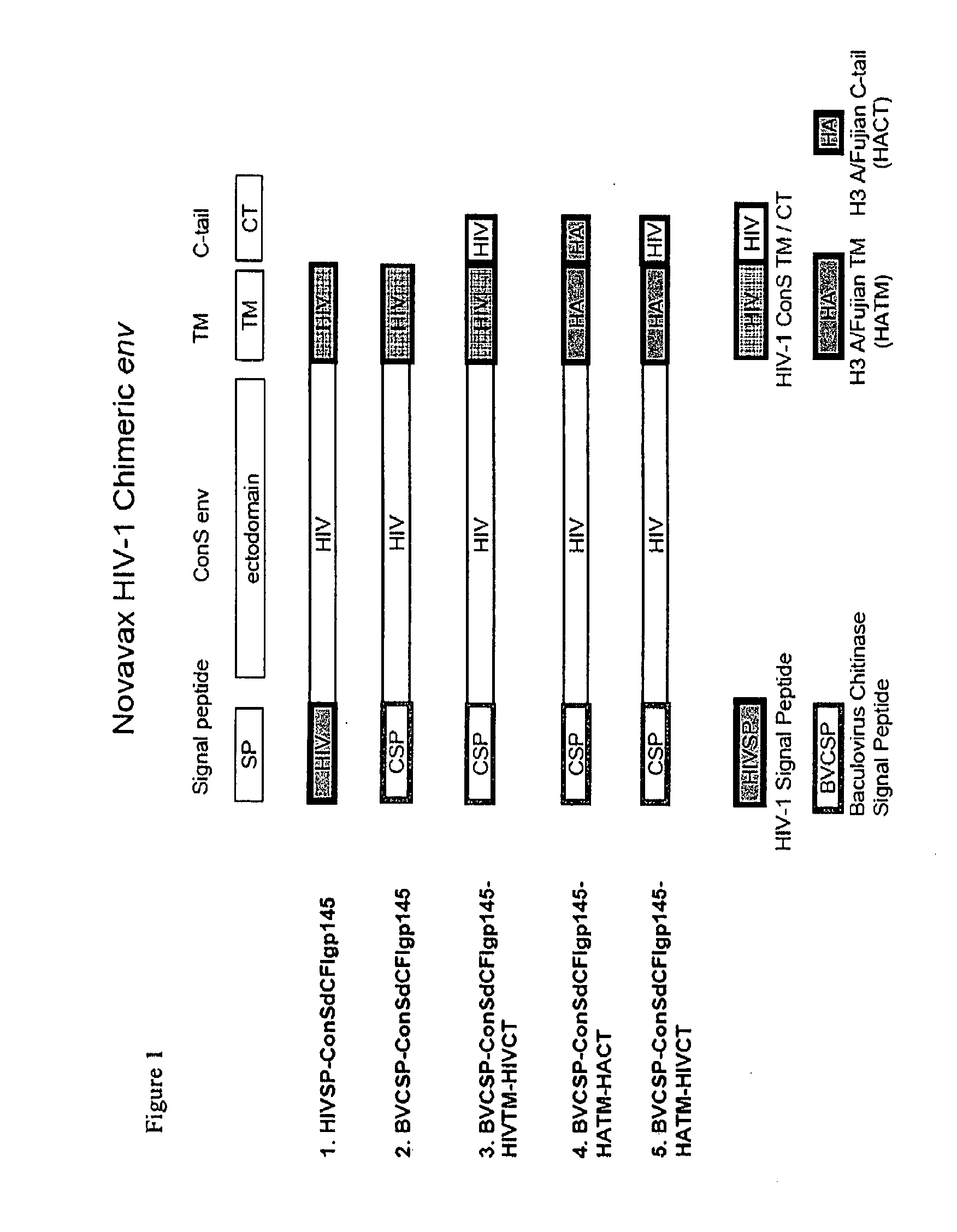
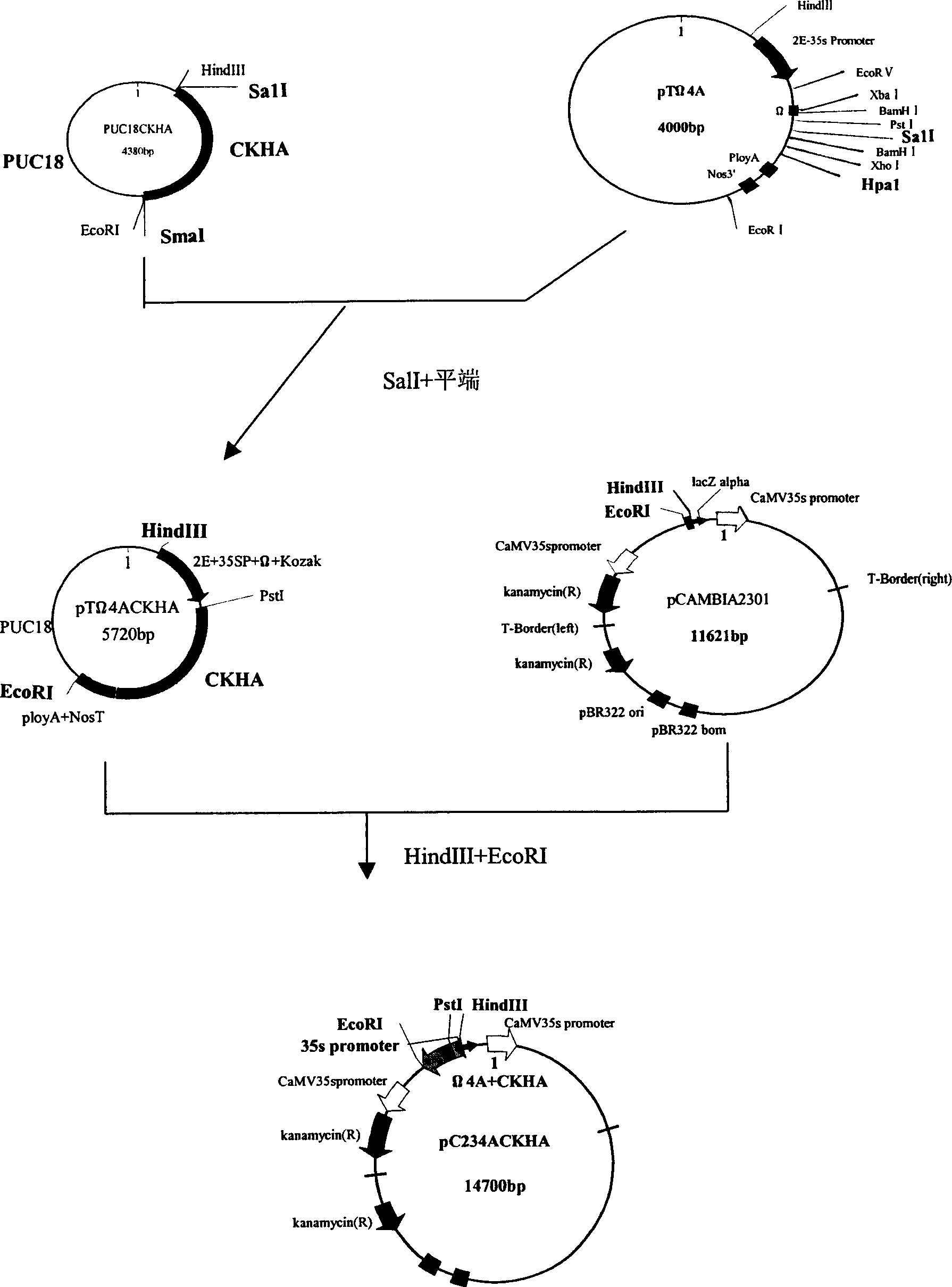
Patents
Literature
159 results about "Hemagglutinin protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
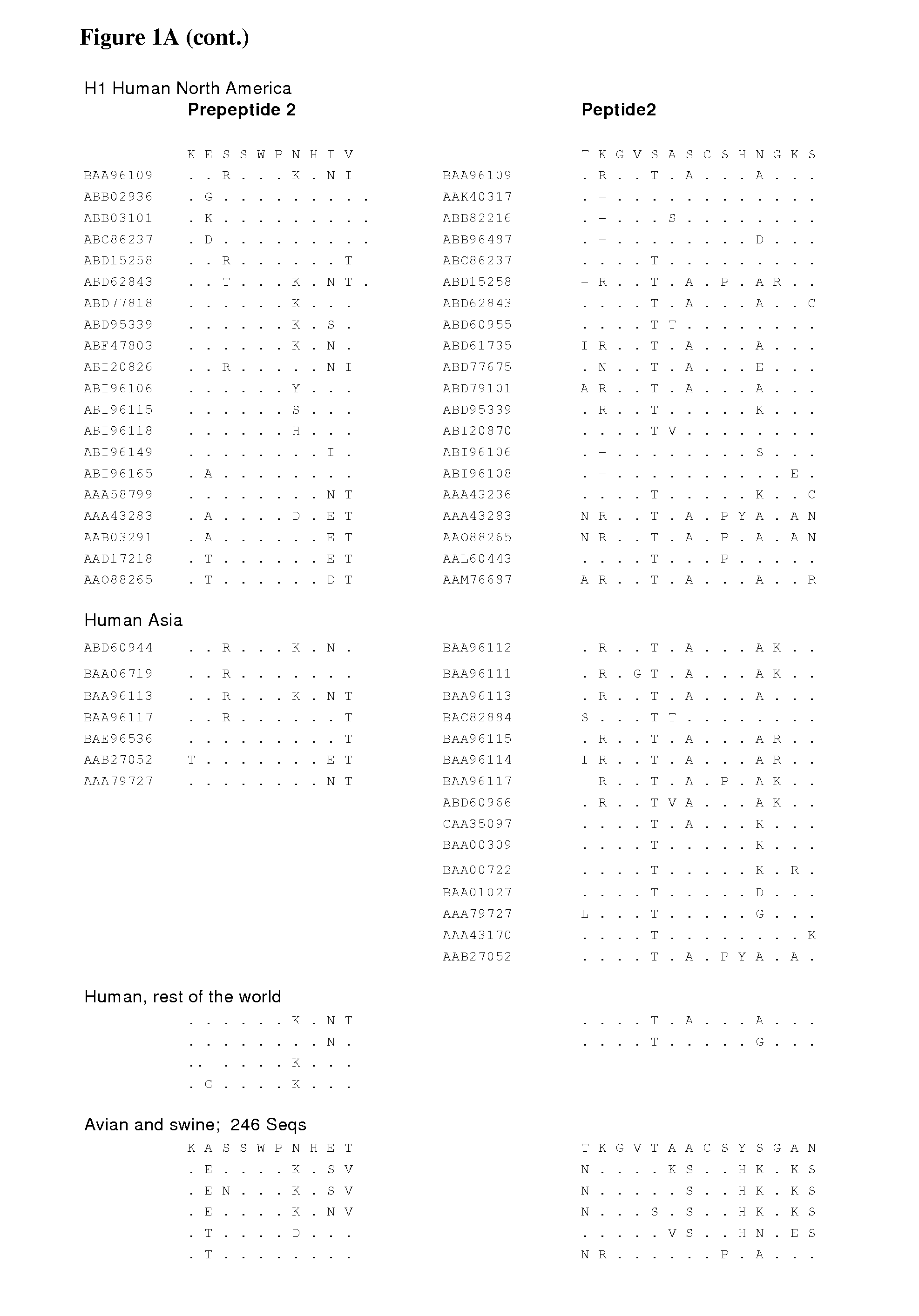
Hemagglutinin is a trimer protein composed of a globular domain and a stem domain, divided along the longitudinal axis of the protein .
