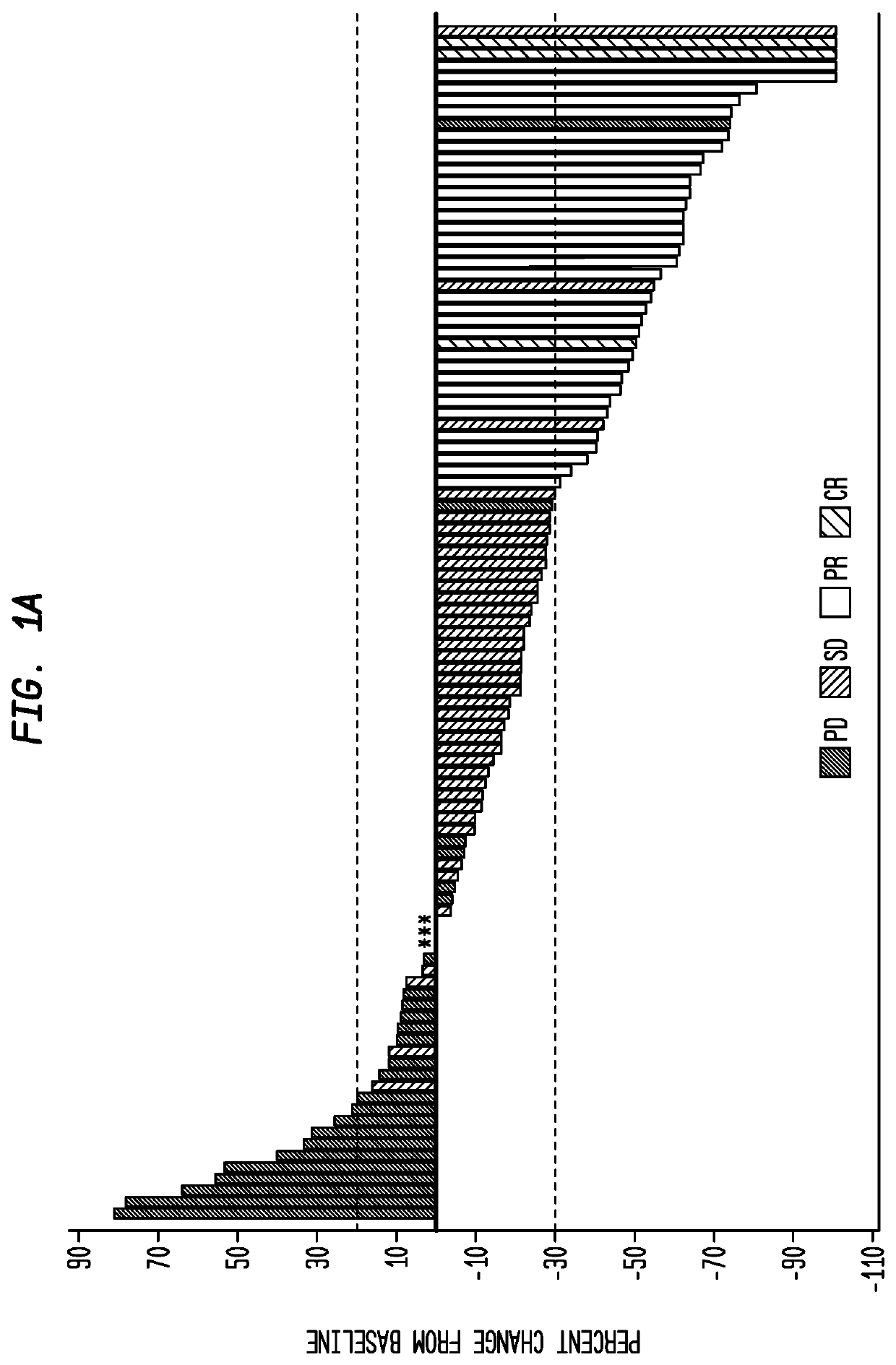
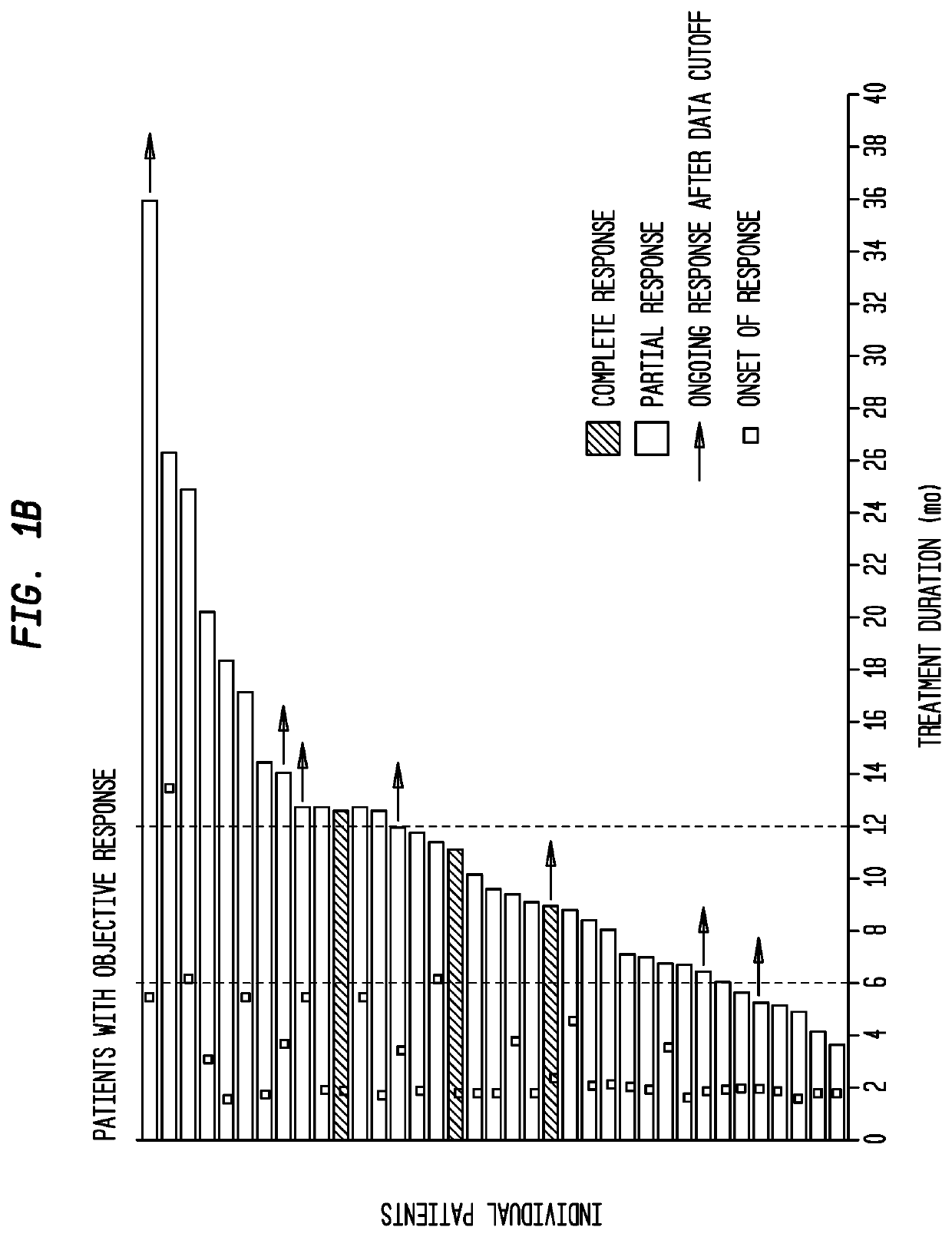
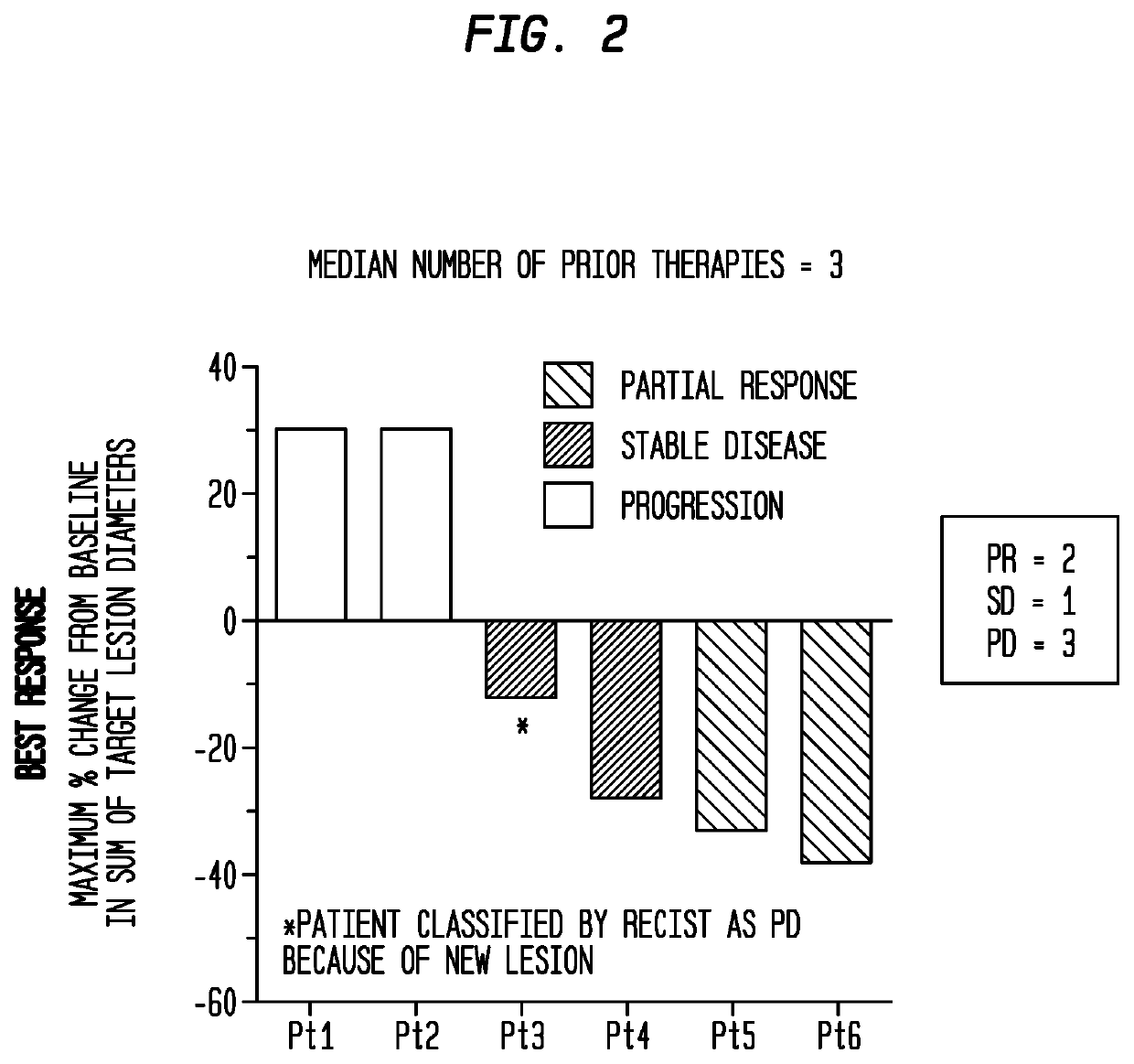
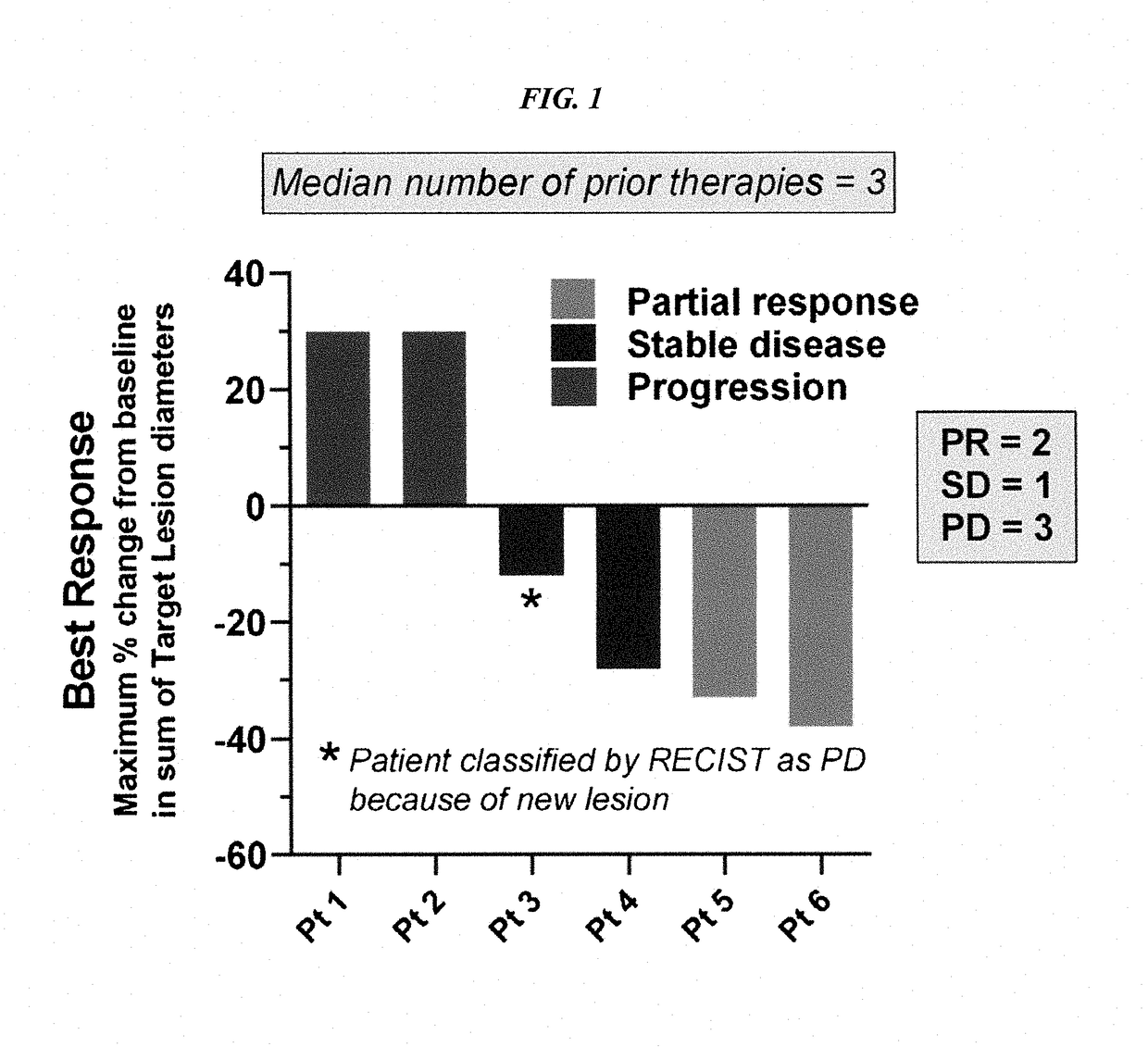
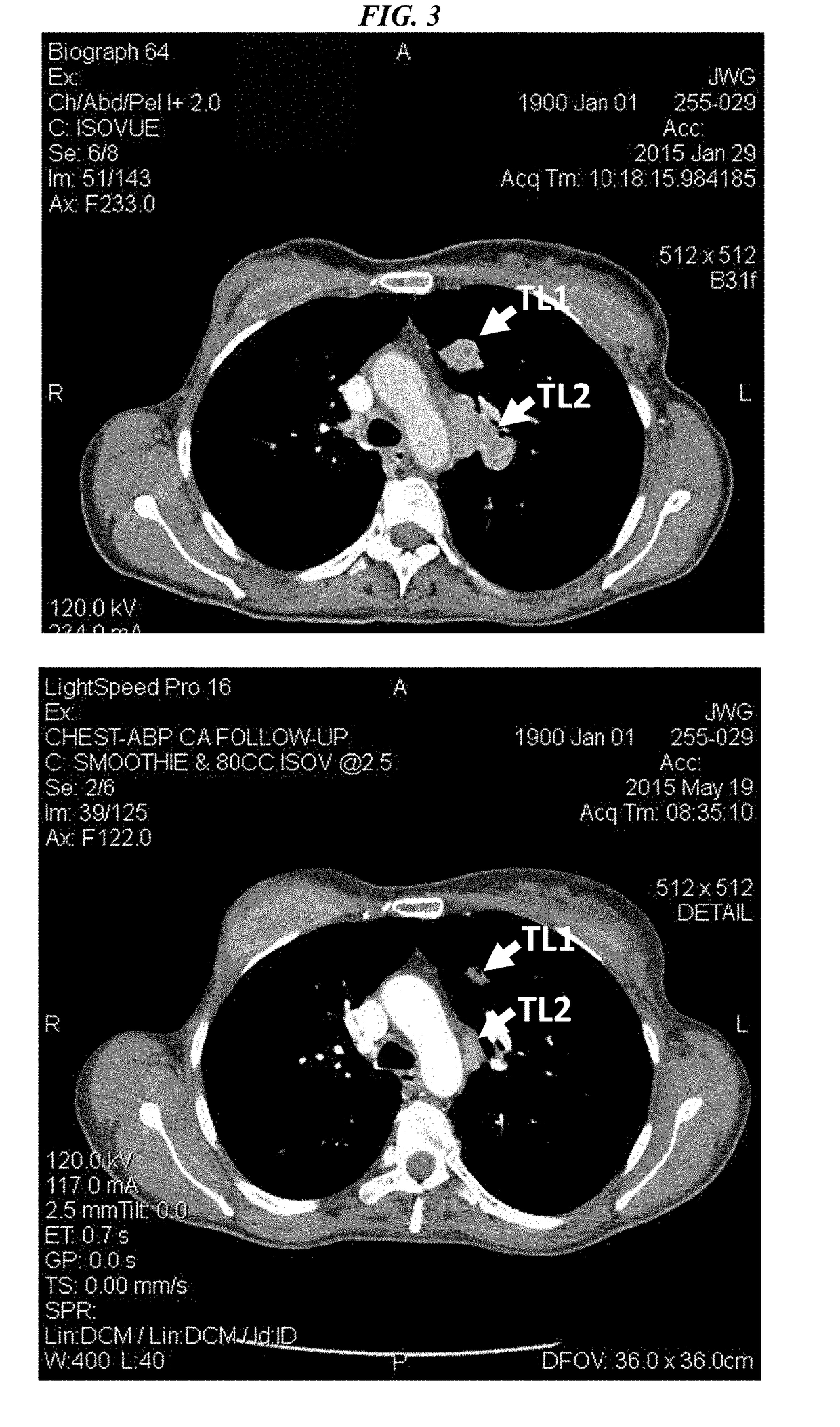
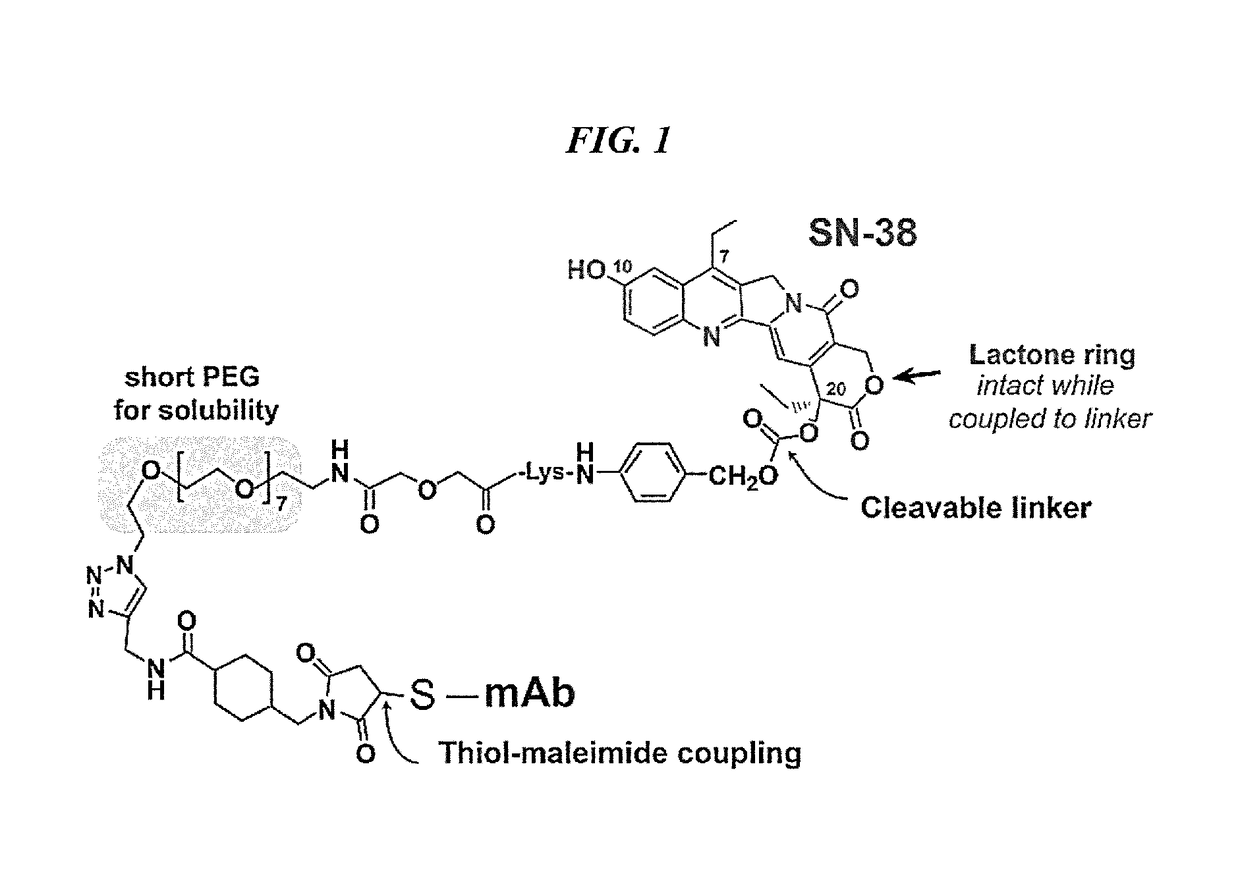
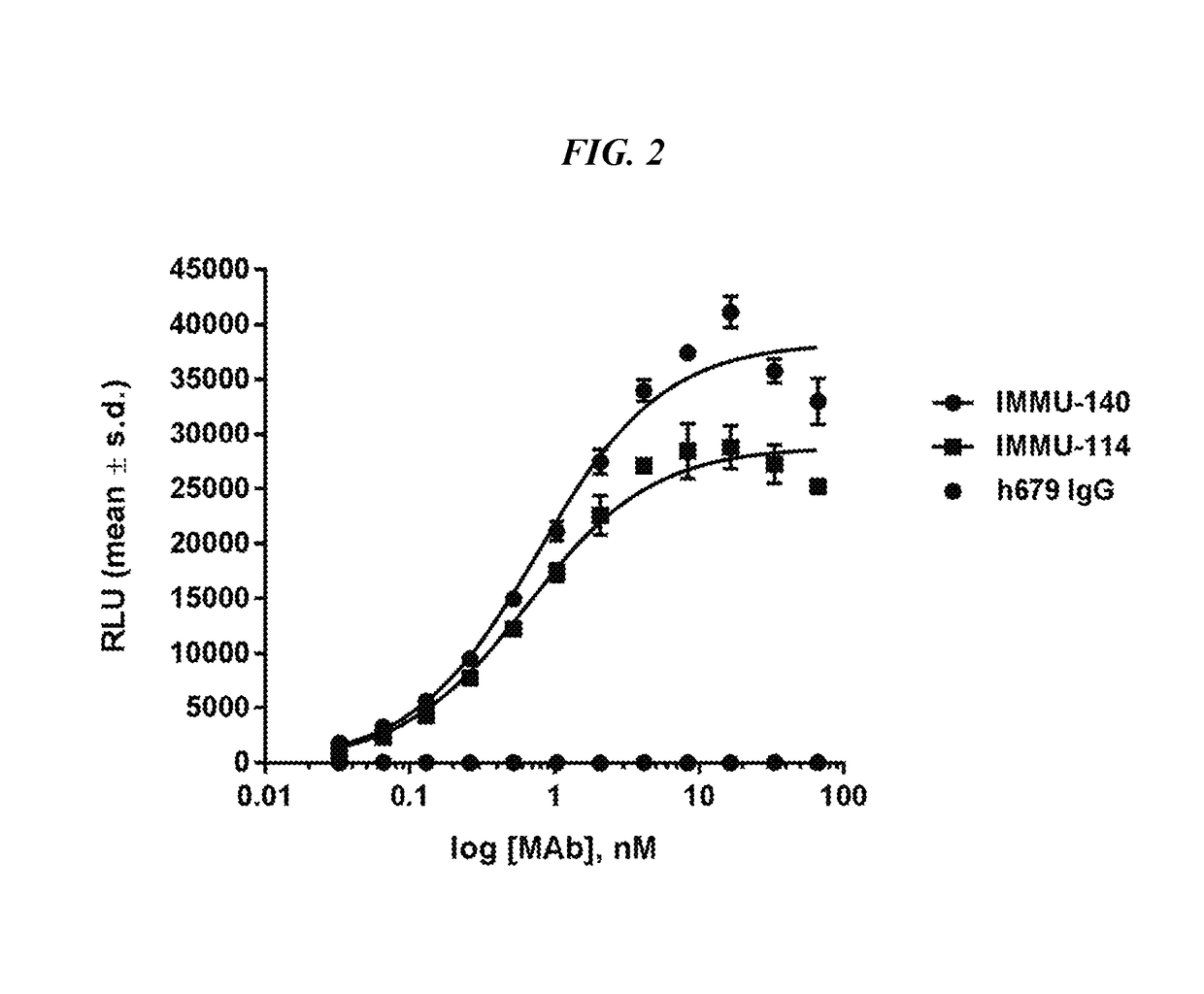
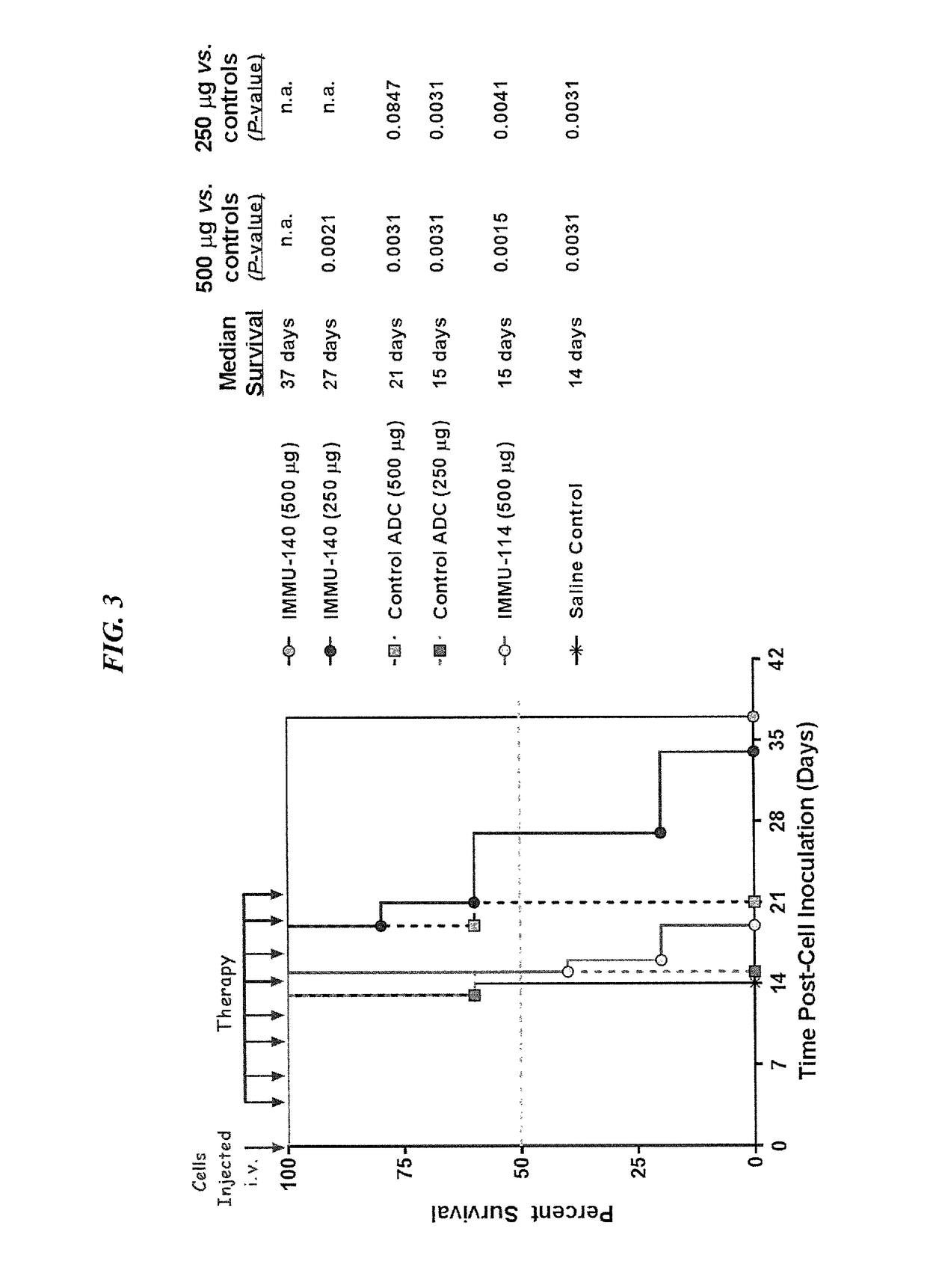
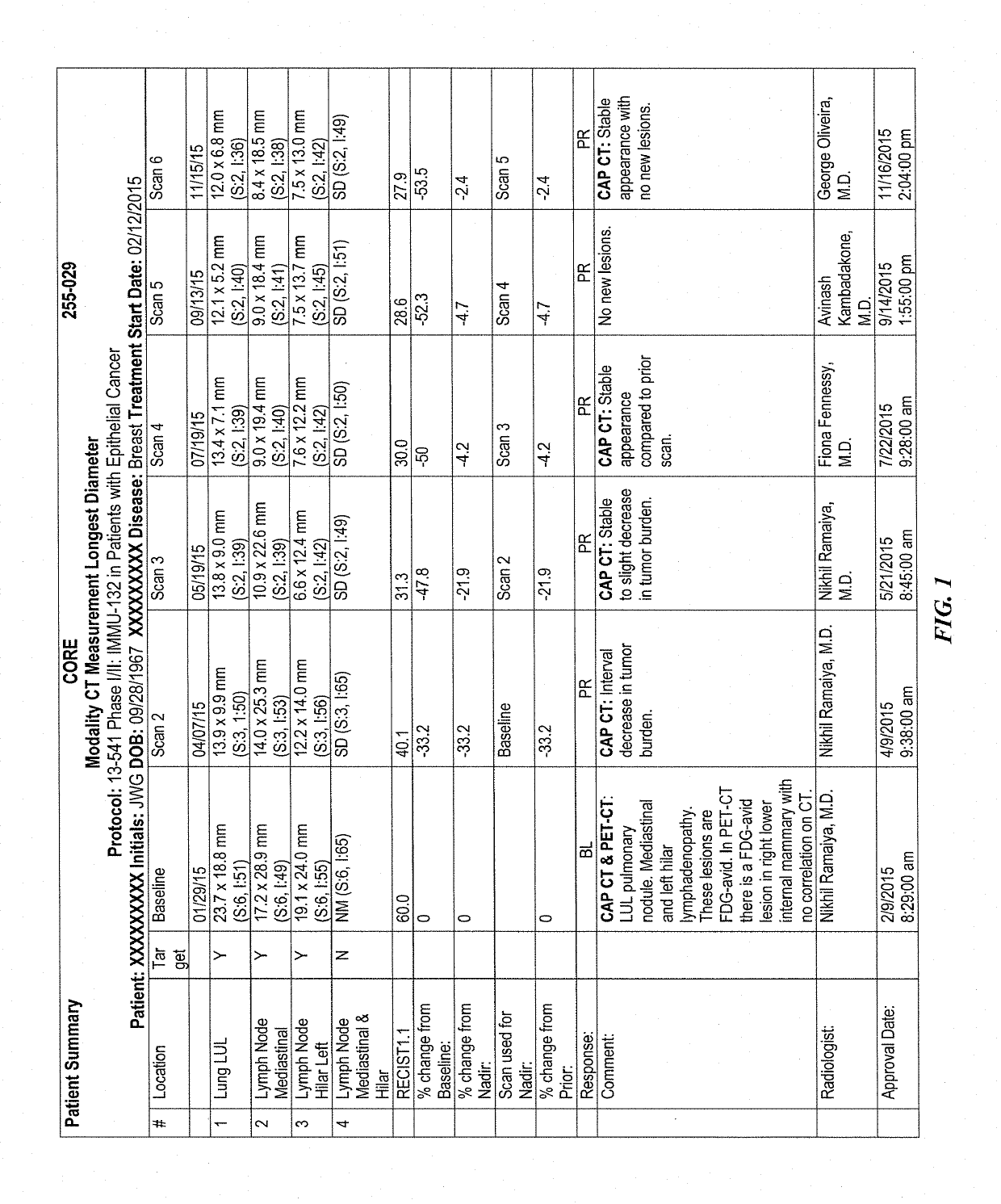
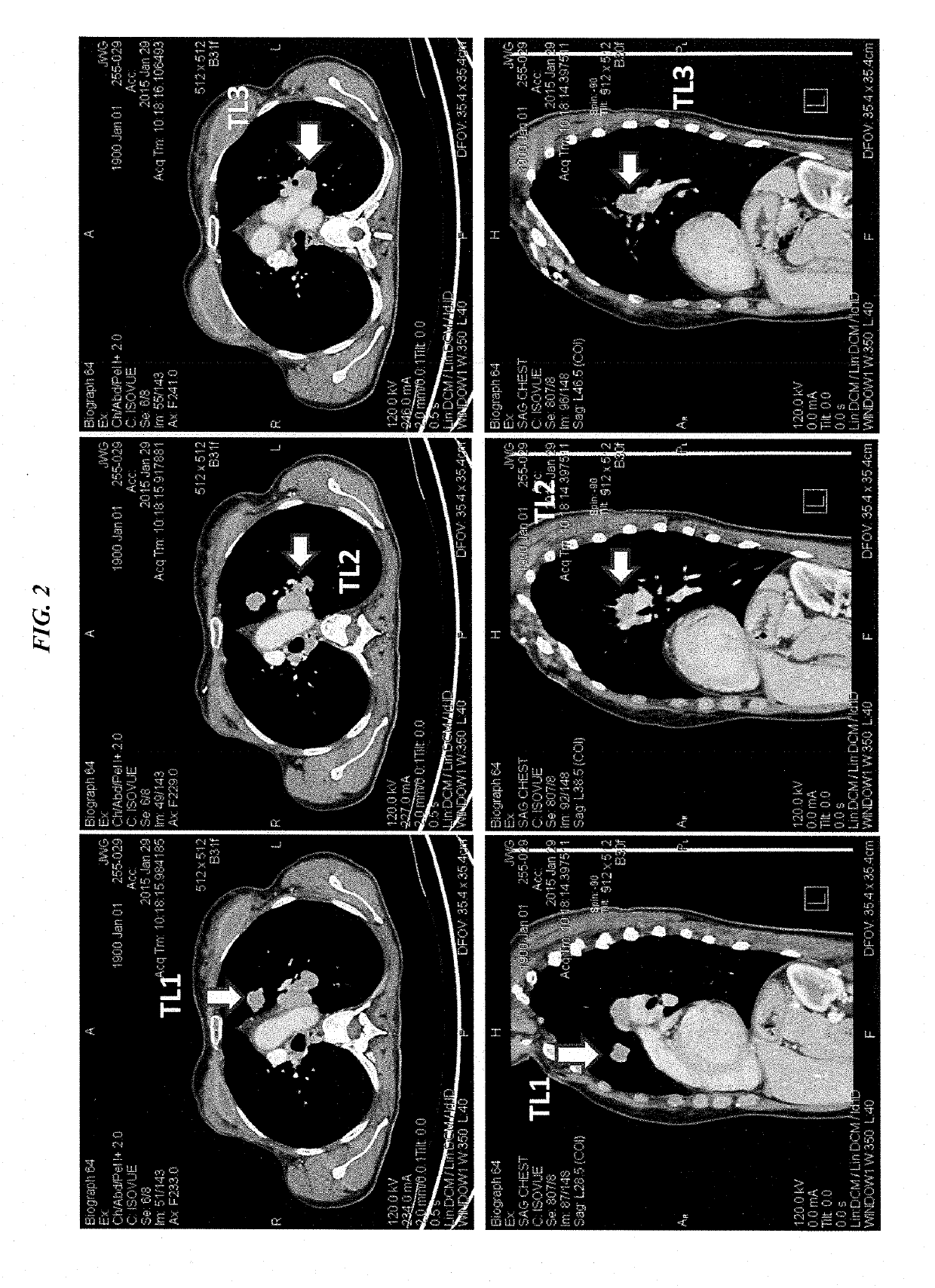
