Antibody neutralizing human infected H7N9 influenza A virus and use thereof
An influenza A virus, antibody technology, applied in the direction of antibodies, antiviral agents, antiviral immunoglobulins, etc., can solve the problem of no marketed antibody drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] Antibody preparation
[0058] Antibodies of the invention can be prepared by any method known in the art. For example, the sequence of the antibody is inserted into the corresponding eukaryotic expression vector and transfected into cells, such as the technology of obtaining antibody by transient expression in 293 cells, and the technology of obtaining antibody by stable expression in CHO cells. High-density cell culture techniques are usually used to obtain high yields of antibodies.
[0059] Antibody purification techniques, including techniques for preparing pharmaceutical grade antibodies, are also known in the art. Antibodies can be purified by centrifugation, filtration, affinity, charge, molecular weight, hydrophobicity and other chromatography methods.
[0060] The preparation methods of the antibody fragments of the present invention are also known in the art, including enzymatic digestion with pepsin or papain, or antibody fragments can be obtained by clonin...
Embodiment 1
[0082] Example 1. Anti-H7N9 virus neutralizing antibody activity and sequence information analysis of rabbit origin
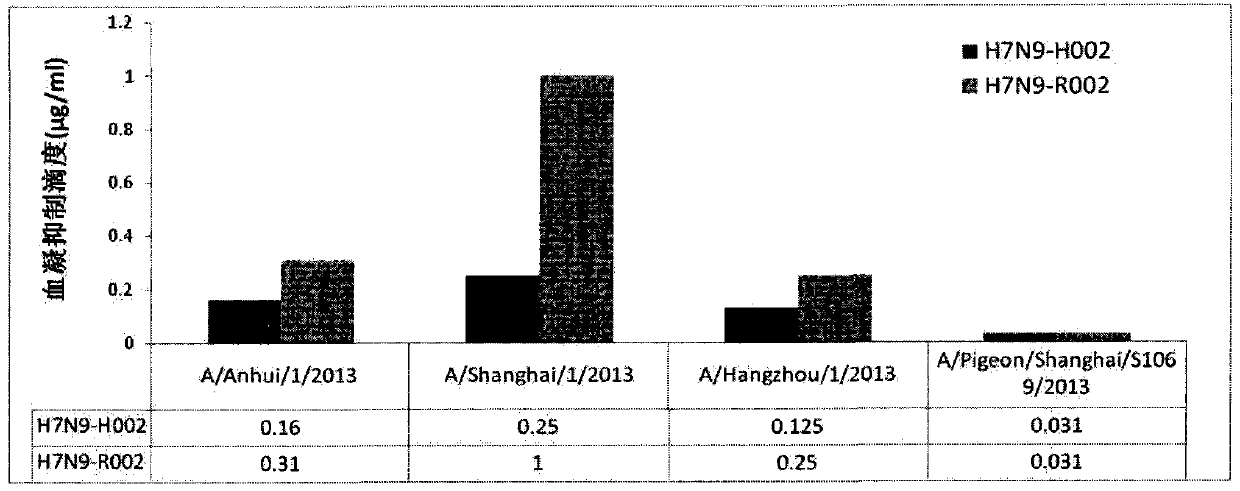
[0083] Before the outbreak of H7N9 virus, the rabbit antibody library of H7N7 (A / Netherlands / 219 / 2003) and H7N9 (A / Anhui / 1 / 2013) hemagglutinin protein was constructed, which was obtained by 293 mammalian transient expression and insect system expression The rabbit antibody library was panned for the hemagglutinin protein of the H7N9 virus (A / Anhui / 1 / 2013), and 30 specific antibodies against the hemagglutinin protein of the human-infected H7N9 virus were screened. The antibody sequence was constructed into a eukaryotic expression vector, and a milligram-scale antibody was produced in the 293 mammalian transient expression system. The binding ability of the antibody to various H7N9 virus hemagglutinin proteins was tested (Table 4). At the same time, the hemagglutination inhibition test was used to detect the activity of the antibody, and the ability of the antibo...
Embodiment 2
[0087] Example 2. Humanized Antibody Has Efficient Virus Neutralizing Activity
[0088] According to the expression level and neutralizing activity of the antibody, the rabbit antibody H7N9-R002 was selected for humanization design to obtain the H7N9-H002 humanized antibody. The humanized transformation technology is based on the most classic CDR transplantation method and is aimed at rabbit source Antibody design. The humanized antibody gene sequence was obtained by whole gene synthesis, and constructed into a eukaryotic expression vector, and antibodies in milligram to gram protein quantities were produced in the 293 mammalian transient expression system. Humanized antibodies that retain the affinity of rabbit antibodies were screened by affinity detection with H7N9 hemagglutinin protein, and the humanized antibody H7N9-H002 with the highest affinity was obtained. The amino acid sequence of the variable region of the light chain of the antibody is SEQ ID NO: 56 , the nucleo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com