H5 subtype avian flu virus hemagglutinin protein monoclonal antibody, and its preparing method and use
This invention relates to a monoclonal antibody capable of combining with H5 subtype avian influenza virus HA protein specifically, the hybridoma cell line secreting said antibody and a preparing method. The invention also relates to a serial test kit for testing H5 subtype avian influenza virus by the antibody and a bit of said antibody in the test sample of the virus and its usage in treatment.
Owner:XIAMEN UNIV
Replikin peptides in rapid replication of glioma cells and in influenza epidemics
Peptides of influenza virus hemagglutinin protein and Plasmodium falciparum malaria antigen, antibodies specific for the peptides, influenza vaccines, malaria vaccines and methods of stimulating the immune response of a subject to produce antibodies to influenza virus or malaria are disclosed. Also disclosed are methods for formulating vaccines for influenza virus.
Owner:BOGOCH SAMUEL +1
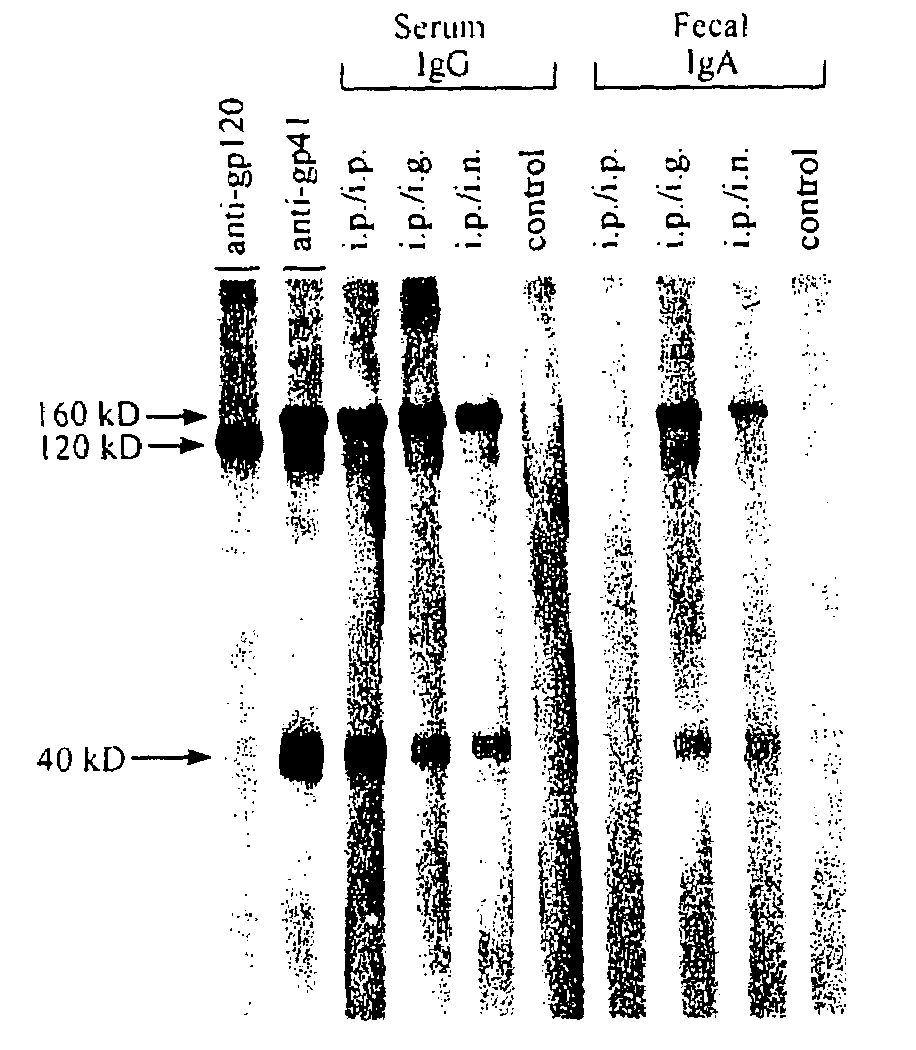
Fusion protein construct and method for inducing HIV-specific serum IgG and secretory IgA antibodies in-vivo
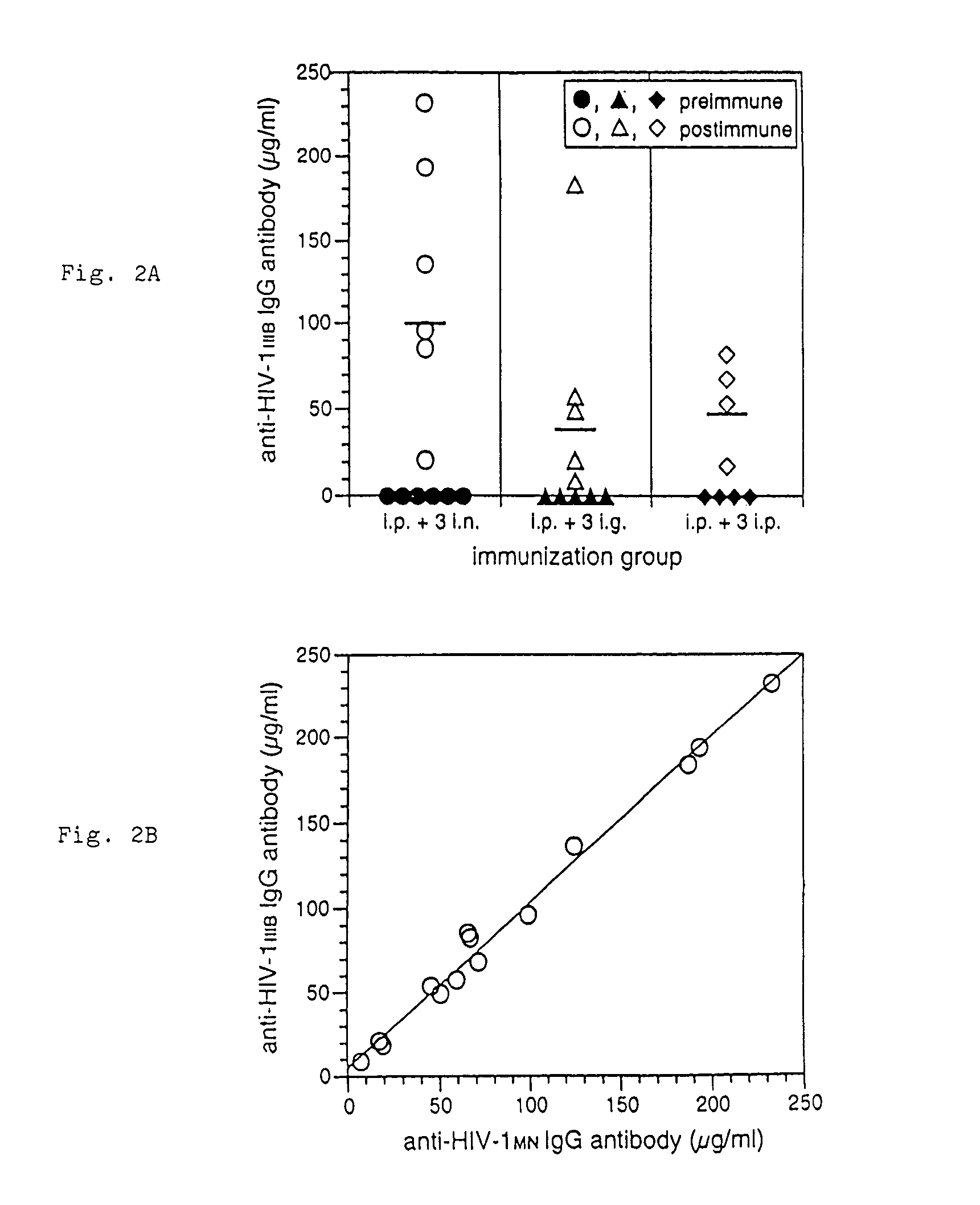
The present invention provides a fusion protein construct (gp41HA) consisting of the ectodomain of the HIV-1IIIB envelope glycoprotein gp41 fused to a fragment of the influenza virus HA2 hemagglutinin protein. Immunization in-vivo via an intraperitoneal prime followed by intranasal or intragastric boosts with gp41HA induces high concentrations of serum IgG antibodies and fecal IgA antibodies that reacted with gp41 in HIV-1IIIB viral lysate and are cross-reactive with gp41 in HIV-1MN lysate. Followup analyses by indirect immunofluorescence showed that both serum IgG and fecal IgA recognized human peripheral blood mononuclear cells infected with either syncytium-inducing (SI) or non-syncytium-inducing (NSI) North American HIV-1 field isolates, but not uninfected cells.
Owner:CHILDRENS MEDICAL CENT CORP
Methods of enhancing protein incorporation into virus like particles
InactiveUS20100143406A1Increase incorporationPolypeptide with localisation/targeting motifSsRNA viruses negative-senseVirus-like particleProtein incorporation
The present invention comprises a method of increasing glycoprotein incorporation on the surface of VLPs, comprising expressing a nucleic acid encoding a chimeric glycoprotein in a host cell, wherein said chimeric glycoprotein comprises the transmembrane domain of an influenza hemagglutinin protein. The invention also embodies specific VLPs comprising said chimeric glycoproteins and methods of inducing immunity in an animal utilizing said VLPs.