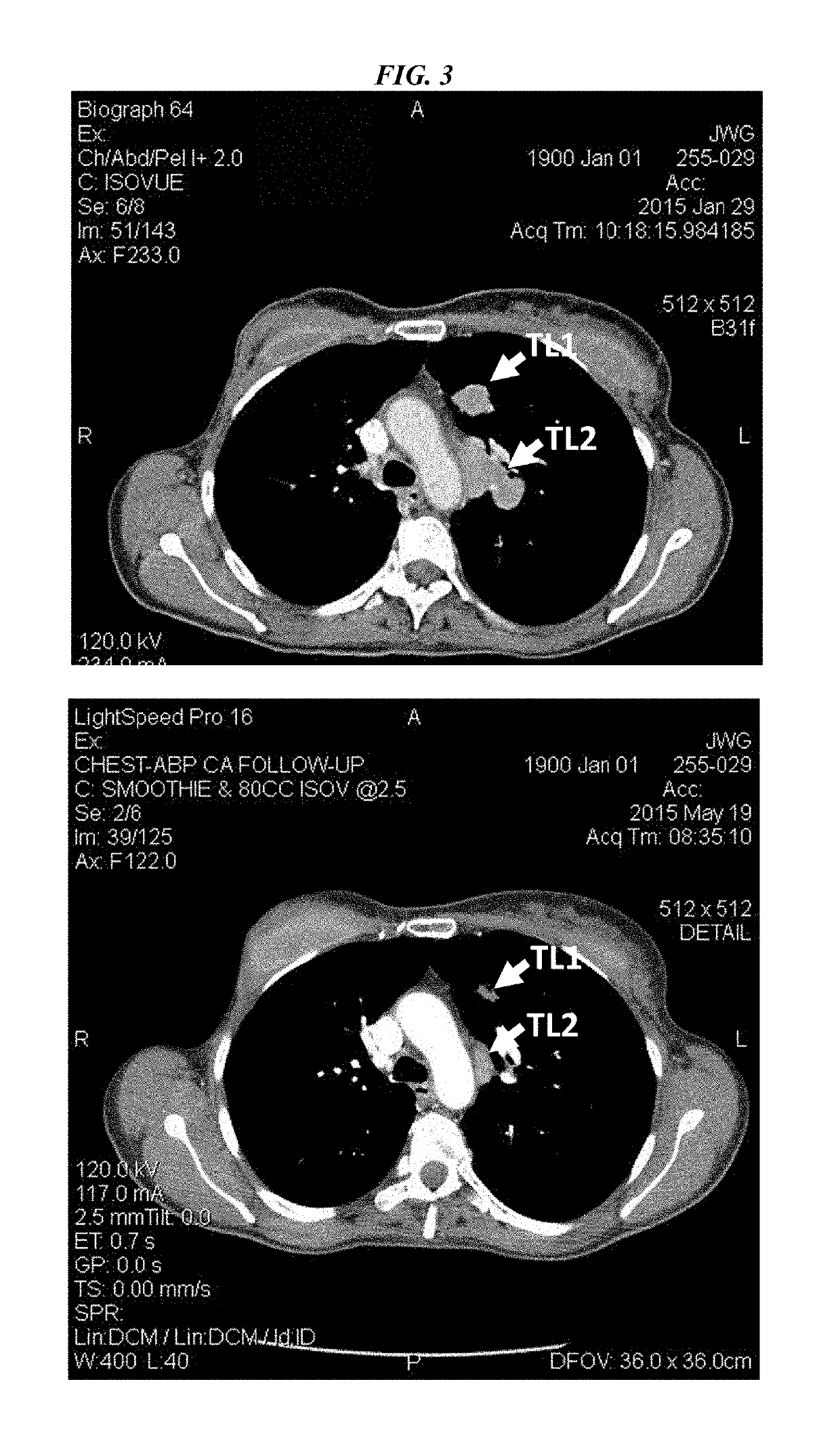
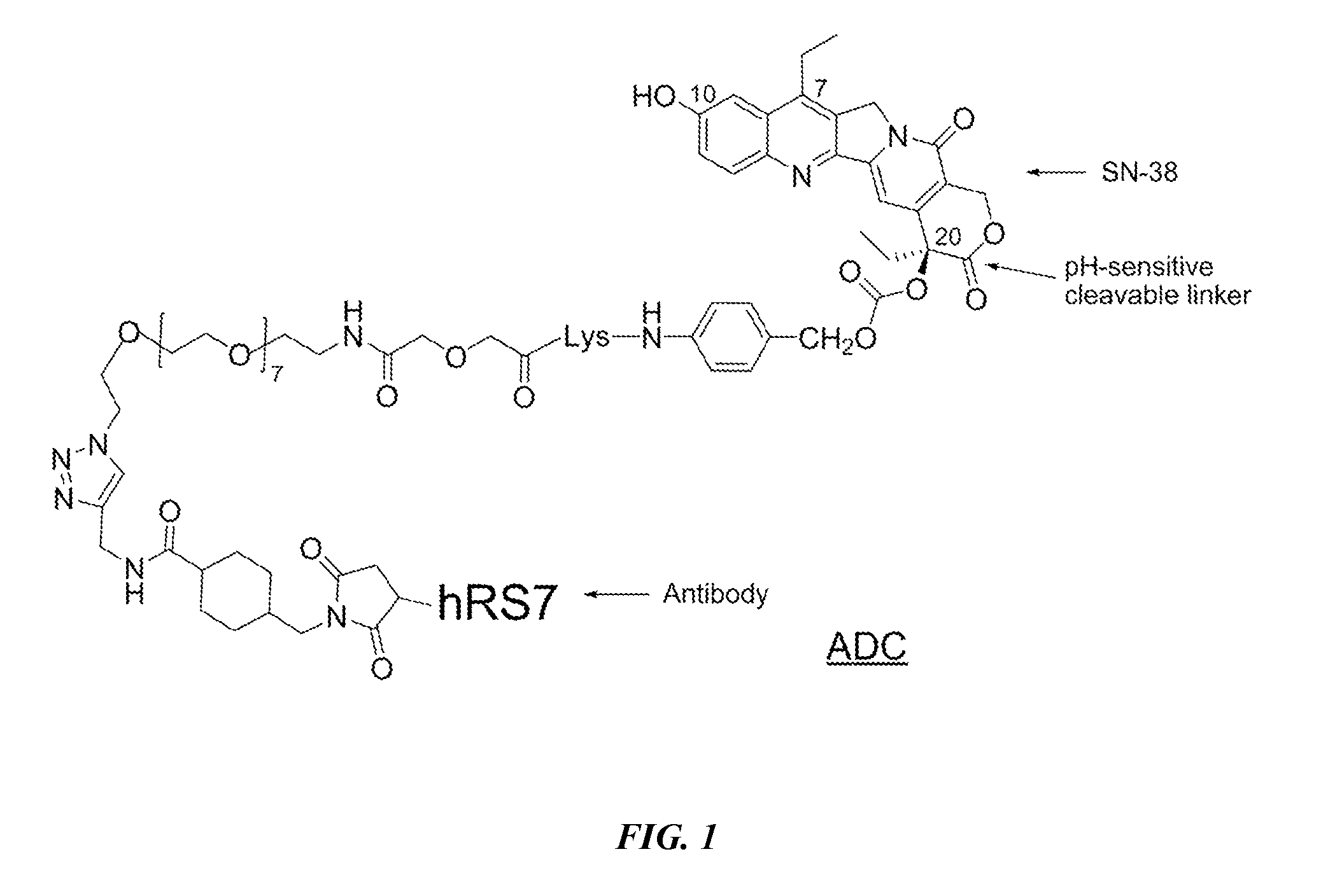
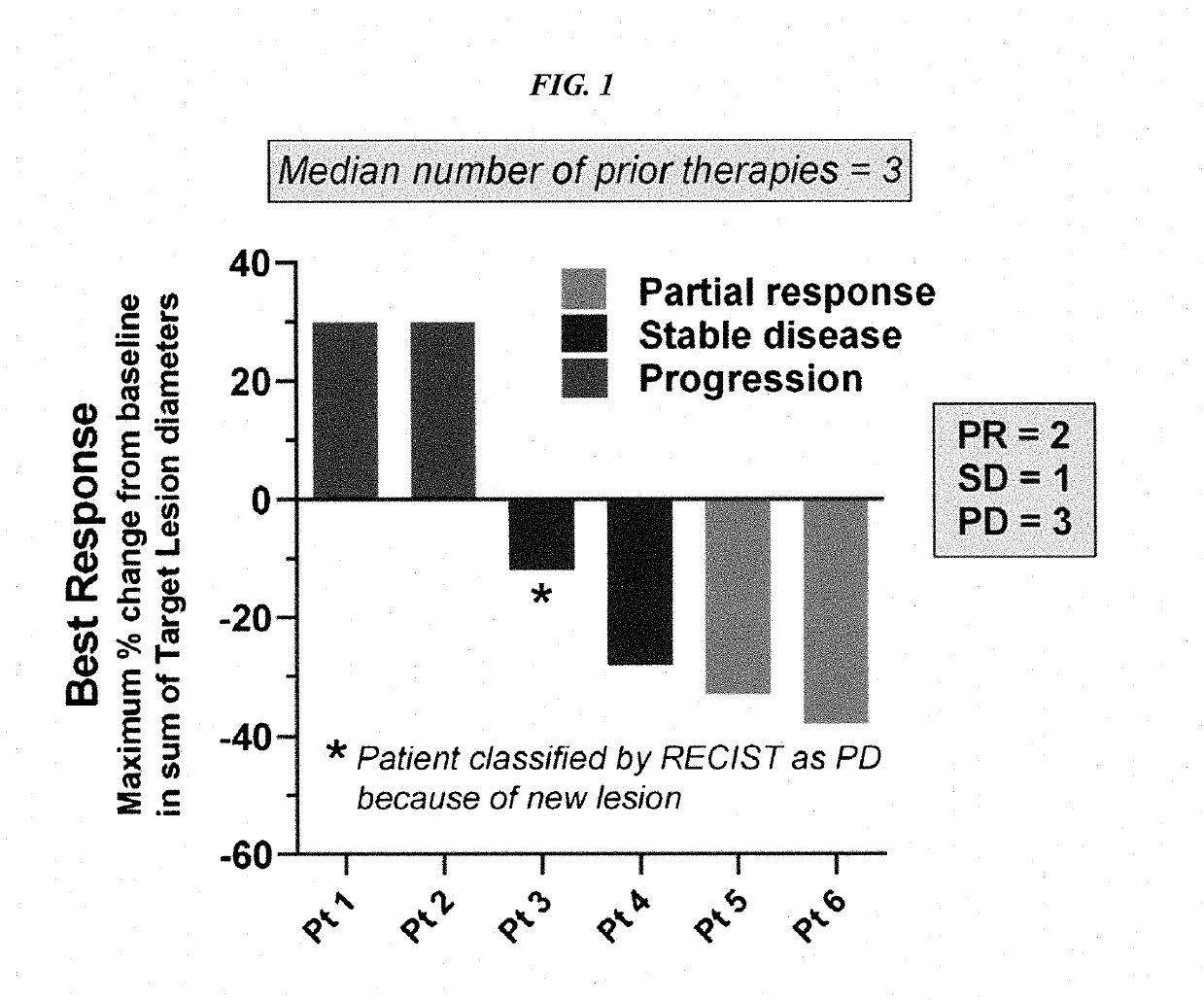
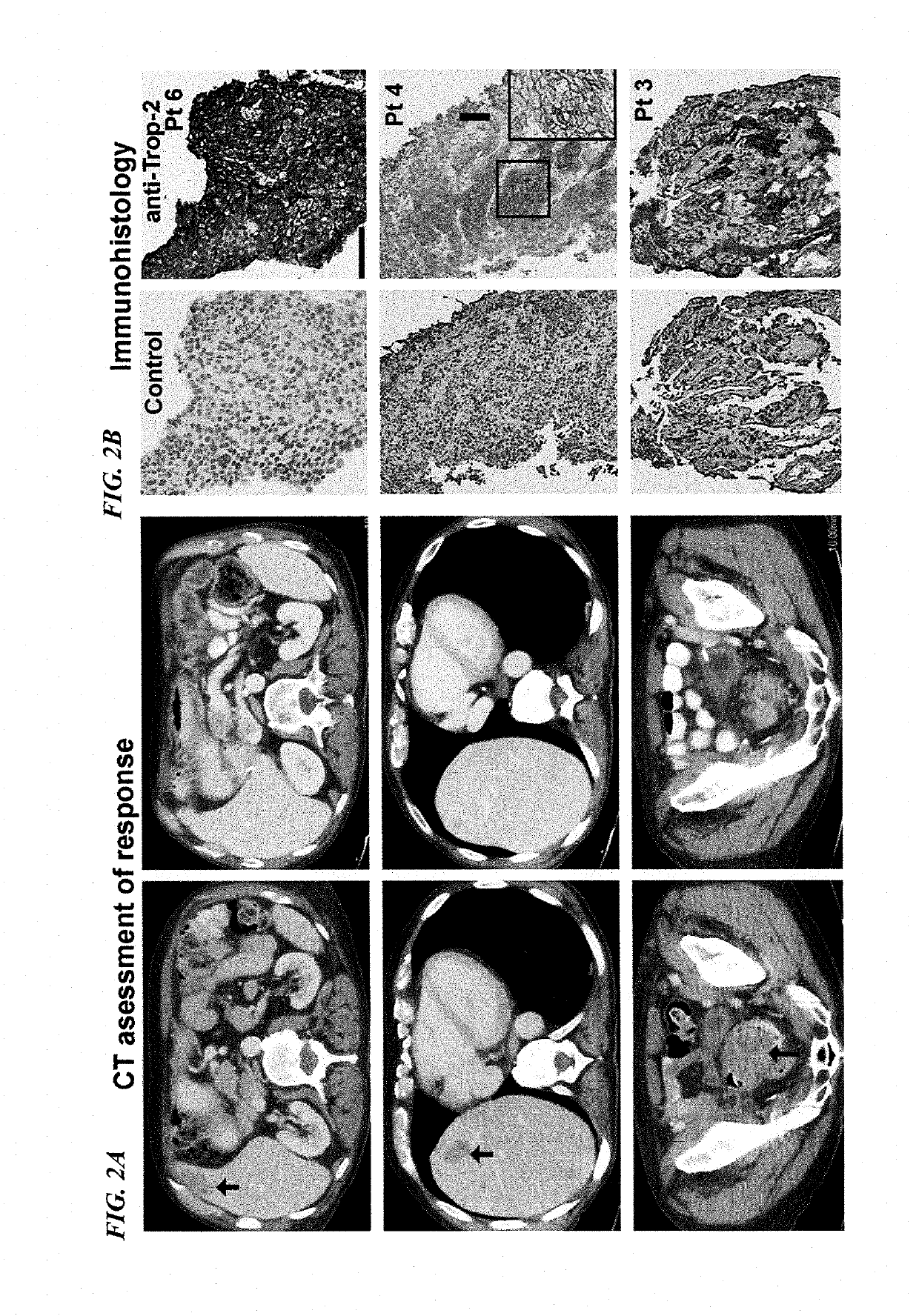
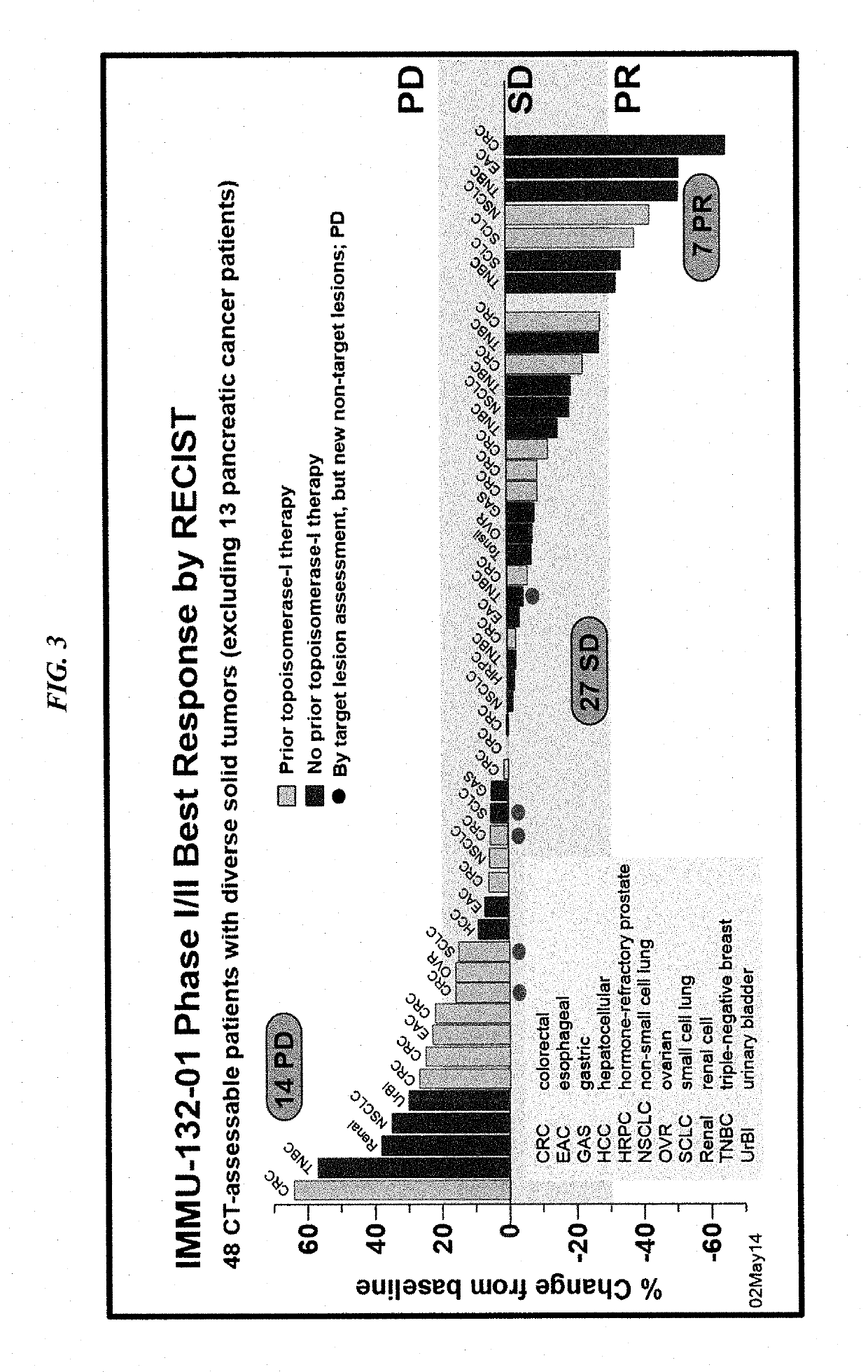
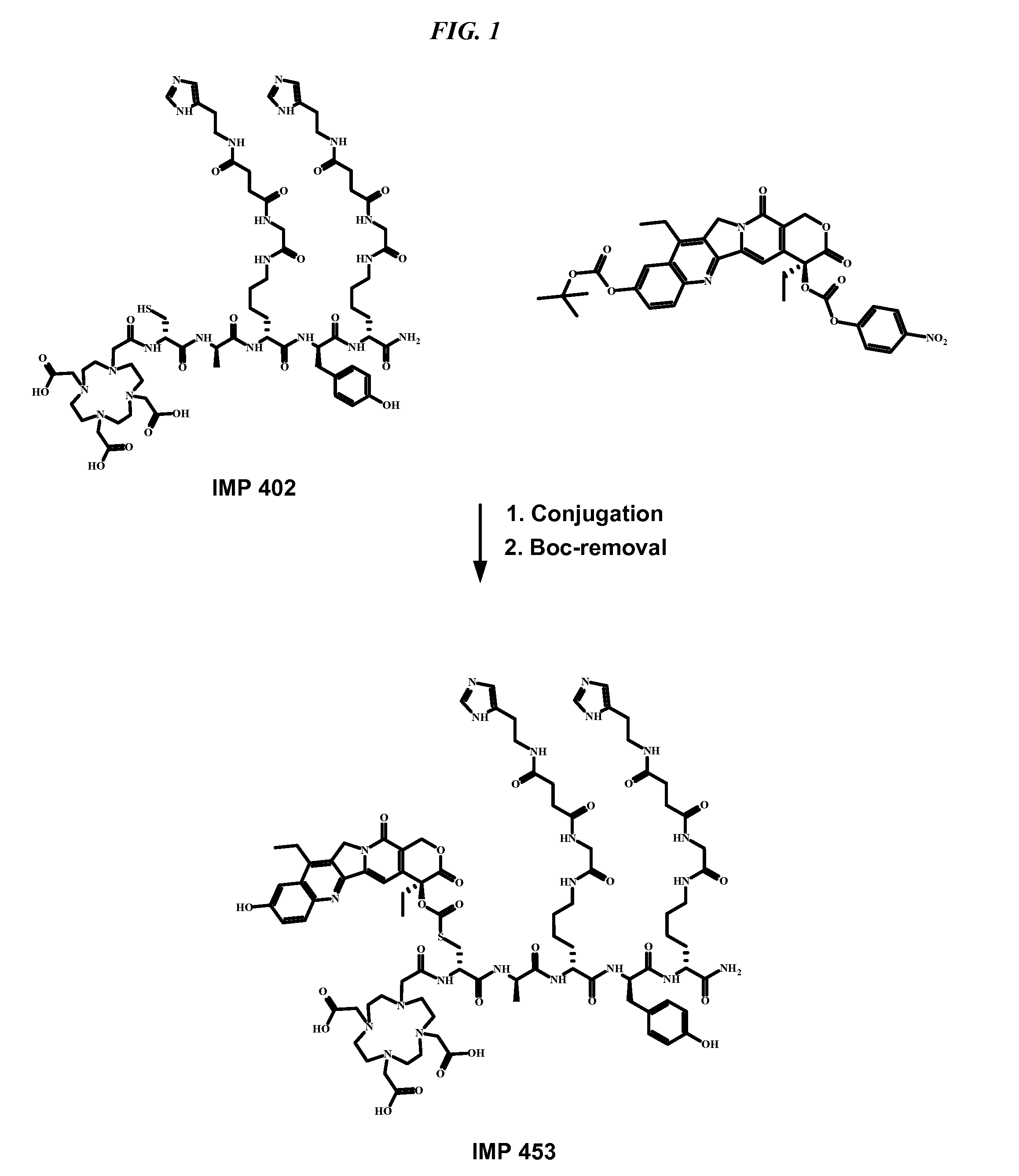
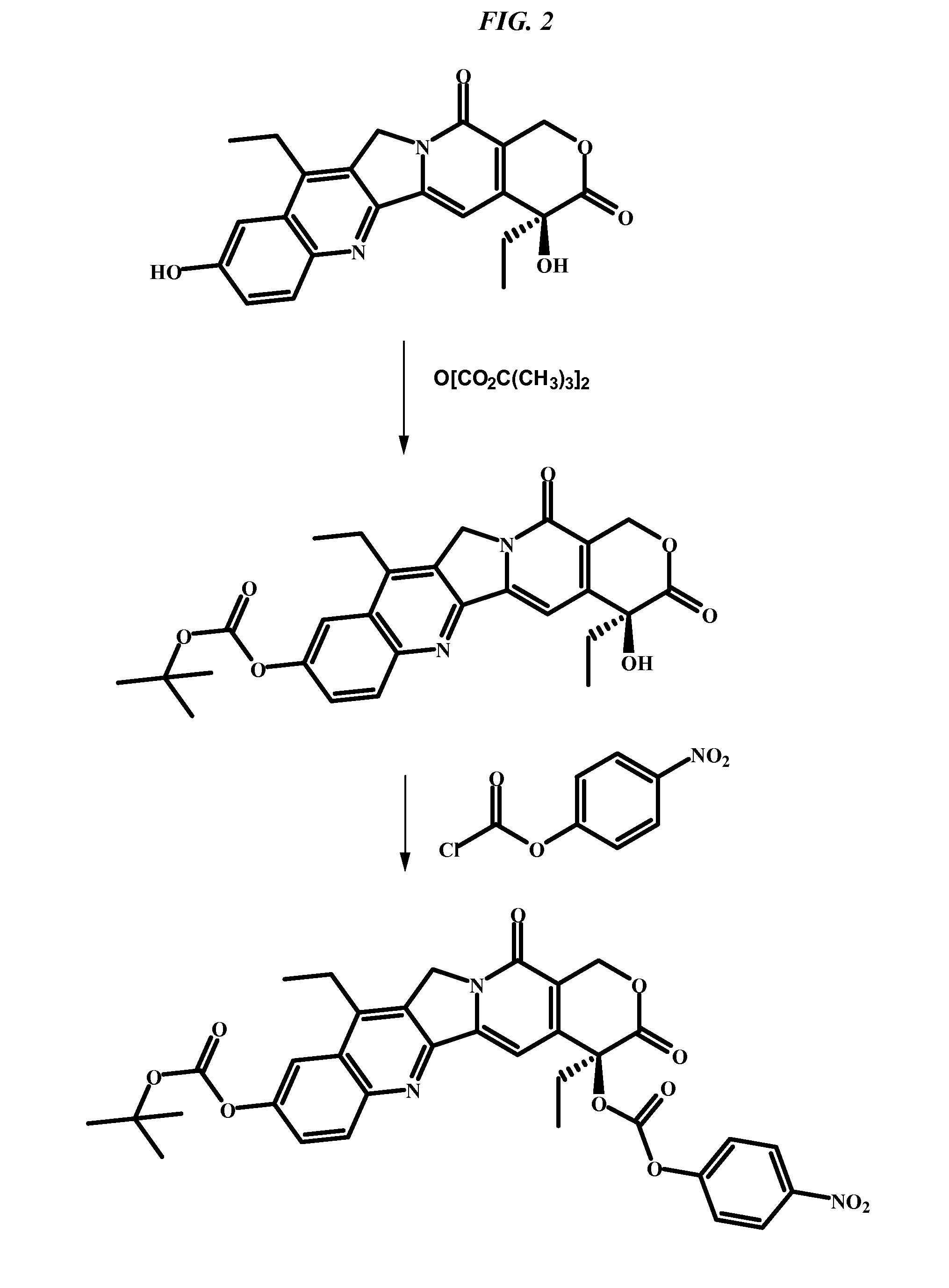
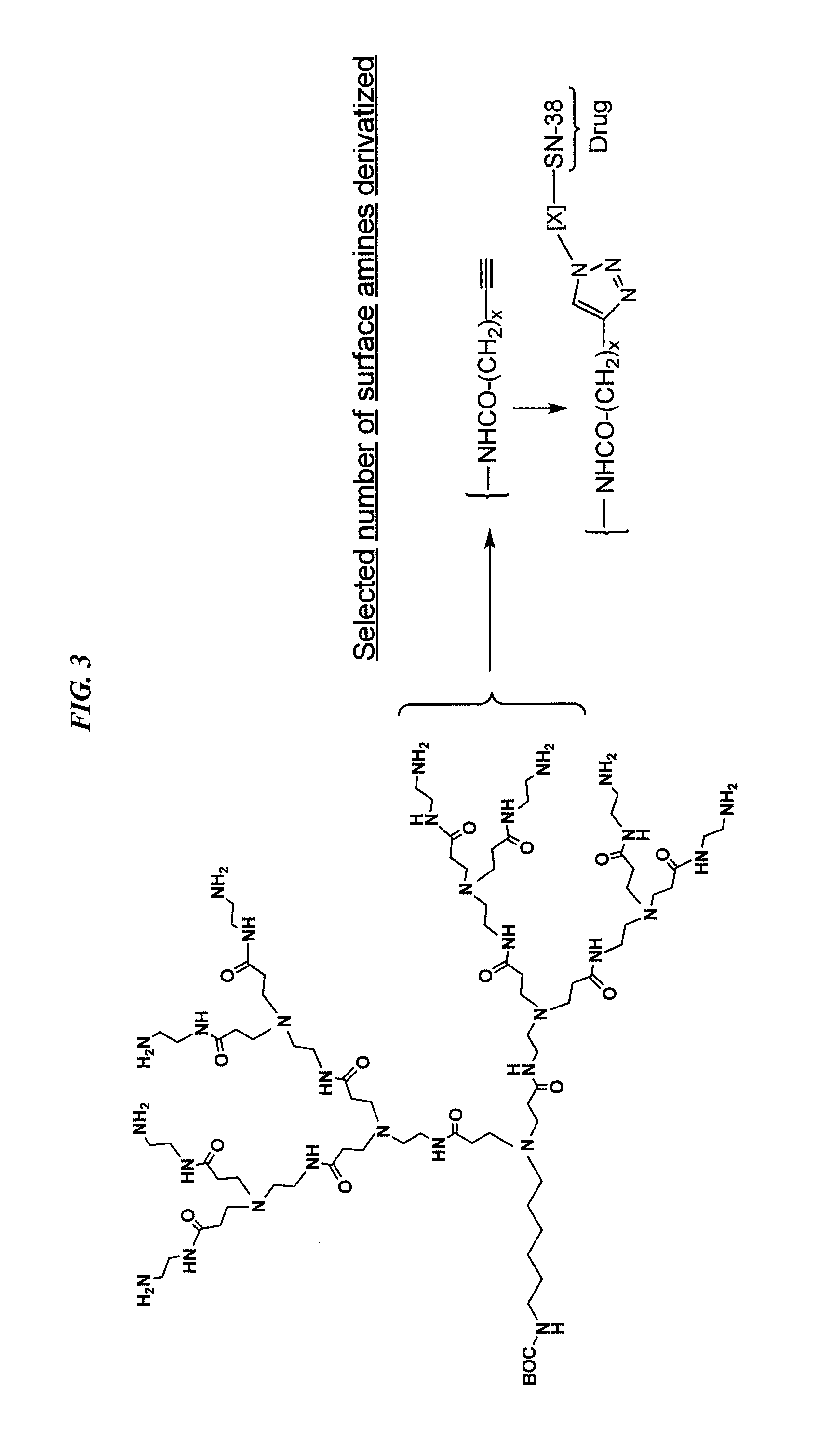
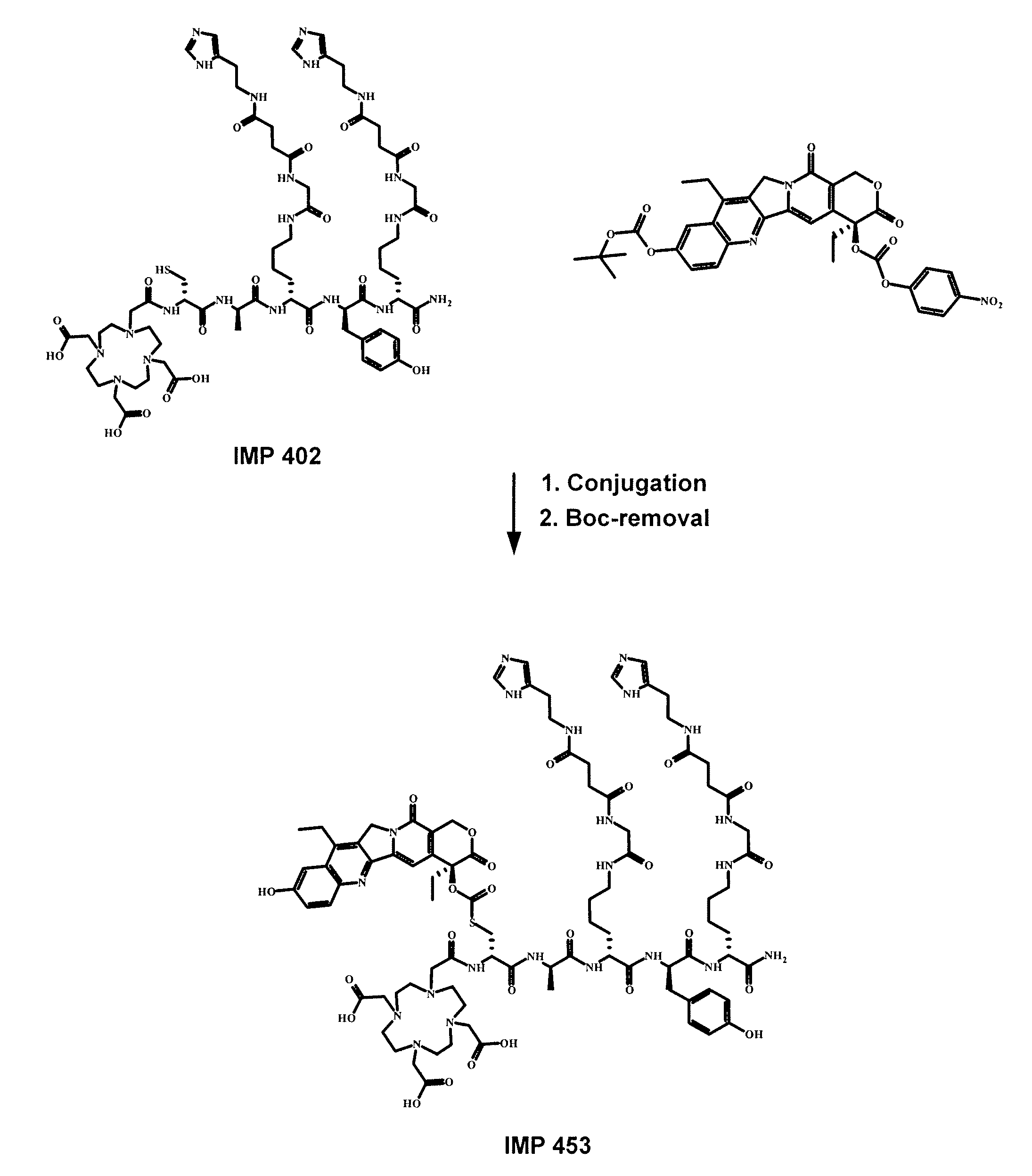
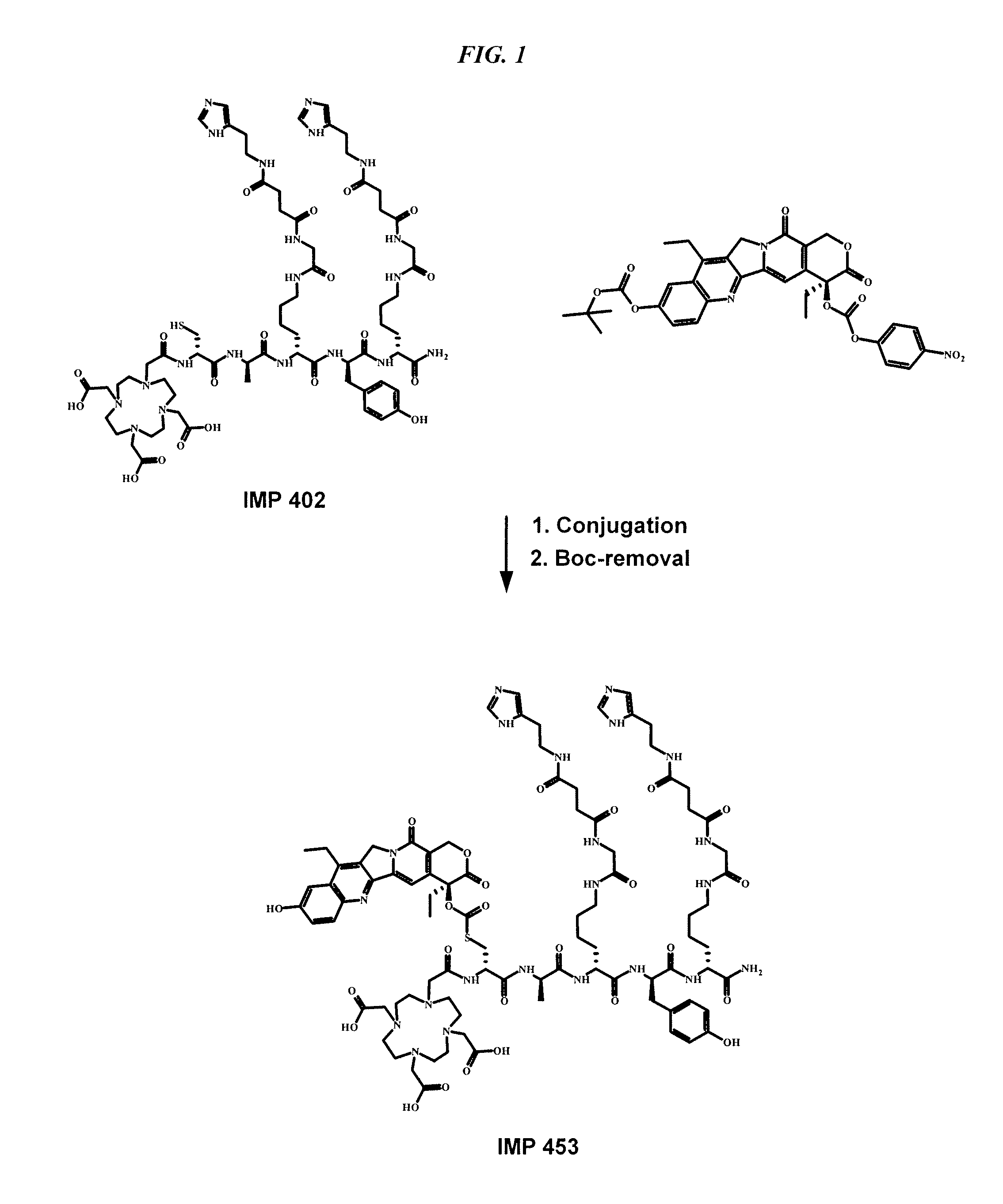
