ELISA kit for H9N2 influenza virus hemagglutinin protein
A technology of hemagglutinin protein and influenza virus is applied in the field of quantitative H9N2 influenza virus hemagglutinin protein double-antibody sandwich ELISA detection kit, which can solve the problems of difficult promotion, quantitative detection and poor specificity of influenza virus subtype detection. , to achieve the effects of high detection sensitivity and accuracy, convenient and easy inspection method, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1H9
[0015] Component preparation of embodiment 1H9N2 influenza hemagglutinin protein ELISA kit
[0016] 1. Preparation of mouse monoclonal antibody:
[0017] 1) Animal immunity
[0018] Balb / c mice were used as immunized animals, and the recombinant H9N2 influenza hemagglutinin protein (product number: 11229-V08H) produced by Sino Biological Technology Co., Ltd. protein. For the first immunization, the immunogen and the same amount of complete Freund's adjuvant were used to make an emulsifier, and the abdomen was injected subcutaneously at multiple points, and after an interval of 2 to 3 weeks, the same dose of the immunogen and the same amount of incomplete Freund's adjuvant were used to make an emulsifier , two times of booster immunization, and three times after the indirect ELISA method was used to measure the serum titer. After the serum titer reached 1:16000, the mouse with the highest titer was selected for a booster immunization once in the peritoneal cavity, and splenoc...
Embodiment 2H9
[0042] The formation of embodiment 2H9N2 influenza hemagglutinin protein ELISA kit
[0043] The assembled ELISA kit contains the following reagents:
[0044] a) mouse monoclonal coating antibody;
[0045] b) HRP-labeled rabbit polyclonal antibody;
[0046] c) H9N2 influenza hemagglutinin protein standard;
[0047] d) Coating buffer: pH9.6, 0.05mol / L carbonate buffer;
[0048] e) Blocking solution: Tris buffer containing 2% bovine serum albumin;
[0049] f) Sample diluent: phosphate buffer containing 0.1% bovine serum albumin;
[0050] g) Washing solution: phosphate buffer containing 0.1% Tween;
[0051] h) Substrate chromogenic solution: composed of chromogenic solution A and chromogenic solution B, chromogenic solution A is hydrogen peroxide or carbamide peroxide, and chromogenic solution B is tetramethylbiphenyl;
[0052] i) Stop solution: 2mol / L sulfuric acid
Embodiment 3H9
[0053] The preparation of embodiment 3H9N2 influenza hemagglutinin protein ELISA kit
[0054] 1. Use orthogonal experiments to explore the optimal antibody combination and working concentration of ELISA kits
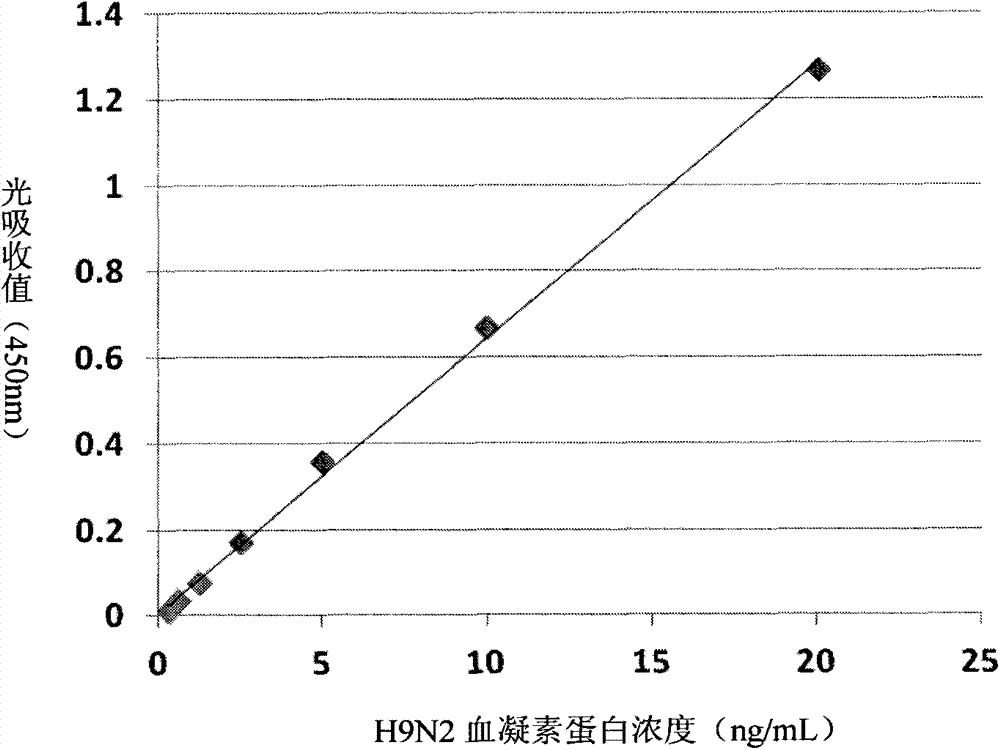
[0055] According to the ultraviolet spectrophotometer method, the concentration of antibody and antigen was determined. Orthogonal test method was used to explore the best combination of antibodies and the best concentration of antibodies to use, dilute different anti-H9N2 influenza hemagglutinin protein monoclonal antibodies to concentrations of 4 μg / ml, 2 μg / ml, 1 μg / ml, recombinant hemagglutinin The protein concentration was diluted to 1000pg / ml, 100pg / ml, 0pg / ml, and the HRP-labeled rabbit polyclonal antibody was diluted to 4μg / ml, 2μg / ml, 1μg / ml, 0.5μg / ml. Considering the background of the blank well and the light absorption value of the positive test well, a mouse monoclonal antibody was selected as the coating antibody, and its optimal working concentration was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com