Owner:NOVAVAX
H9 subtype avian influenza virus isolate and vaccine composition prepared thereby
ActiveCN103789272AGood effectImprove immune efficiencyMicroorganism based processesAntiviralsHemagglutininAvian influenza virus
The invention discloses an avian influenza virus isolate belonging to an H9 subtype. An amino acid sequence of an HA1 structural domain of hemagglutinin has the following characteristic sites: 69-bit P, 180-bit A, 221-bit N and 236-bit R; the vaccine composition prepared by the H9 subtype avian influenza virus isolate with the following characteristic sites has good immune efficiency, and is superior to the vaccine prepared by the strain in the prior art in effect, cross protection can be provided for a popular wild strain, significant cross immunogen features are displayed, and the avian influenza virus isolate has a good application prospect in the aspect of preventing and treating poultry cross immune protection.
Owner:PU LIKE BIO ENG +1
Gene encoding hemagglutinin protein of H5 avian influenza virus and its application
ActiveCN1632124AHigh level of immune responseImproving immunogenicityViral antigen ingredientsAntibody ingredientsHemagglutininHighly pathogenic
The present invention relates to an artificially synthesized gene optiHA containing codons for chicken partial tropism. Its reading frame contains 1707 bp nucleotides and encodes a total of 568 amino acids. The gene is compatible with H5 subtype highly pathogenic avian influenza virus A / Goose / GuangDong / 1 / 96(H5N1)[GD / 1 / 96(H5N1)]hemagglutinin (HA) gene has a nucleotide homology rate of 70%, an amino acid homology rate of 100%, and encodes the H5 subtype Hemagglutinin (HA) protein of avian influenza virus GD / 1 / 96 (H5N1). The invention also relates to the application of the gene as an immunogenic gene of H5 subtype influenza DNA vaccine and other genetic engineering vaccines.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Kit for detecting peste des petits ruminants virus hemagglutinin protein antibody and application method of kit
InactiveCN105158480AGood response specificityHigh sensitivitySsRNA viruses negative-senseVirus peptidesAntigenCrop livestock
The invention provides a kit for detecting a peste des petits ruminants virus hemagglutinin protein (H protein) antibody. The kit takes recombinant protein of extracellular region sequence code of peste des petits ruminants virus hemagglutinin protein gene as an envelope antigen. The invention further provides an application method of the kit. The kit can quickly specifically detect the peste des petits ruminants virus H protein antibody in serum, and is good in reaction specificity and high in sensitivity. The operation is simple; the cost is low; and the reaction result is stable and reliable, and is easy to observe. The kit is suitable for monitoring the vaccine immunity effect of import and export immunity, food sanitation, and a livestock farm of peste des petits ruminants.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Peptide vaccine for influenza virus
The invention provides peptide epitopes for use in the prevention and / or treatment of influenza or for the development of such treatment or vaccine against influenza. The invention also relates to a method for evaluating the potential of a chemical entity, such as an antibody, to bind to a peptide epitope derived from the divalent sialoside binding site of hemagglutinin protein of influenza virus, and to conjugates containing one or more such peptide epitopes. The peptide epitopes of the invention are cyclic peptides comprising a 7-mer peptide derived from H1, H3 or H5 hemagglutinin of influenza virus. The 7-mer peptide has a sequence corresponding to the loop sequence at positions 220-226 of X31-hemagglutinin.
Owner:GLYKOS FINLAND
Turkey herpesvirus vectored recombinant containing avian influenza genes
InactiveUS20080241188A1Easy to distinguishEasy to detectSsRNA viruses negative-senseVectorsVaccinationElisa kit
The present invention provides a recombinant turkey herpesvirus modified by the presence of the cDNA encoding the hemagglutinin protein of avian influenza virus under a promoter. A poultry vaccine comprising the recombinant turkey herpesvirus described in the present invention can induce serological responses that may be easily detected by the hemagglutination inhibition assay but not by commercially available diagnostic ELISA kits; thus enabling easy differentiation between vaccination and field infection.
Owner:ZEON CORP +1
Modified influenza hemagglutinin proteins and uses thereof
The present invention is generally directed to modified influenza hemagglutinin (HA) proteins and methods for making and using them, including their use in immunogenic compositions such as vaccines for the treatment and / or prevention of influenza infection.
Owner:NOVAVAX
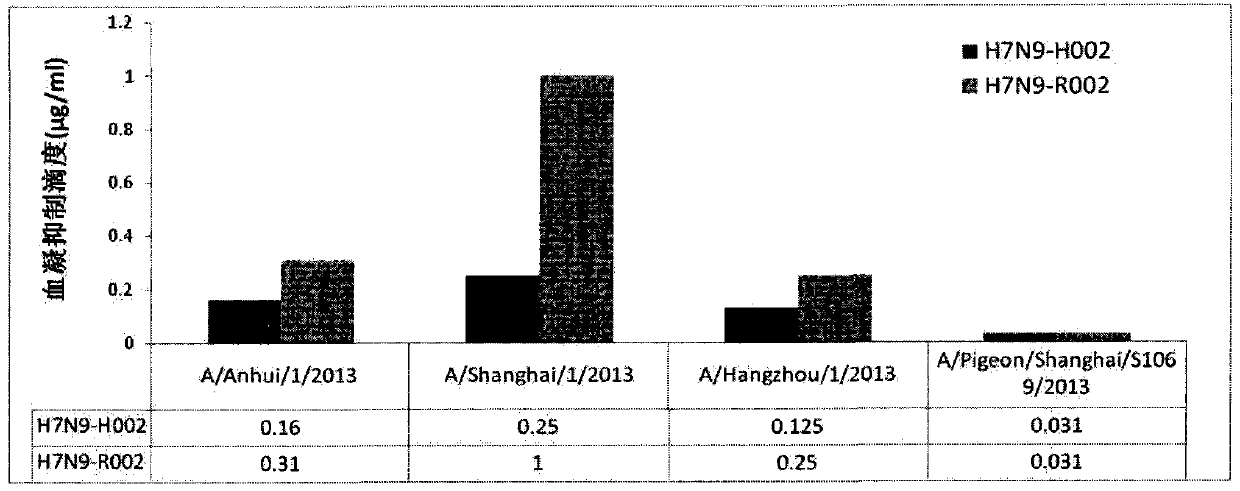
Antibody neutralizing human infected H7N9 influenza A virus and use thereof
ActiveCN104892753AGenetic material ingredientsBiological material analysisAntibody fragmentsInfluenza virus A hemagglutinin
Provided is a neutralising antibody which binds to and neutralises an H7 type influenza A virus. The nucleotide sequences of the light and heavy chain variable regions of the antibody are at least 75% identical to the nucleotide sequence of any one of SEQ ID NO: 38-55, 58 or 59, and the antibody can neutralise a human infection H7N9 influenza A virus haemagglutinin protein. Also provided is an efficient expression method for integrating an antibody into a cell such as a CHO cell, and a use for the antibody, a related haemagglutinin protein antigen binding fragment and an epitope in the diagnosis, treatment and prevention of human infection H7N9 influenza A virus infection.
Owner:SINO CELL TECH INC
Novel DNA sequences, vectors and proteins of avian influenza hemagglutinin
InactiveUS20090106864A1Wide range of activitiesBroad protectionSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininHeterologous
The subject invention provides novel amino acid sequences (including a consensus sequence) of the Avian Influenza A virus hemagglutinin protein. These newly constructed genes are designed to provide a broader spectrum of activity across the serotype family thus providing a basis for a vaccine that has broad heterologous disease protection. The novel genes have been further improved by the addition of strategic glycosylation sites into the amino acids sequences that they encode. These genes can also, optionally, be codon optimized for plant expression, inserted into the appropriate vector and cloned into plants for expression. Polypeptides produced by recombinant host cells or transgenic plants can also be used as source of antigen for the formulation of vaccines for the control of influenza in susceptible individuals. Additionally, transgenic plant material may also be used as source of antigen for the formulation of vaccines for the control of influenza in susceptible individuals.
Owner:DOW AGROSCIENCES LLC
Structure and application of influenza virus hemagglutinin protein binding polypeptide
InactiveCN102268072ACytopathic inhibitionNo obvious toxicityPeptide/protein ingredientsPeptidesDiseaseHemagglutinin
Belonging to the technical field of biomedicine, the invention relates to a sequence and structure of a polypeptide able to specifically bind with influenza virus hemagglutinin protein, and application of the polypeptide in anti-influenza viruses. By expressing purified influenza virus hemagglutinin protein and screening a random peptide library with a phage display technology, a polypeptide specifically bound with influenza virus hemagglutinin and equipped with sequences numbered 1-18 can be obtained. As a hemagglutinin-binding peptide can hinder the combination of hemagglutinin and a host cell receptor, so the influenza virus can be inhibited from infecting the host cell. Thus, the invention also conducts an anti-influenza virus activity study to the hemagglutinin-binding peptide selected from the phase peptide library, and finds that a polypeptide H17, with a sequence of NH2-SHGRITFAYFAN-COOH, can effectively inhibit the influenza virus from infecting the host cell and is of small toxicity. Therefore, the hemagglutinin-binding peptide of the invention and the H17 polypeptide therein with an anti-influenza virus activity are expected to become novel treatment medicaments for treating diseases caused by influenza virus infection and reducing the hazards of diseases caused by influenza viruses.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Recombinant modified vaccinia virus measles vaccine
InactiveUS20110052627A1Improve toleranceMinor side effectsSsRNA viruses negative-senseViral antigen ingredientsHemagglutinin proteinVaccinia
The invention concerns methods, compositions and kits for use in preparing a medicament and vaccine for measles virus comprising an Attenuated Modified Vaccinia Virus Ankara (MVA) strain encoding hemagglutinin protein, fusion protein, and nucleoprotein of measles virus (MVA-Measles). The recombinant virus induced superior cellular and humoral responses to the measles virus when compared to Measles vaccine Rouvax®. Both T cell and B cell immune responses to the recombinant MVA were observed not only in adult animals, but also in newborn and juvenile animals. Results in adult humans showed that MVA-Measles induces a strong immune response, is safe and well tolerated.