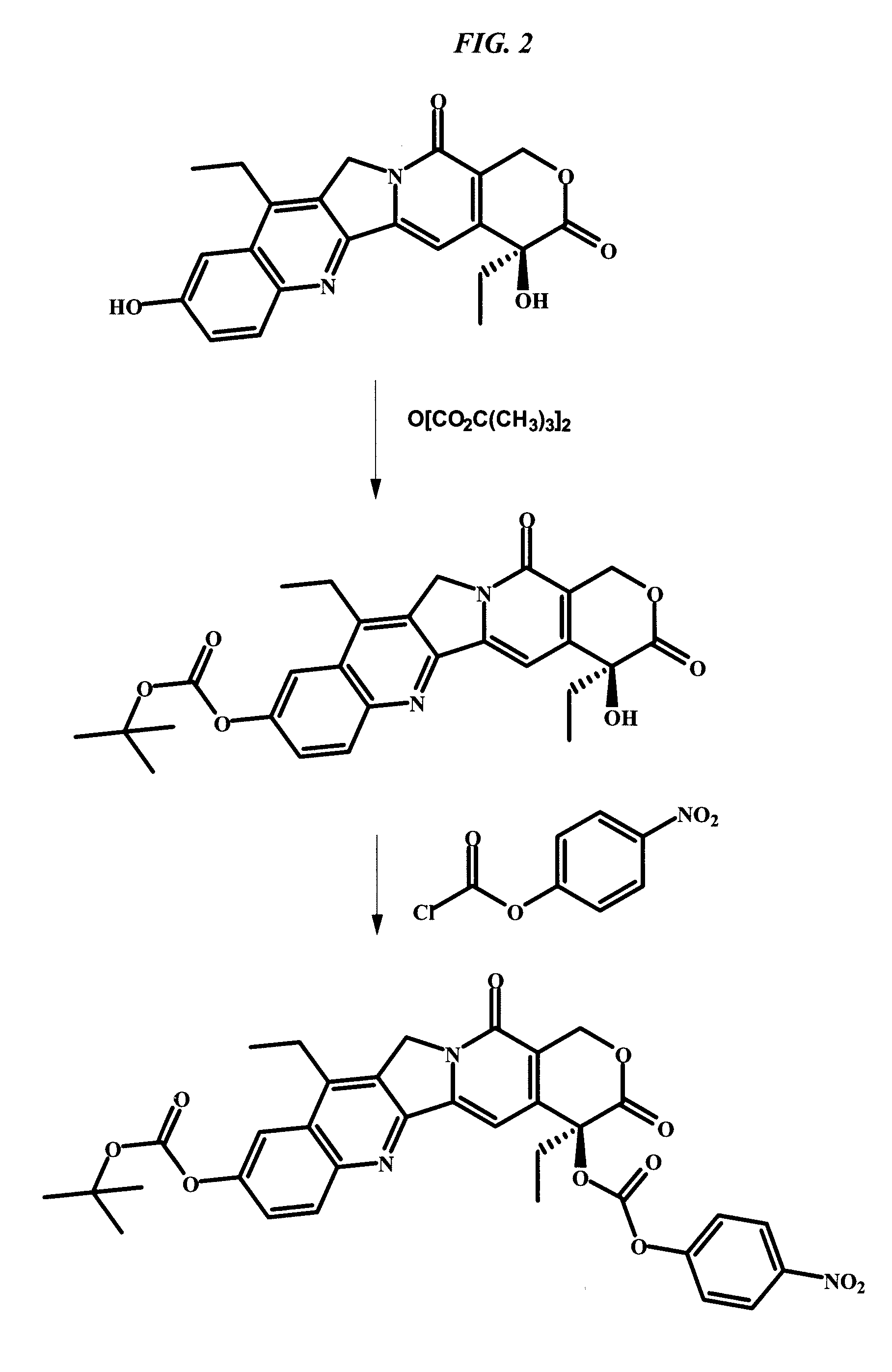
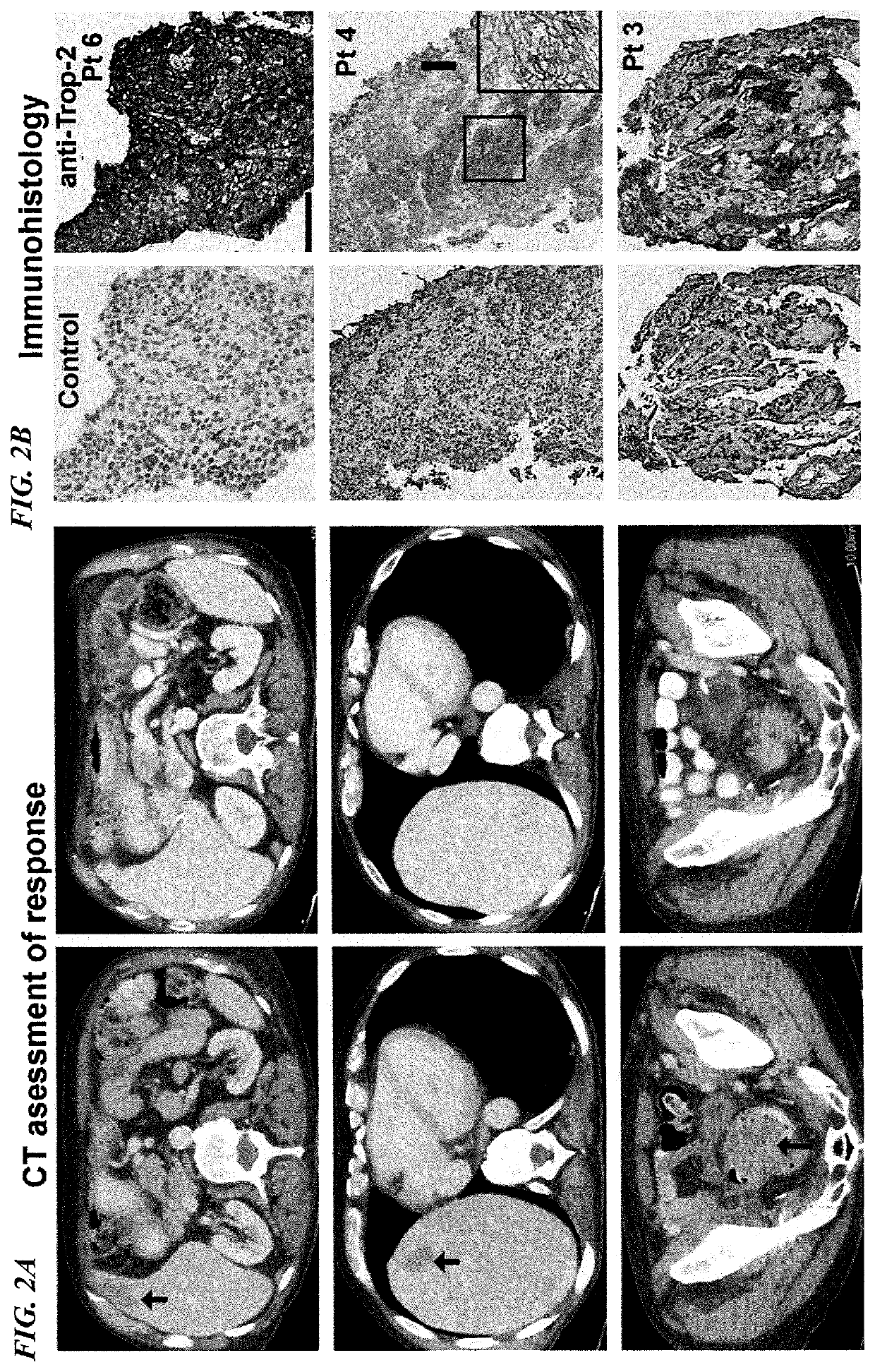
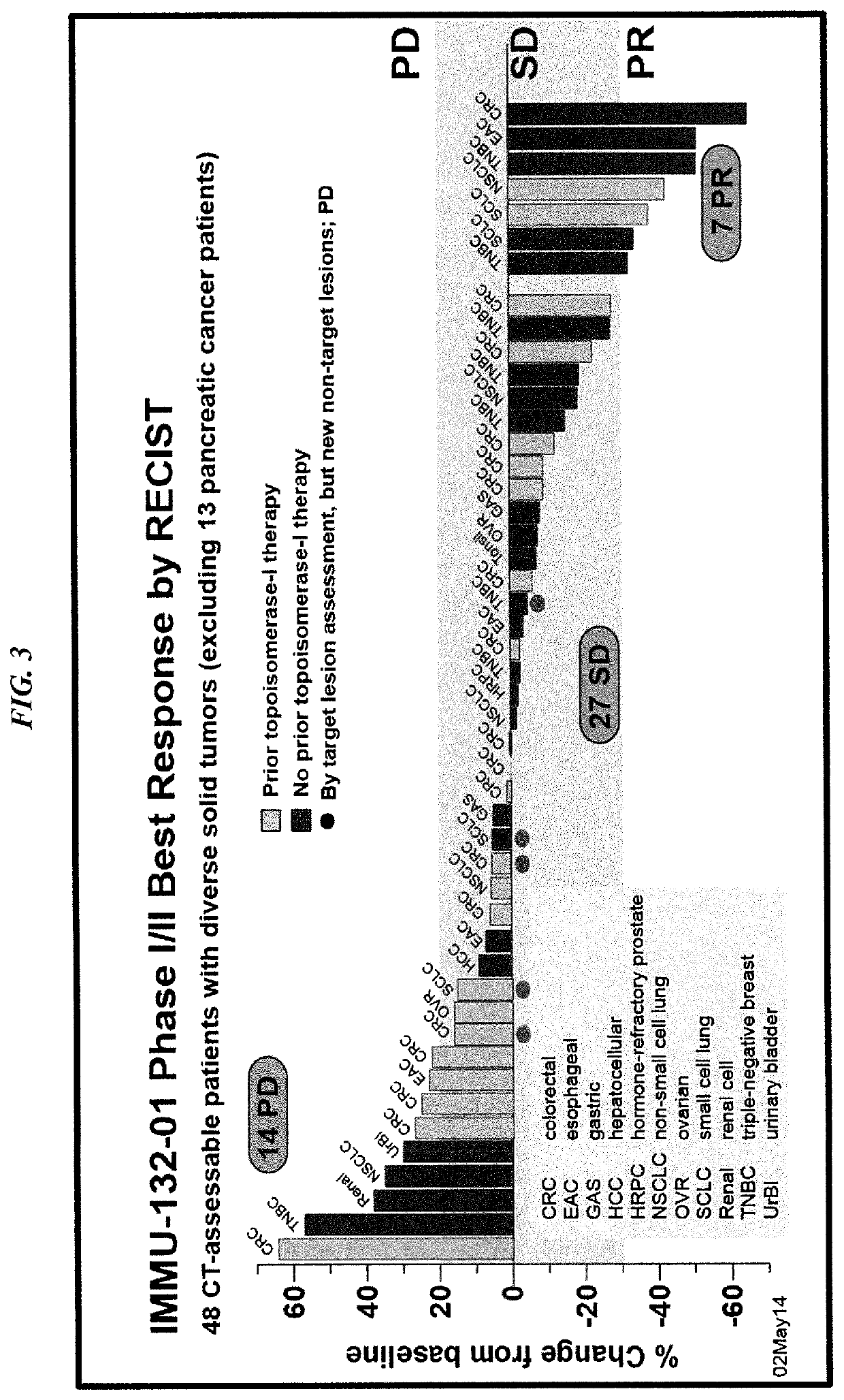
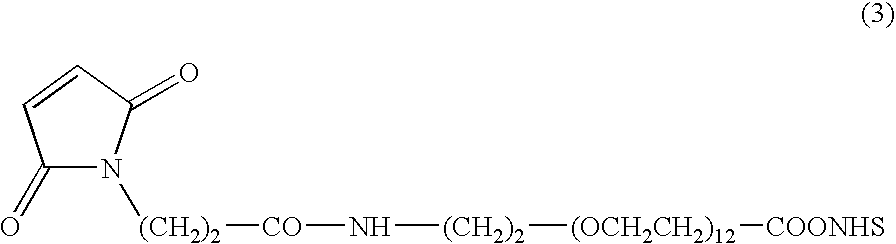Patents
Literature
33results about How to "Reducing certain severe side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
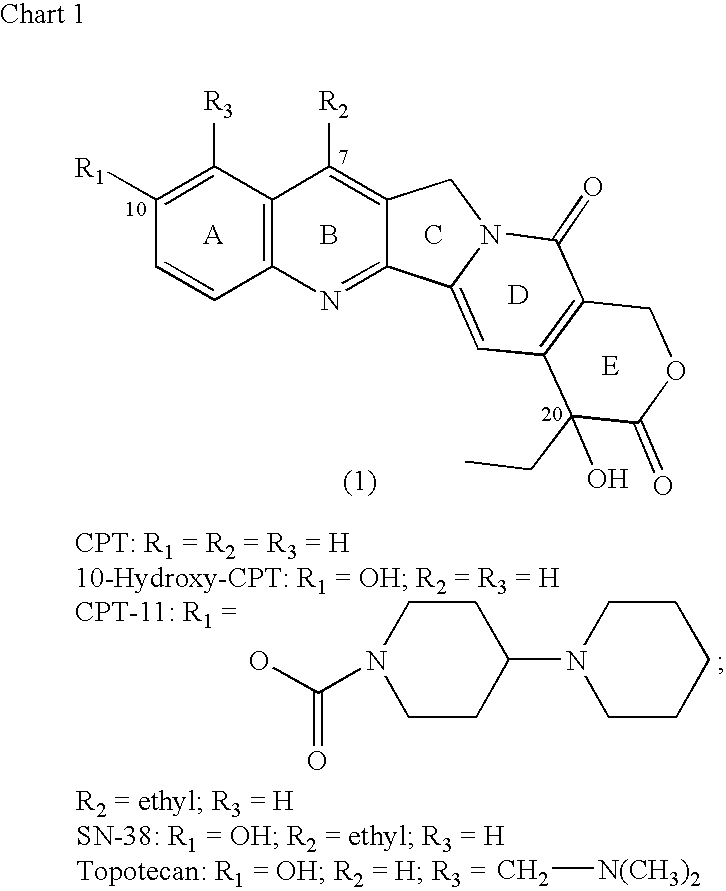
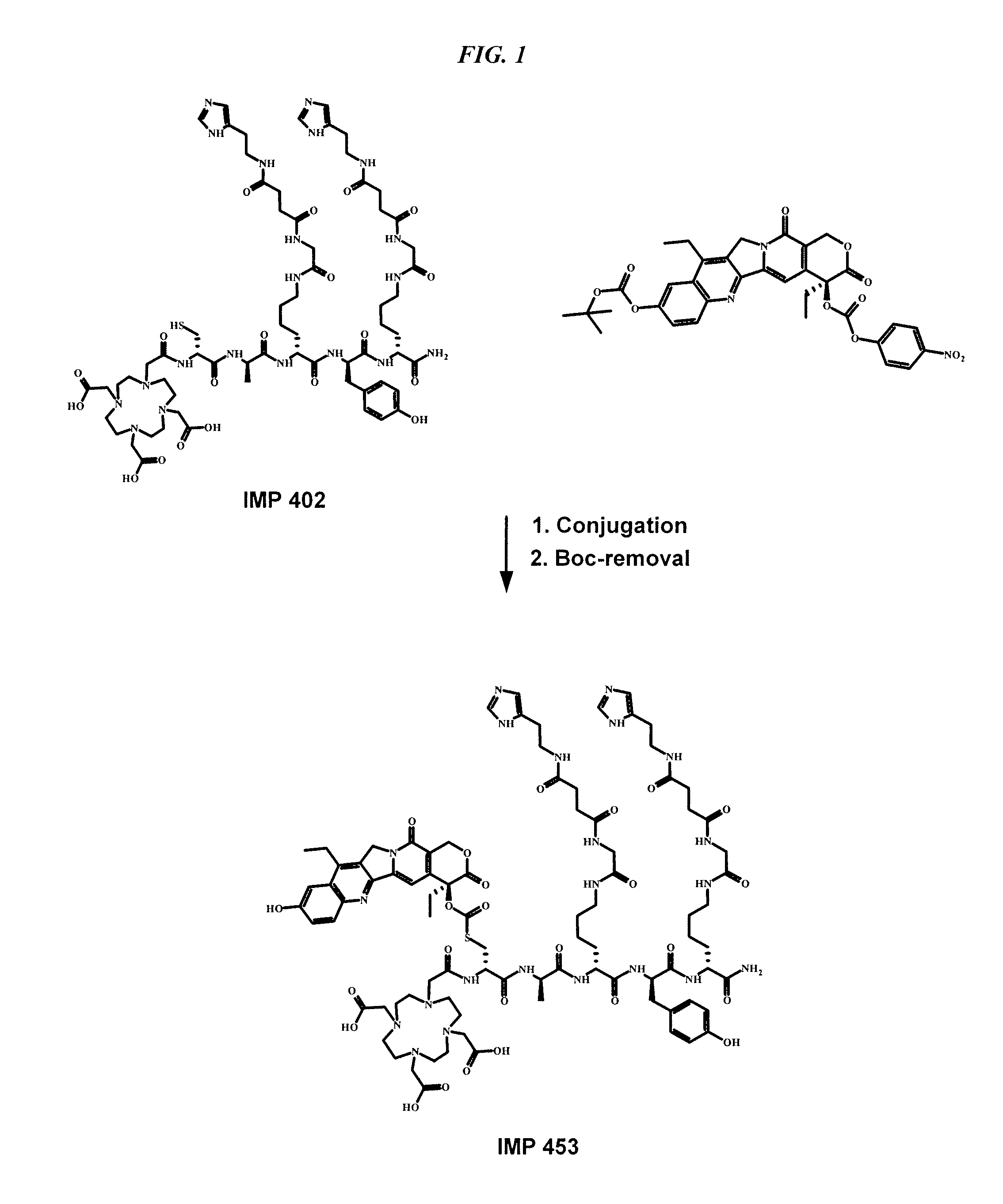
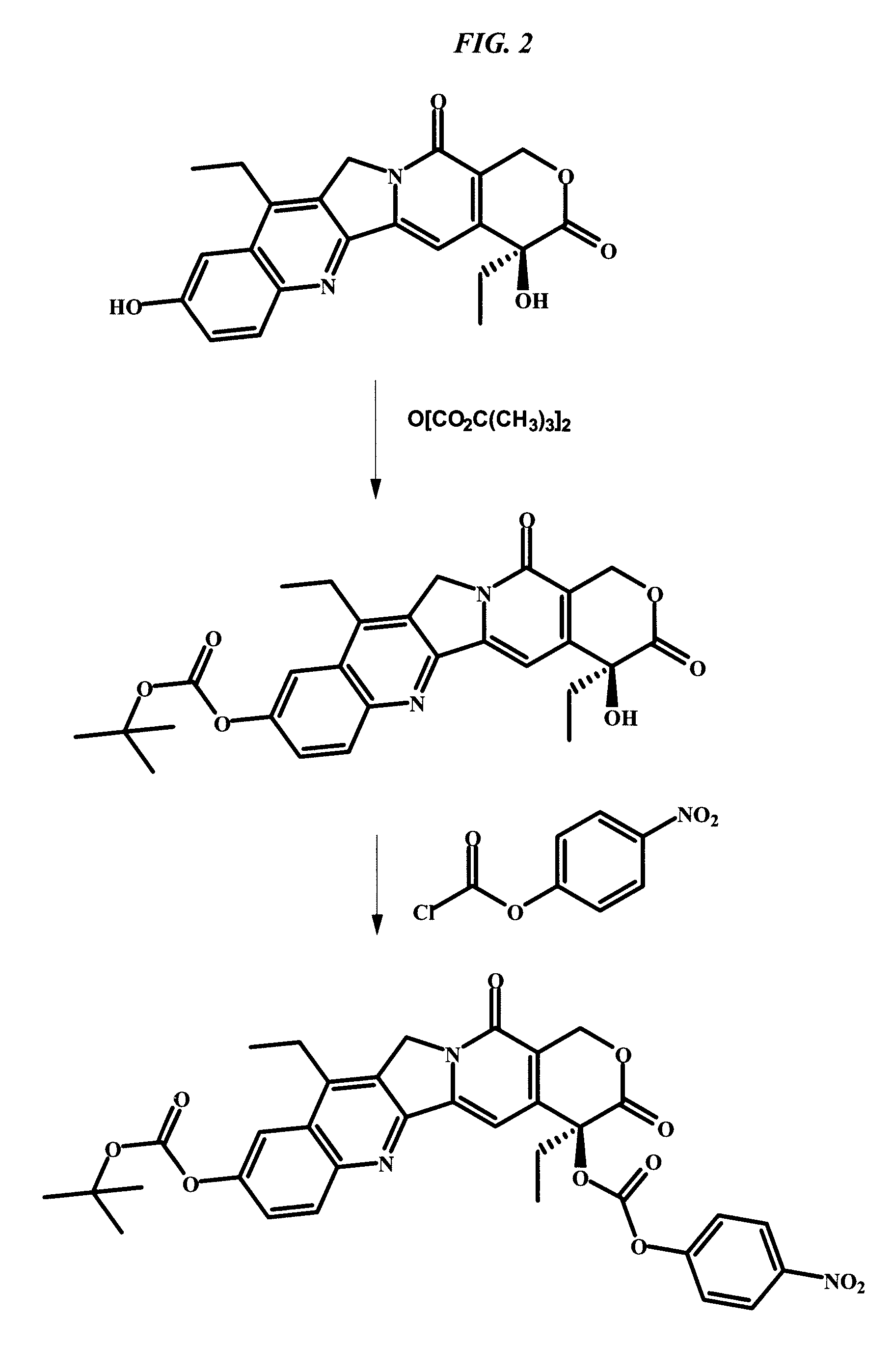
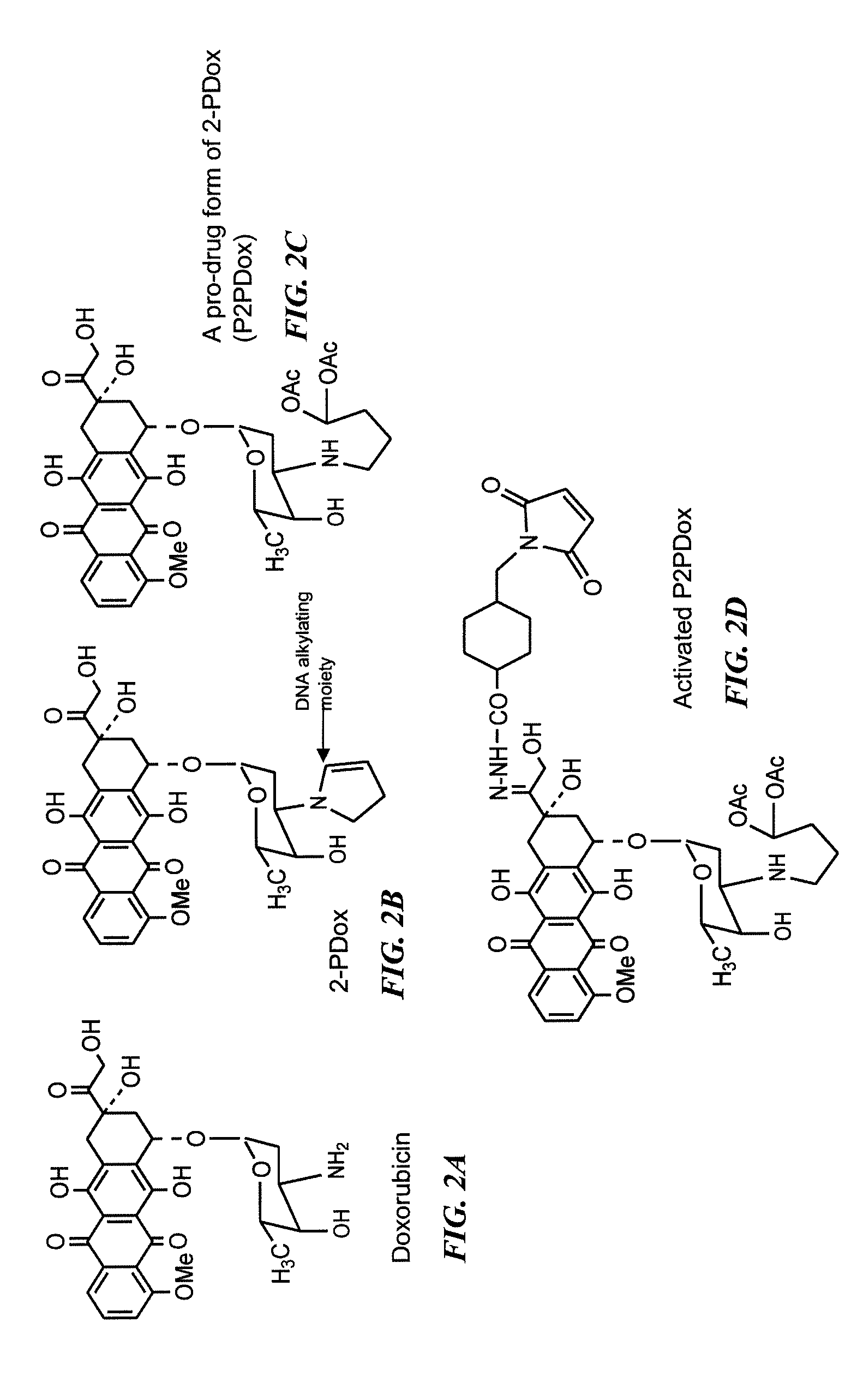