Owner:BAVARIAN NORDIC AS
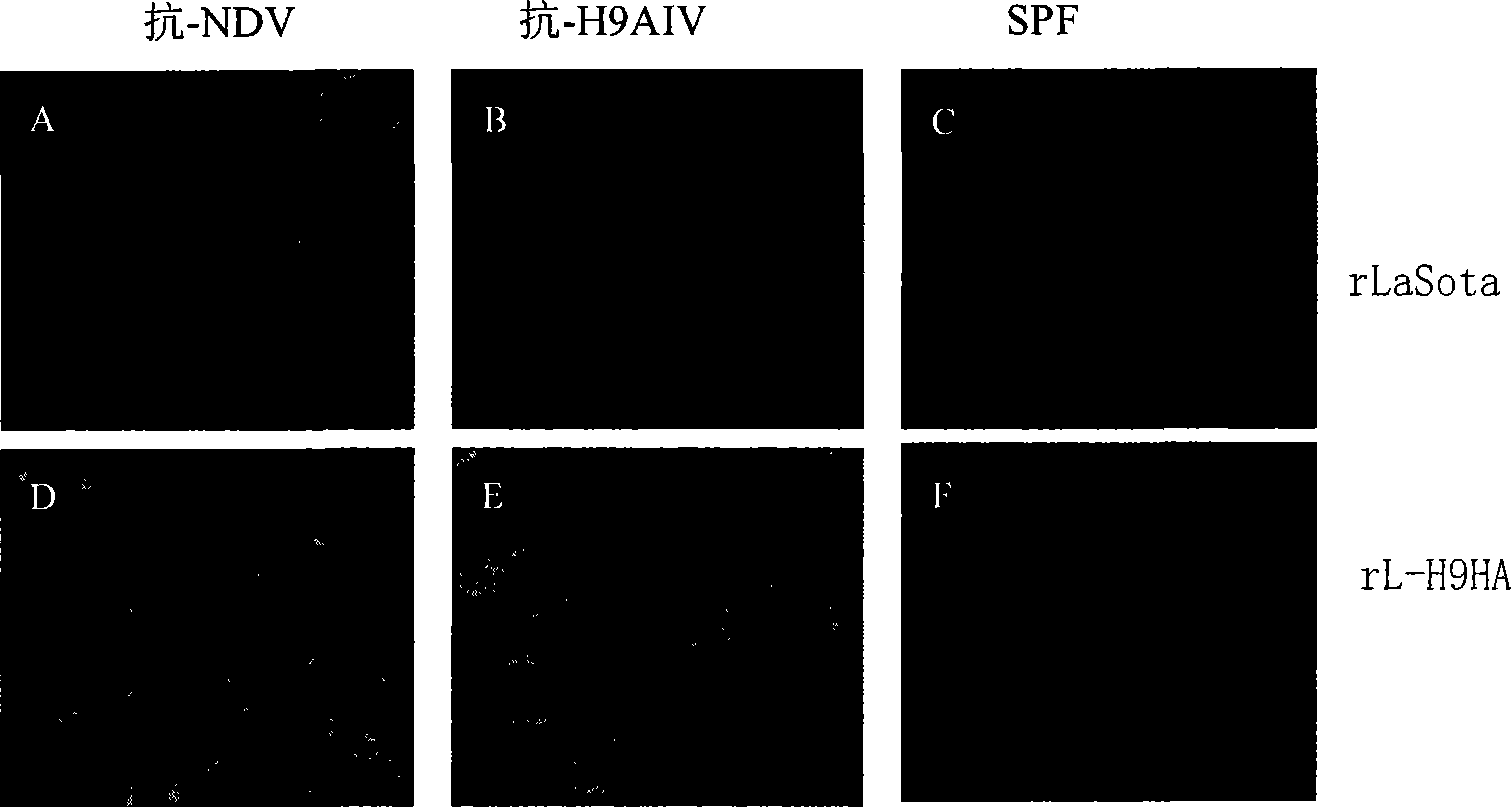
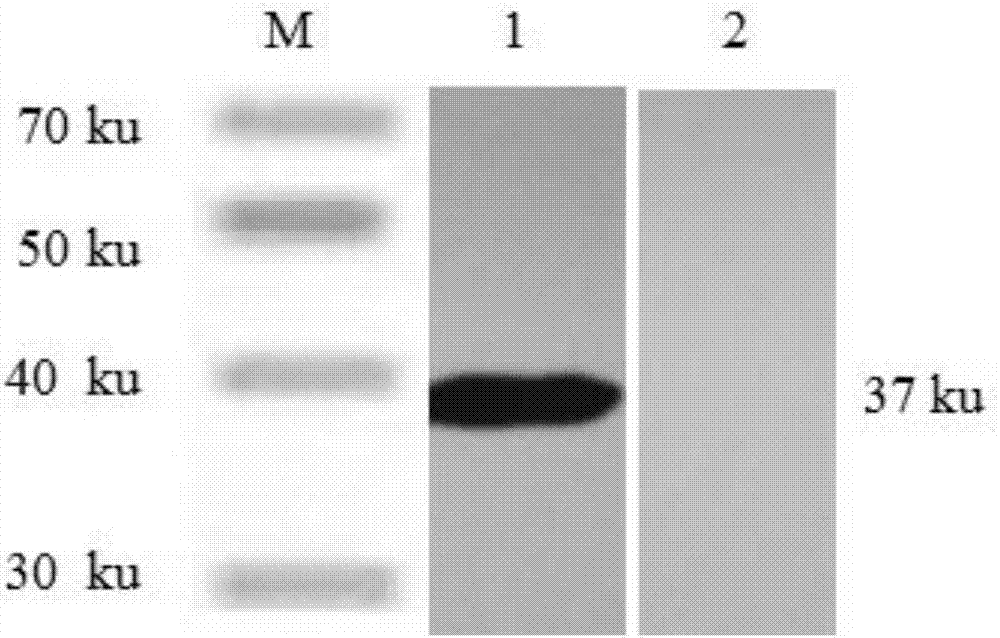
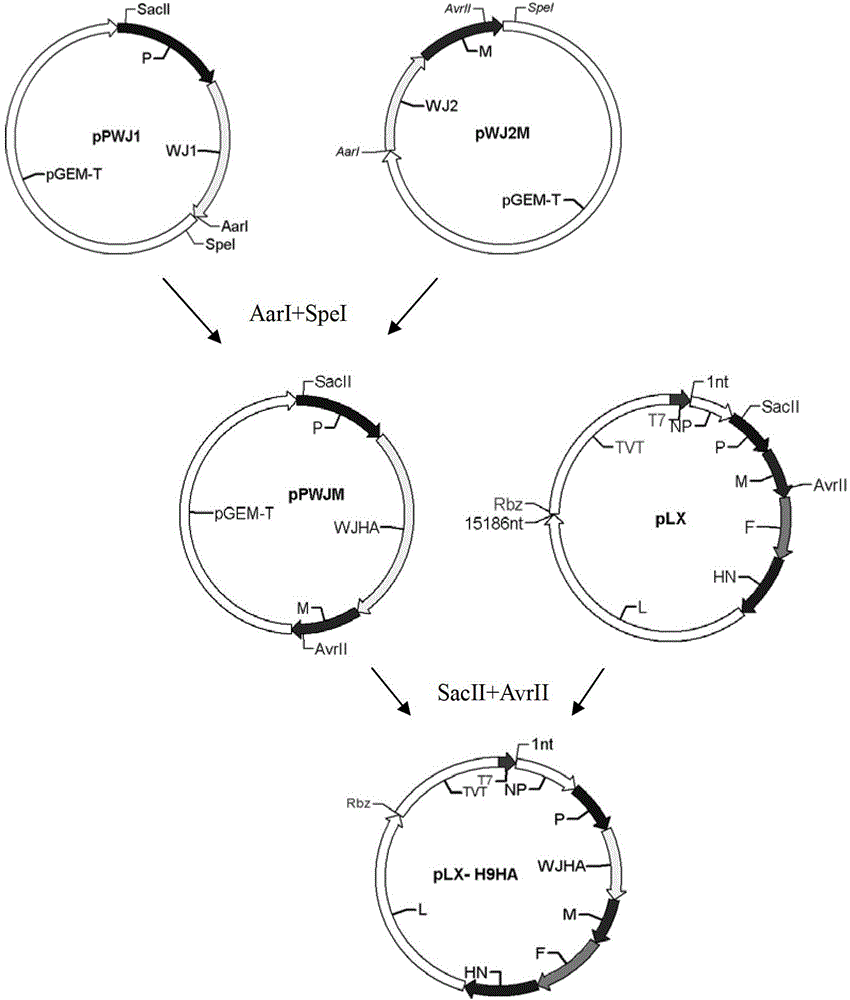
Recombined newcastle disease virus LaSota attenuated vaccine strain for expressing avian influenza virus H9 subtype HA protein
The invention relates to a recombinant newcastle pestilence LaSota HCLV expressing the bird flu virus H9 subtype hemagglutinin (HA) protein, in particular the recombinant newcastle pestilence LaSota is rL-H9HA. The invention further discloses a method for preparing the recombinant newcastle pestilence LaSota HCLV and the application of the HCLV in preparing a bivalent vaccine for preventing bird flu and newcastle pestilence.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Broad spectrum monoclonal antibody for identification of influenza virus hemagglutinin protein HA1 structural domains
The invention relates to an antibody for identification of epitopes on influenza virus hemagglutinin protein HA1 structural domains, a cell strain generating the antibody and the use of the antibody and the cell strain. The antibody is can cross the HA subtype for specific binding of the hemagglutinin (HA) protein HA1 structural domains of H1 subtype (including seasonal H1N1 and new H1N1) and H5 subtype influenza virus. Therefore, the invention also relates vaccines or pharmaceutical compositions which comprise the antibody and are used for prevention and / or treatment of H1 subtype and H5 subtype influenza virus infection and / or diseases (such as the flu) induced by the H1 subtype and H5 subtype influenza virus.
Owner:XIAMEN UNIV
Avianinflu virus H5 subtype emulsion agglutination kit and its use
ActiveCN1978634AStrong specificityHigh sensitivityImmunoglobulins against virusesFused cellsPositive controlHemagglutinin protein
This invention discloses a latex agglutination kit to quickly detect H5 subtype of avian influenza virus. The anti monoclonal antibodies of H5 subtype of avian influenza virus hemagglutinin protein were coupled to the surface of carboxyl polystyrene latex particles using water-soluble carbodiimide, the methods about detection of H5 subtype of avian influenza virus were established, the latex agglutination detection kits of H5 subtype of avian influenza virus were prepared supplemented by other matching reagents. This kit was made up of box body and the body latex diagnostic reagents in the box, sample handling liquid A, sample handling liquid B, the positive control samples and negative control samples. This invention kit can directly detect the H5 subtype avian flu virus, with high specificity, high sensitivity, simple and rapid diagnosis, and other significant advantages.
Owner:HUAZHONG AGRI UNIV
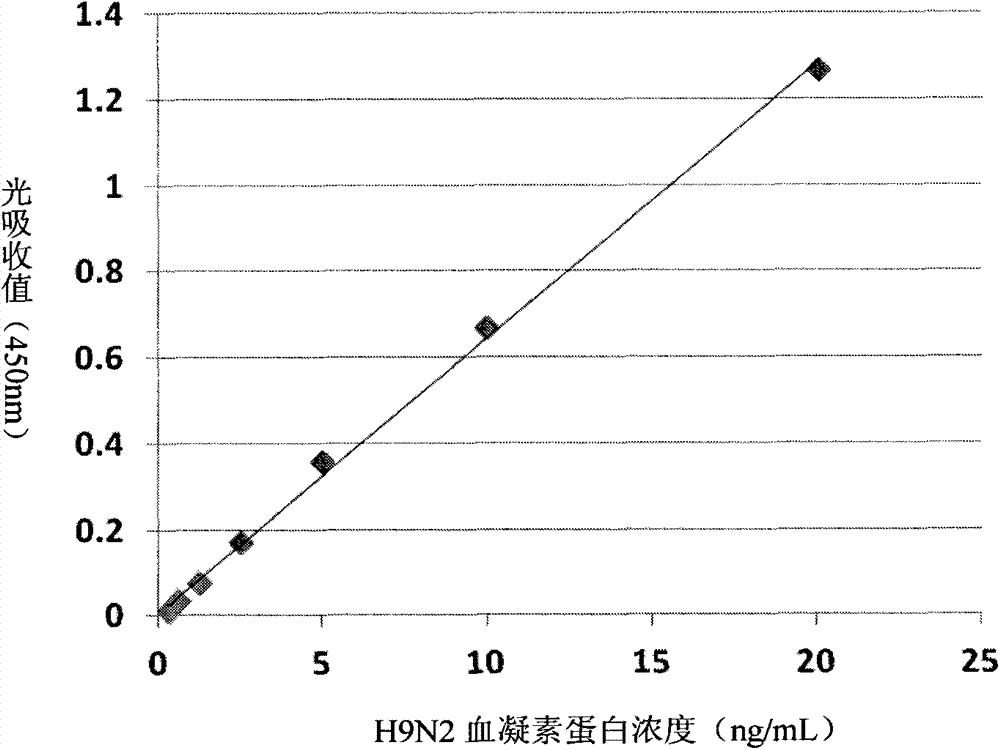
ELISA kit for H9N2 influenza virus hemagglutinin protein
ActiveCN104749372AQuick checkThe inspection method is convenient and easyBiological material analysisBiological testingElisa kitVirus influenza
The invention discloses a double-antibody sandwich ELISA kit for H9N2 influenza A virus hemagglutinin protein. The kit comprises a solid phase vector coated with a monoclonal antibody, a rabbit polyclonal antibody labeled by horseradish peroxidase, an H9N2 hemagglutinin protein standard substance, a sample diluting liquid, a washing liquid, a substrate coloring liquid and a reaction terminating liquid. The kit is good in sensitivity, capable of performing quantitative detection for the H9N2 influenza virus hemagglutinin protein and specifically recognizing H9N2 influenza A viruses, and free of cross reactions with hemagglutinin protein of other main subtypes comprising H1N1, H2N2, H3N2, H5N1 and H7N7 of the influenza A viruses and hemagglutinin protein of influenza B viruses. The kit is simple to operate and capable of rapidly detecting a large number of samples simultaneously, can be used for supporting fundamental research of the H9N2 influenza viruses, and has important significance for performing epidemiologic study on influenza viruses.
Owner:北京义翘神州科技股份有限公司
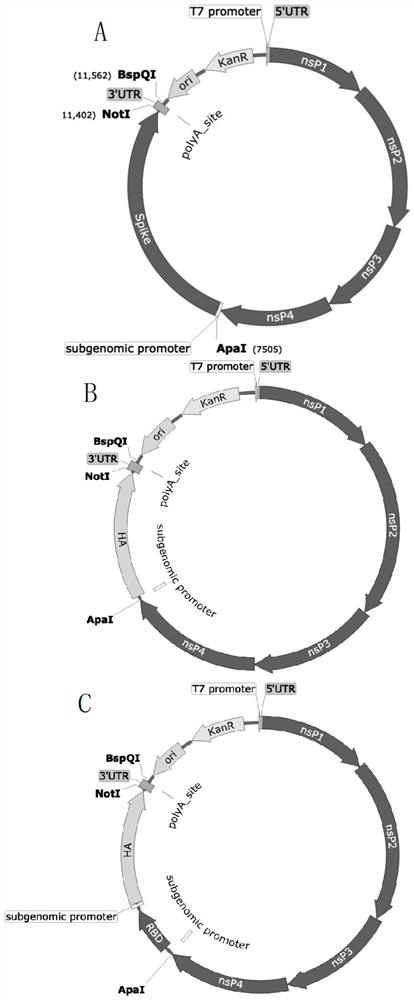
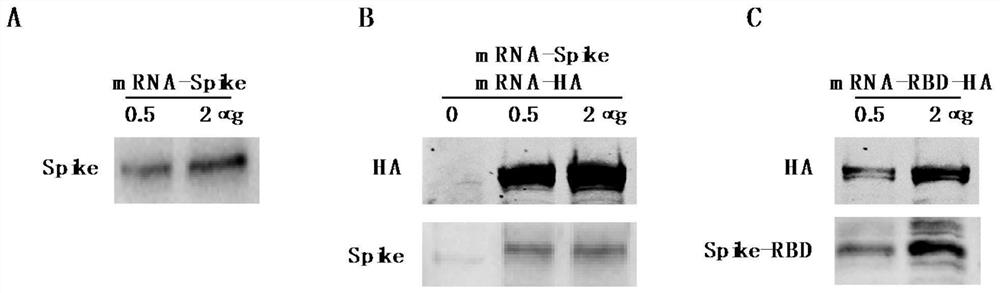
MRNA-based combined vaccine against coronavirus and influenza virus and preparation method of mRNA-based combined vaccine
PendingCN112546211ARelieve painImproving immunogenicitySsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutinin proteinLiposome
The invention discloses an mRNA-based combined vaccine against coronavirus and influenza virus and a preparation method of the mRNA-based combined vaccine. The mRNA-based combined vaccine comprises aliposome-coated mRNA-Spike and liposome-coated mRNA-HA mixed combined vaccine and a liposome-coated mRNA-RBD-HA combined vaccine. mRNA is designed according to the genome of the venezuelan equine encephalomyelitis virus TC83 in the alpha virus family. The mRNA-Spike contains a Spike protein gene of the novel coronavirus pneumonia, the mRNA-HA contains a hemagglutinin protein gene of the influenza,and the mRNA-Spike contains a Spike protein gene of the nnoval coronavirus pneumonia and a hemagglutinin protein gene of the influenza. By using one mRNA, the antigen of the coronavirus and the antigen of the influenza virus can be expressed at the same time, high immunogenicity is achieved, the pain of a patient can be relieved, and the immune effect of injection of a single virus vaccine can beachieved.