Camptothecin-binding moiety conjugates
ActiveUS20060193865A1High affinityReducing certain severe side effectsAntibacterial agentsNervous disorderAntibody fragmentsCamptothecin
The invention relates to therapeutic conjugates with improved ability to target various diseased cells containing a targeting moiety (such as an antibody or antibody fragment), a linker and a camptothecin as a therapeutic moiety, and further relates to processes for making and using the said conjugates.
Owner:IMMUNOMEDICS INC
Immunoconjugates with an intracellularly-cleavable linkage
InactiveUS7999083B2Reducing certain severe side effectsReceive treatment wellAntibacterial agentsOrganic active ingredientsIntracellularAntibody fragments
Owner:IMMUNOMEDICS INC
Camptothecin-Binding Moiety Conjugates
ActiveUS20080166363A1Reducing certain severe side effectsReceive treatment wellAntibacterial agentsAntimycoticsAntibody fragmentsCamptothecin
The invention relates to therapeutic conjugates with improved ability to target various diseased cells containing a targeting moiety (such as an antibody or antibody fragment), a linker and a camptothecin as a therapeutic moiety, and further relates to processes for making and using the said conjugates.
Owner:IMMUNOMEDICS INC
Immunoconjugates with an Intracellularly-Cleavable Linkage
ActiveUS20100104589A1Reducing certain severe side effectsReceive treatment wellAntibacterial agentsOrganic active ingredientsAntibody fragmentsChemistry
Owner:IMMUNOMEDICS INC
Camptothecin-binding moiety conjugates
ActiveUS7591994B2Reducing certain severe side effectsReceive treatment wellAntibacterial agentsAntimycoticsAntibody fragmentsCamptothecin