Owner:IMMORNA (HANGZHOU) BIOTECHNOLOGY CO LTD
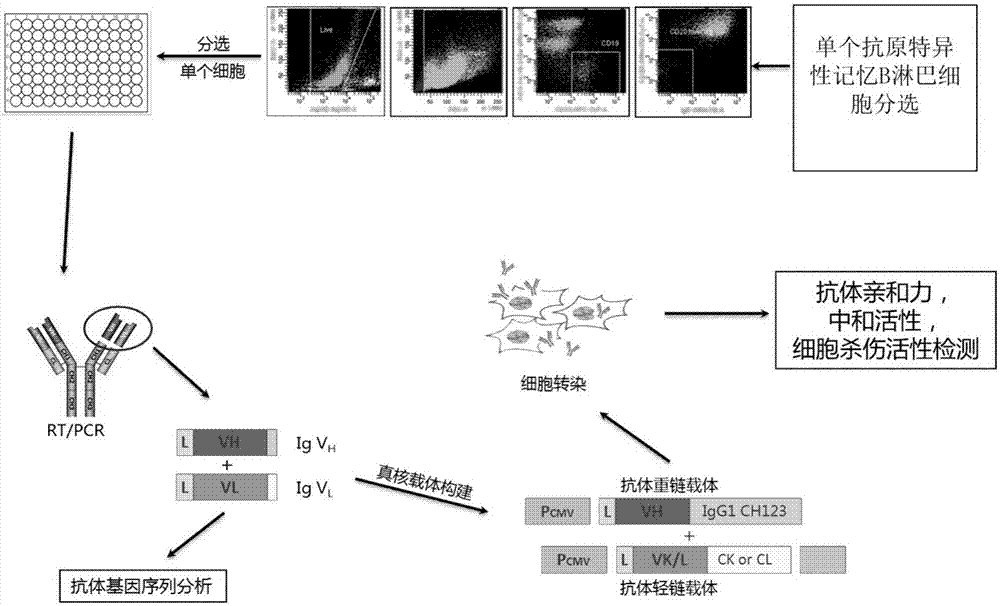
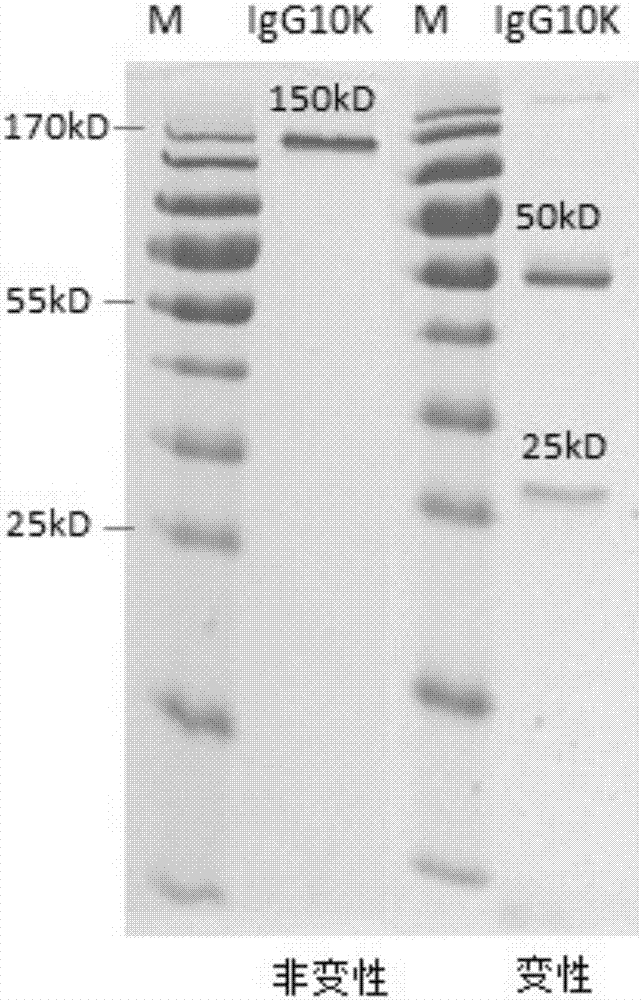
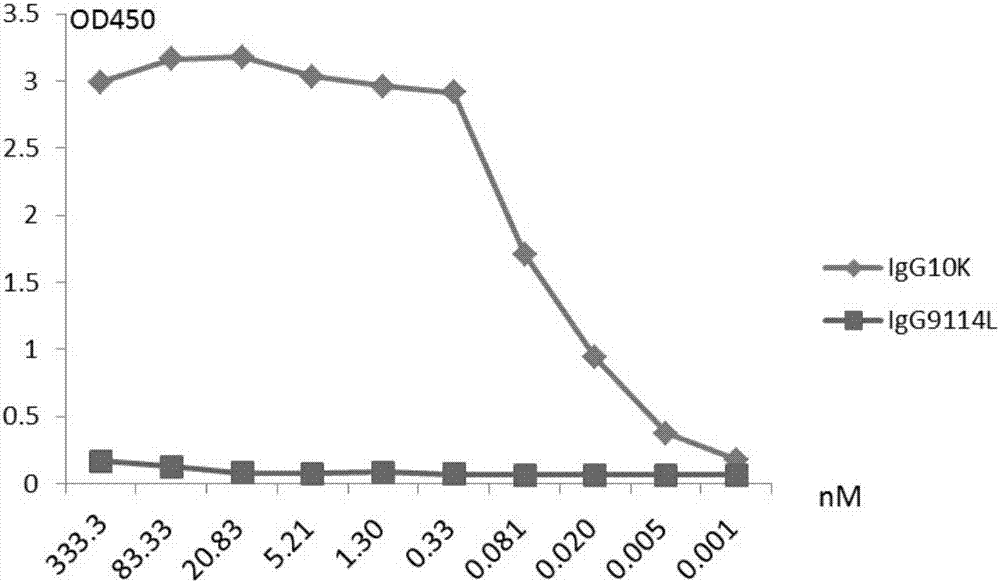
Humanized anti-H7N9 avian influenza virus high-affinity antibody 10K and application thereof
InactiveCN107056938AEfficient combinationImmunoglobulins against virusesAntiviralsHeavy chainEffector cell
The invention discloses a humanized anti-H7N9 avian influenza virus high-affinity antibody 10K filtered and obtained based on a single cell separation technology, the amino acid sequences of light chain and heavy chain variable regions of the antibody are shown as SEQ ID No. 2 and SEQ ID No. 5 respectively. The high-affinity specificity of the antibody is combined with H7N9 avian influenza virus 7 type hemagglutinin protein, and can mediate the kill and wound (ADCC) of effector cells using NK cells as main parts for H7N9 influenza virus infected cells. The antibody 10K can be used for therapeutic development of highly pathogenic avian influenza infection, and also can be used for development of H7N9 influenza virus antigen dectection reagents.
Owner:深圳普兰达科技有限公司
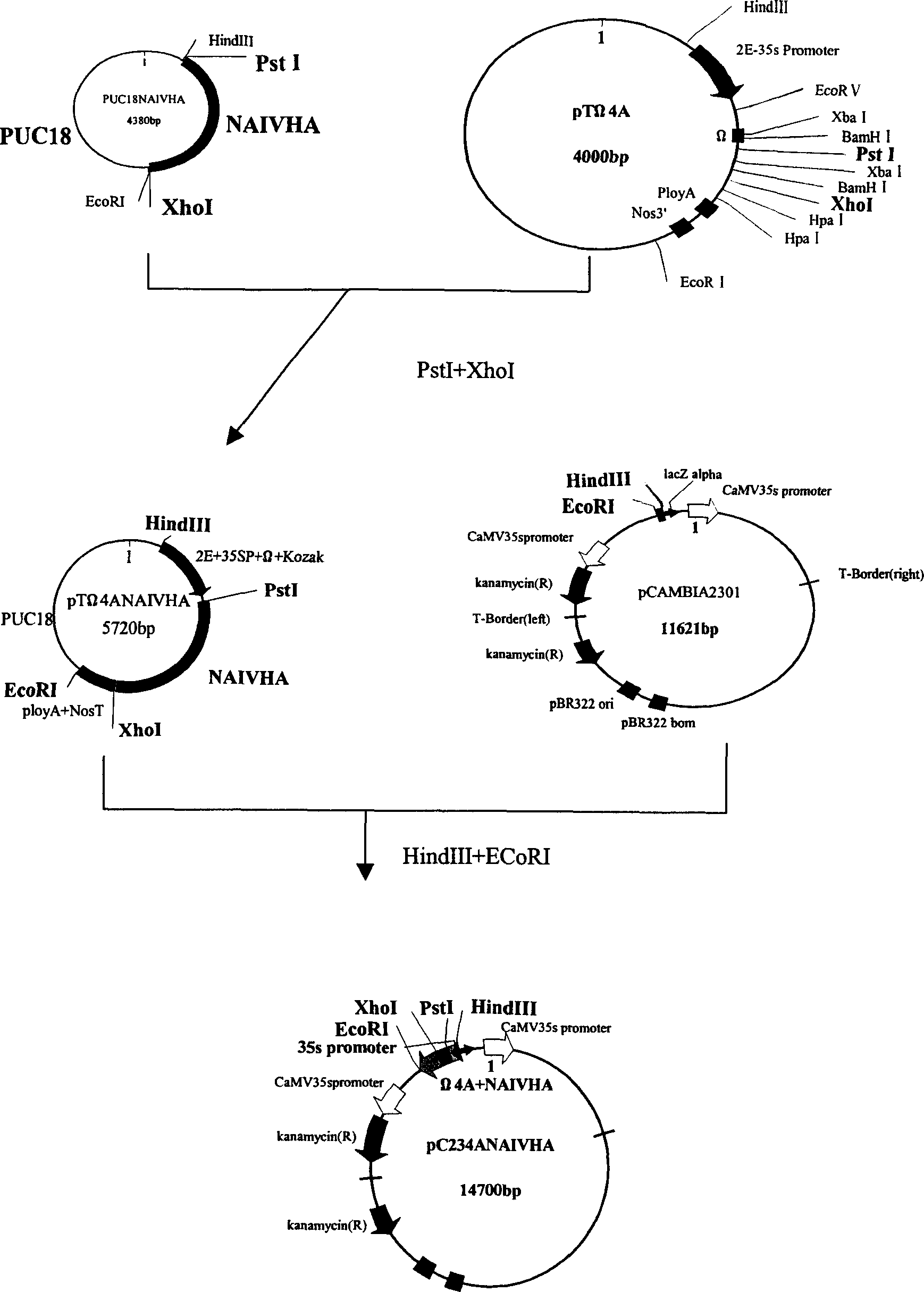
Coding hemaagglutinin gene of poultry influenza virus, plant expressing carrier and application thereof
InactiveCN1861793AHigh expressionImproving immunogenicityViral antigen ingredientsFermentationHemagglutininFowl
This invention discloses a new coding fowl influenza hemagglutinin protein gene VAIVHA and the construction, conversion and expression of the plant carrier. This gene can be highly expressed in the plant, and the expression value can be improved more than 40 times than the wild fowl influenza hemagglutinin gene. The gene transfer plant can be directly feed on the animal, or the protein extraction and purified protein immunize the animal, and the protection result of it is good. This shows that the gene plant has the ability to prevent the fowl influenza.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI +2
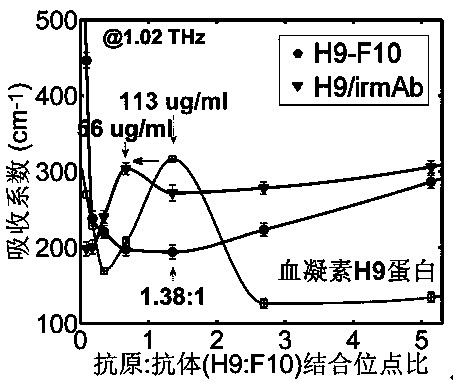
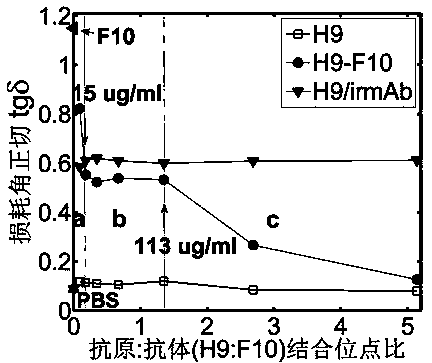
Terahertz time-domain spectroscopy-based unmarked hemagglutinin detection method
ActiveCN104215776AHigh detection sensitivityImprove detection efficiencyBiological testingHemagglutininTime domain
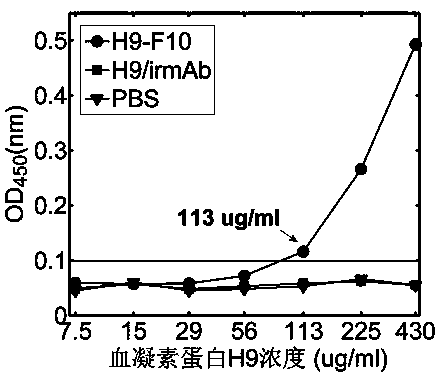
The invention discloses a terahertz time-domain spectroscopy-based unmarked hemagglutinin detection method which comprises the following steps: a. dissolving a sample into a PBS buffer solution to prepare an antigen solution H9 with different concentration, and reacting a monoclonal antibody F10 with the antigen solution H9 according to an equal volume ratio to obtain a reactant; b. incubating the reactant at room temperature for a certain period of time, then shaking, and staying overnight under a low-temperature condition, thereby forming an antigen-antibody complex structure; c. placing the complex into a detection box, vertically placing the detection box at the focal spot of a terahertz light beam, and recording concentration-related terahertz intensity absorption spectrum; and d. according to the concentration-related terahertz intensity absorption spectrum, judging whether an absorption peak exists, and if not, detecting hemagglutinin in the sample.
Owner:SHENZHEN UNIV
Preparation method and application of newcastle disease virus living-vector vaccine through gene recombination of canine distemper attenuated vaccine strains F and H
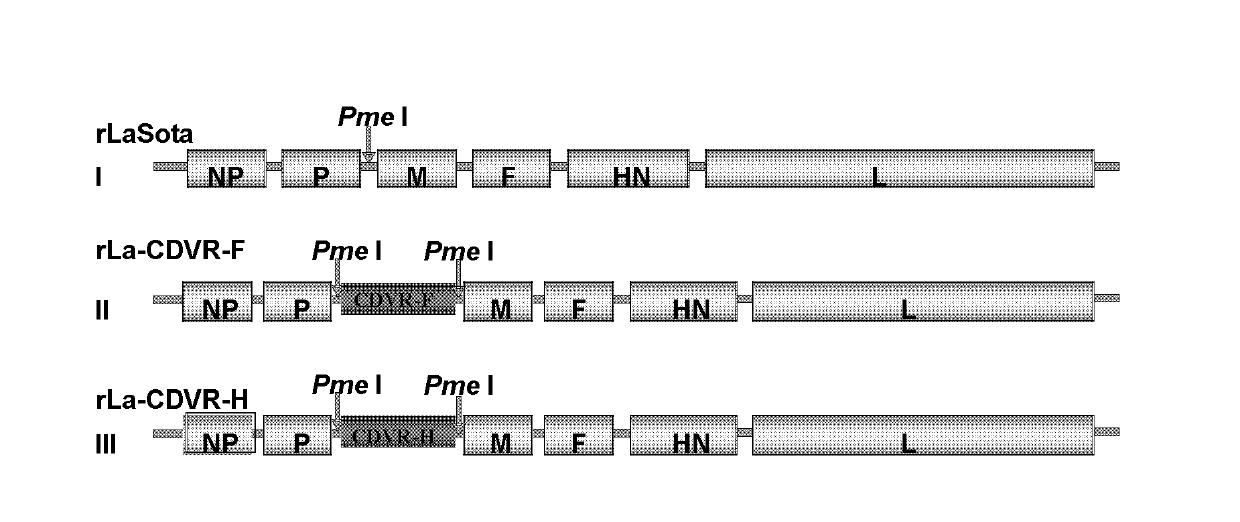
InactiveCN102816741AHigh growth titerReduce pathogenicityViral antigen ingredientsMicroorganism based processesCanine distemper virus CDVVector vaccine
The invention relates to a recombination newcastle disease LaSota attenuated vaccine for expressing canine distemper virus fusion protein (F) or canine distemper virus hemagglutinin protein (H). Particularly, the recombination newcastle disease LaSota attenuated vaccine is rLa-CDVR-F or rLa-CDVR-H. The invention further discloses a method for preparing the recombination newcastle disease LaSota attenuated vaccine and application of the recombination newcastle disease LaSota attenuated vaccine in preparation of vaccines / kits.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +2
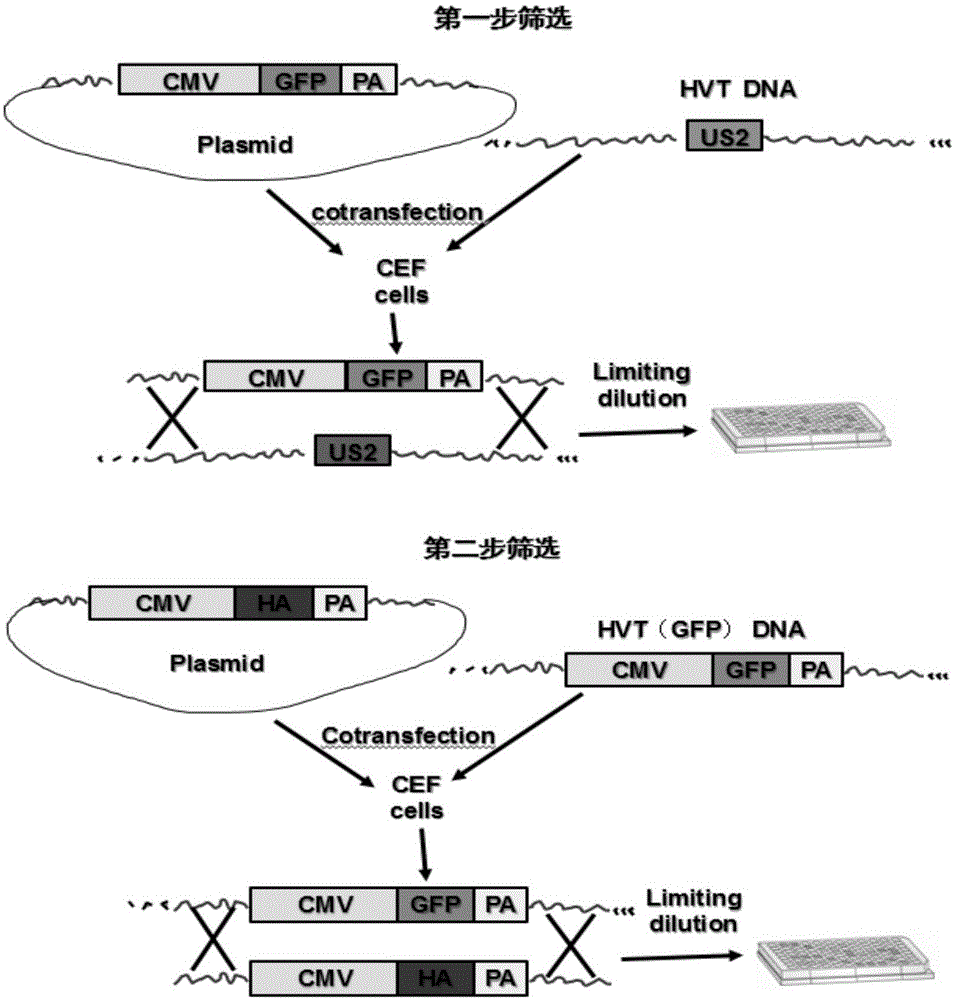
RHVT (recombinant Herpesvirus of Turkey)-H9HA (H9 hemagglutinin) and construction method thereof
InactiveCN105002146AMicroorganism based processesViruses/bacteriophagesHemagglutininAvian influenza virus
The invention relates to an rHVT (recombinant Herpesvirus of Turkey)-H9HA (H9 hemagglutinin) for expressing H9 subtype AIV (avian influenza virus) HA protein and a construction method thereof. The collection number of a vaccine strain rHVT-H9HA is CGMCC No: 10907. A GFP (green fluorescent protein) expression cassette is separated from a carrier pEGFP-C1 (plasmid enhanced green fluorescent protein) and is inserted into an HVT genome, and a recombinant virus rHVT-GFP is obtained. Through homologous recombination, a GFP gene of the rHVT-GFP is replaced with an HA gene of H9N2 subtype AIV epidemic strain A / Chicken / Jiangsu / WJ57 / 2012, a recombinant virus without fluorescence is selected, and the rHVT-H9HA for stably expressing the H9 subtype AIV HA gene is obtained. The recombinant virus strain is low in cost, good in safety, long in immunity period and suitable for large-scale production of vaccine and can be used for making the vaccine.
Owner:YANGZHOU UNIV
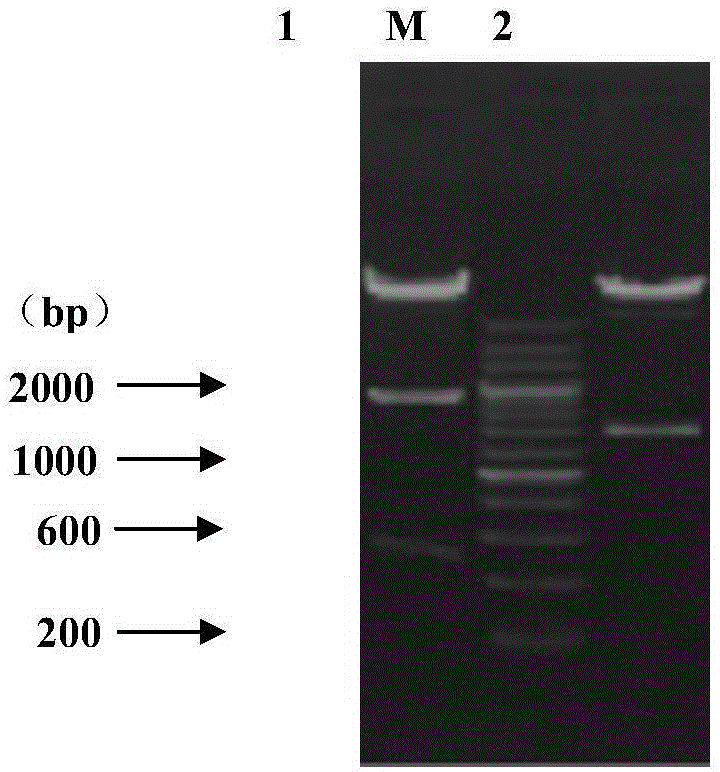
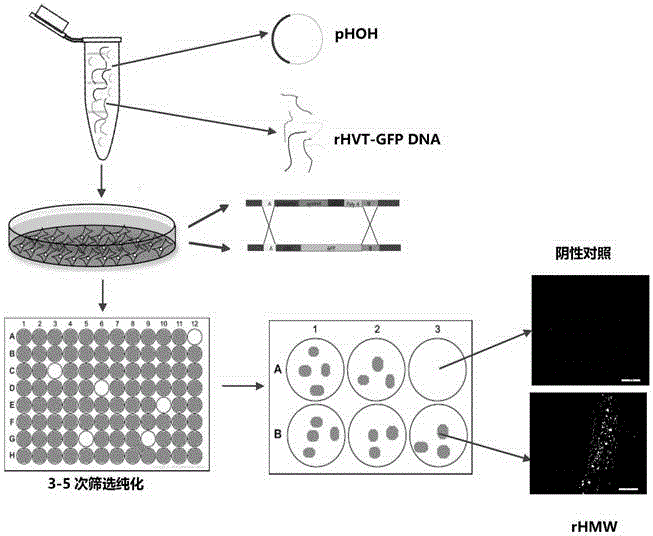
Recombinant turkey herpesvirus strain rHMW for expressing H7N9 subtype avian influenza virus chimeric hemagglutinin and construction method of recombinant turkey herpesvirus strain rHMW
The invention belongs to recombinant viral vector vaccines in the technical fields of molecular biology and biology, and in particular relates to a recombinant turkey herpesvirus strain rHMW for expressing H7N9 subtype avian influenza virus chimeric hemagglutinin and a construction method of the recombinant turkey herpesvirus strain rHMW. The recombinant turkey herpesvirus strain rHMW has a collection number of CGMCC NO. 12984. The recombinant virus can be used for preparing vaccines capable of preventing H7N9 subtype avian influenza viruses. The recombinant turkey herpesvirus strain rHMW disclosed by the invention has obvious effects for preventing and controlling infectious diseases of poultry and achieves huge social benefits and economic benefits.
Owner:YANGZHOU UNIV
Combination vaccine
The present invention relates to a vaccine, especially a combination vaccine providing at least a first and a second antigenic function, the combination vaccine comprising at least one RNA encoding at least one or more proteins or fragments, variants or derivatives of proteins awarding antigenic function, wherein the first antigenic function being a Fusion (F) protein or a fragment, variant or derivative of a Fusion (F) protein derived from the virus family Paramyxoviridae and the second antigenic function being an Hemagglutinin (HA) protein or a fragment, variant or derivative of an Hemagglutinin (HA) protein derived from the virus family Orthomyxoviridae. Furthermore, the present invention is directed to a kit or kit of parts comprising the components of said combination vaccine and to said combination vaccine for use in a method of prophylactic or therapeutic treatment of diseases, particularly in the prevention or treatment of infectious diseases like RSV and influenza.
Owner:CUREVAC SE
Colloidal gold immunochromatograohic assay detection test strip based on NDV (Newcastle Disease Virus) hemagglutinin protein monoclonal antibody
InactiveCN103937751AImmunoglobulins against virusesTissue cultureProtein.monoclonalNewcastle disease virus NDV
The invention relates to the field of bioengineering, and particularly relates to a newly prepared hybridoma cell strain for resisting NDV (Newcastle Disease Virus)hemagglutinin protein. A monoclonal antibody with a broad spectrum neutralization effect on NDV is obtained by using the cell strain, and an immune colloidal gold test strip for quickly detecting a newcastle disease virus is developed by using the antibody. By adopting the test paper disclosed by the invention, the result can be obtained within 5 minutes by sampling once, and the test paper has the characteristics of being simple and rapid, specific and sensitive. A more practical tool and method are provided for rapid diagnosis of a newcastle disease by development of the test strip, and the colloidal gold immunochromatograohic assay detection test strip is applicable to primary veterinary stations and small and medium-sized farms.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Indirect ELISA (Enzyme-linked Immuno Sorbent Assay) kit for detecting type A haemophilus paragallinarum antibody as well as detection method and application thereof
InactiveCN107219364AEasy to manufactureEasy to purifyBiological material analysisColor/spectral properties measurementsElisa kitAntiendomysial antibodies
The invention discloses an indirect ELISA kit for detecting Haemophilus paragallinarum type A antibody, a detection method and an application thereof, and relates to the technical field of biological detection. The ELISA kit of the present invention includes: coated microplate, washing solution, serum diluent, substrate chromogenic solution, rabbit anti-chicken enzyme-labeled secondary antibody, stop solution, type A Hpg standard positive serum and type A Hpg standard negative serum Serum; wherein, the coated microtiter plate uses the recombinant hemagglutinin protein of Haemophilus paragallinarum type A as the coated antigen. The ELISA kit of the present invention is used to detect the antibody of Haemophilus paragallinarum type A, and has the advantages of high efficiency, good sensitivity, specificity and repeatability, and the kit is easy to operate, fast and low in cost, and is suitable for clinical applications and promote.
Owner:YANGLING VOCATIONAL & TECHN COLLEGE +1
Newcastle disease virus rLX/H9HA and construction method and application thereof
InactiveCN103146751ASuitable for mass productionImprove reproductive performanceMicroorganism based processesVector-based foreign material introductionVaccine manufacturingHemagglutinin
The invention discloses a recombination newcastle disease virus rLX / H9HA of H9 subtype avian influenza virus hemagglutinin albumen. A preservation number is CGMCCNo: 6652. According to the method, a reverse genetic operation platform of an established newcastle disease virus (DVA) weak poison LX strain is used, an HA gene of an H9N2 subtype avian influenza virus (AIV) epidemic strain A / Chicken / Jiangsu / WJ57 / 2012 is inserted in a genome overall length transcription carrier pLX of the LX strain, and thus a recombination newcastle disease virus genome overall length cDNA clone Plx-H9HA containing a H9N2 subtype AIVHA gene. A recombinant virus rLX / H9HA obtained through transfection on a chick embryo has high breeding geometric mean titer, can still express HA albumen stably through continuous passing of multiple generations, is suitable for mass production of vaccines, and can be used for vaccine manufacturing.
Owner:YANGZHOU UNIV
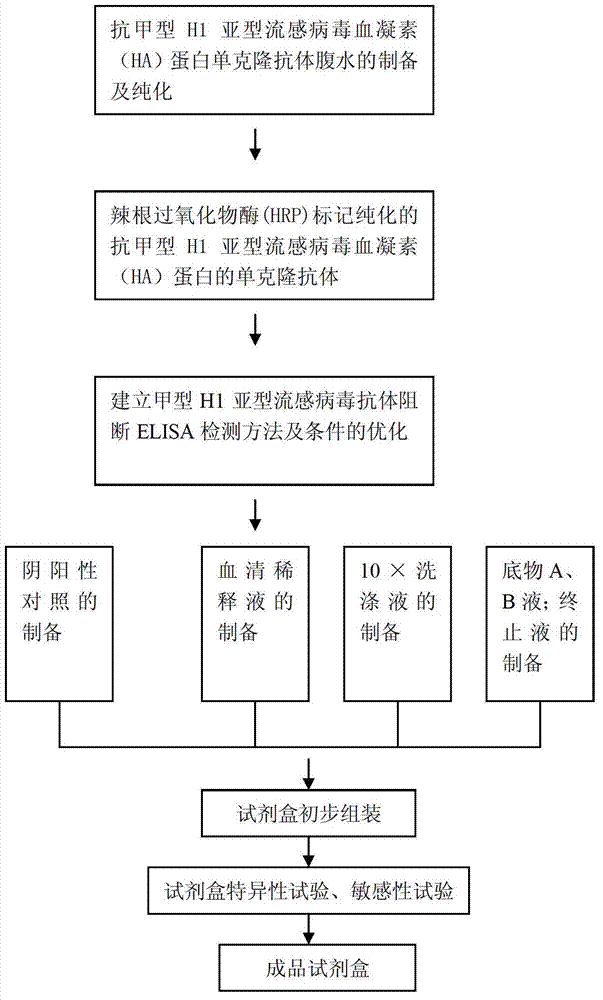
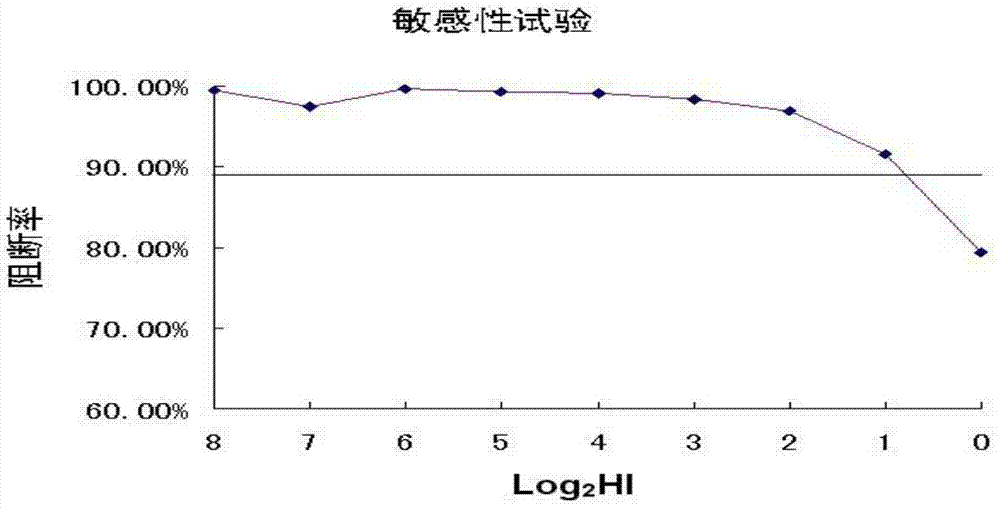
A-type H1 subtype influenza virus antibody blocking ELISA kit and applications thereof
InactiveCN103592436AStrong specificityHigh sensitivityImmunoglobulins against virusesMaterial analysisElisa kitPositive control
The invention discloses an A-type H1 subtype influenza virus antibody blocking ELISA kit. The kit comprises: a) an A-type H1 subtype influenza virus A-Influ / JML-F9; b) a monoclonal antibody with strong specificity of an A-type H1 subtype influenza virus-resistant hemagglutinin protein; c) an antibody blocking ELISA core kit prepared from the monoclonal antibody of the A-type H1 subtype influenza virus-resistant hemagglutinin protein; and d) a sample diluting liquid, 10 times of a washing liquid, a substrate liquid A, a substrate liquid B, a reaction stop solution, a positive control sample and a negative control sample in the kit. The invention also discloses applications of the A-type H1 subtype influenza virus antibody blocking ELISA kit in A-type H1 subtype influenza virus antibody detection. The kit has strong specificity, high sensitivity, short detection time, easy and practical operation, and no need of being operated by a professional person. The kit is good in stability and long in retention period, and solves a problem of the A-type H1 subtype influenza virus antibody detection.
Owner:HUAZHONG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com