The invention relates to therapeutic conjugates with improved ability to target various diseased cells containing a targeting moiety (such as an antibody or antibody fragment), a linker and a camptothecin as a therapeutic moiety, and further relates to processes for making and using the said conjugates.
Owner:IMMUNOMEDICS INC
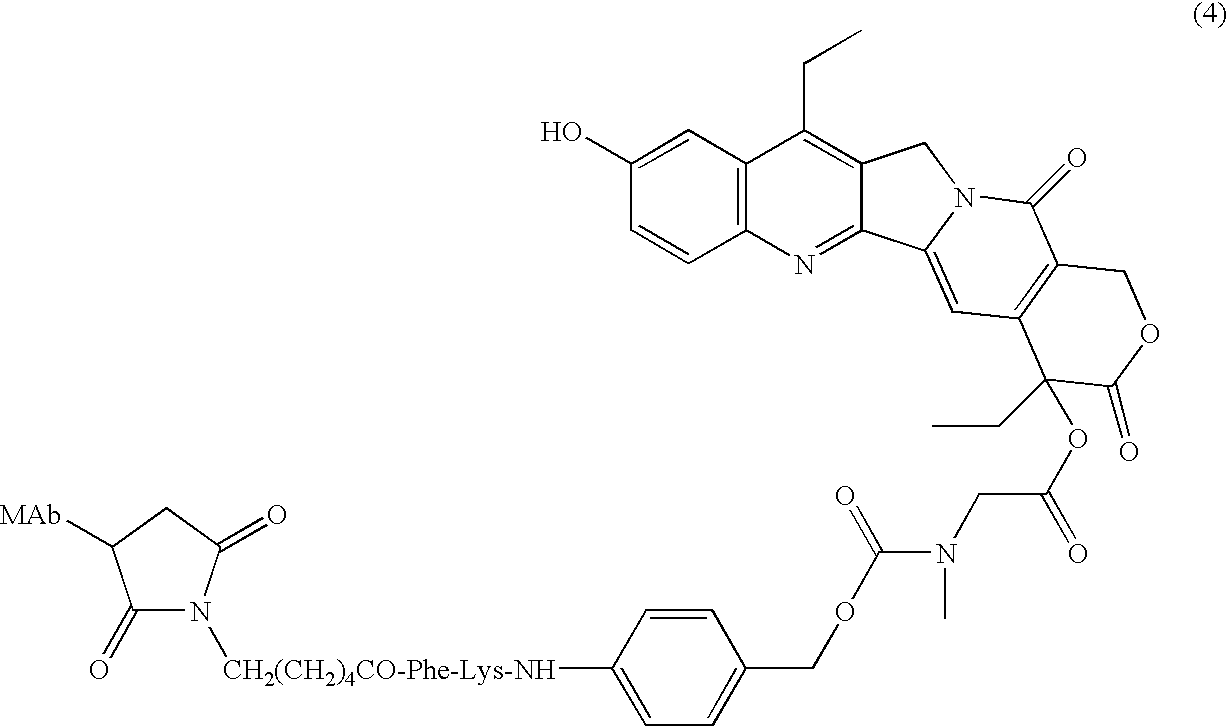
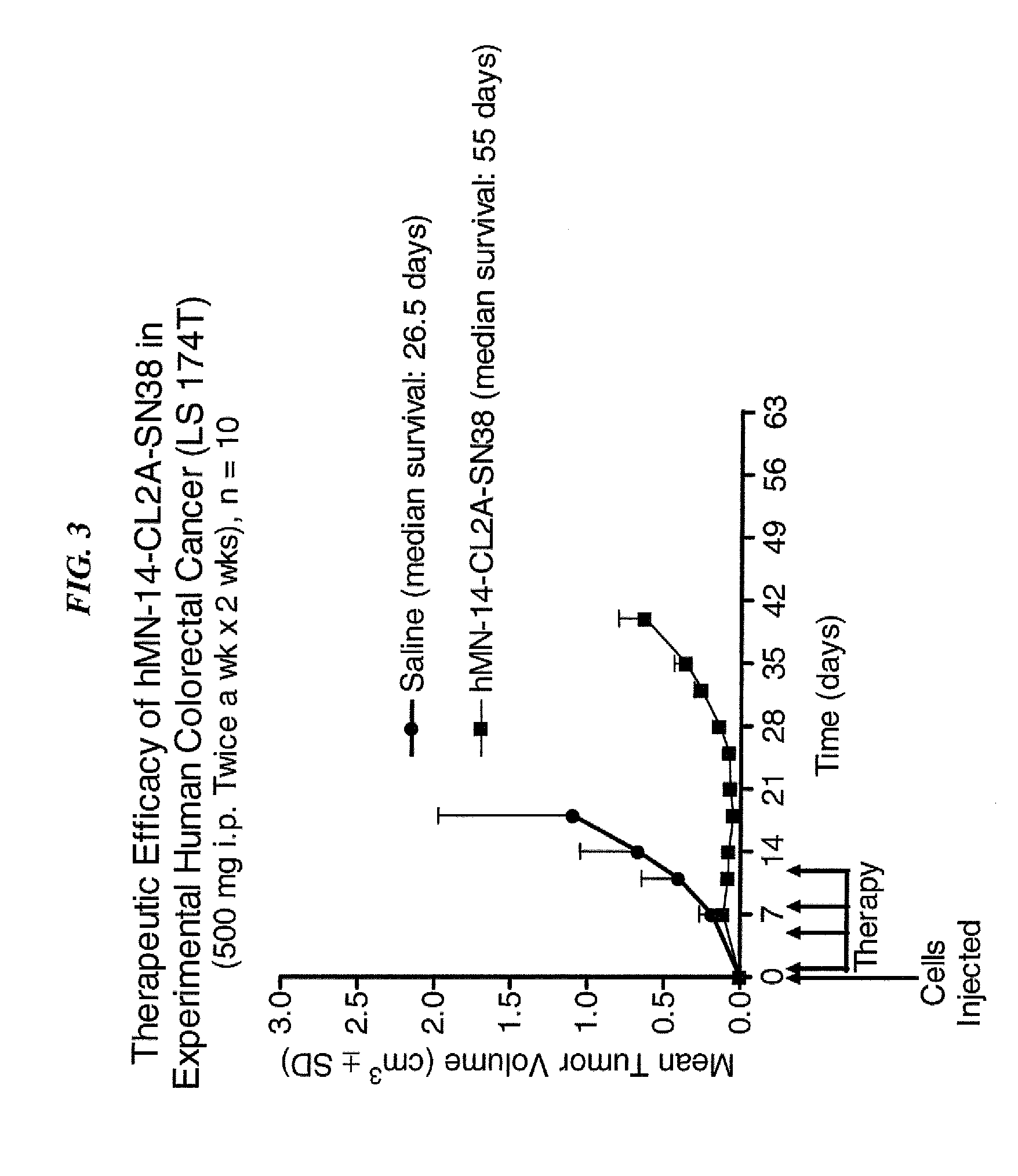
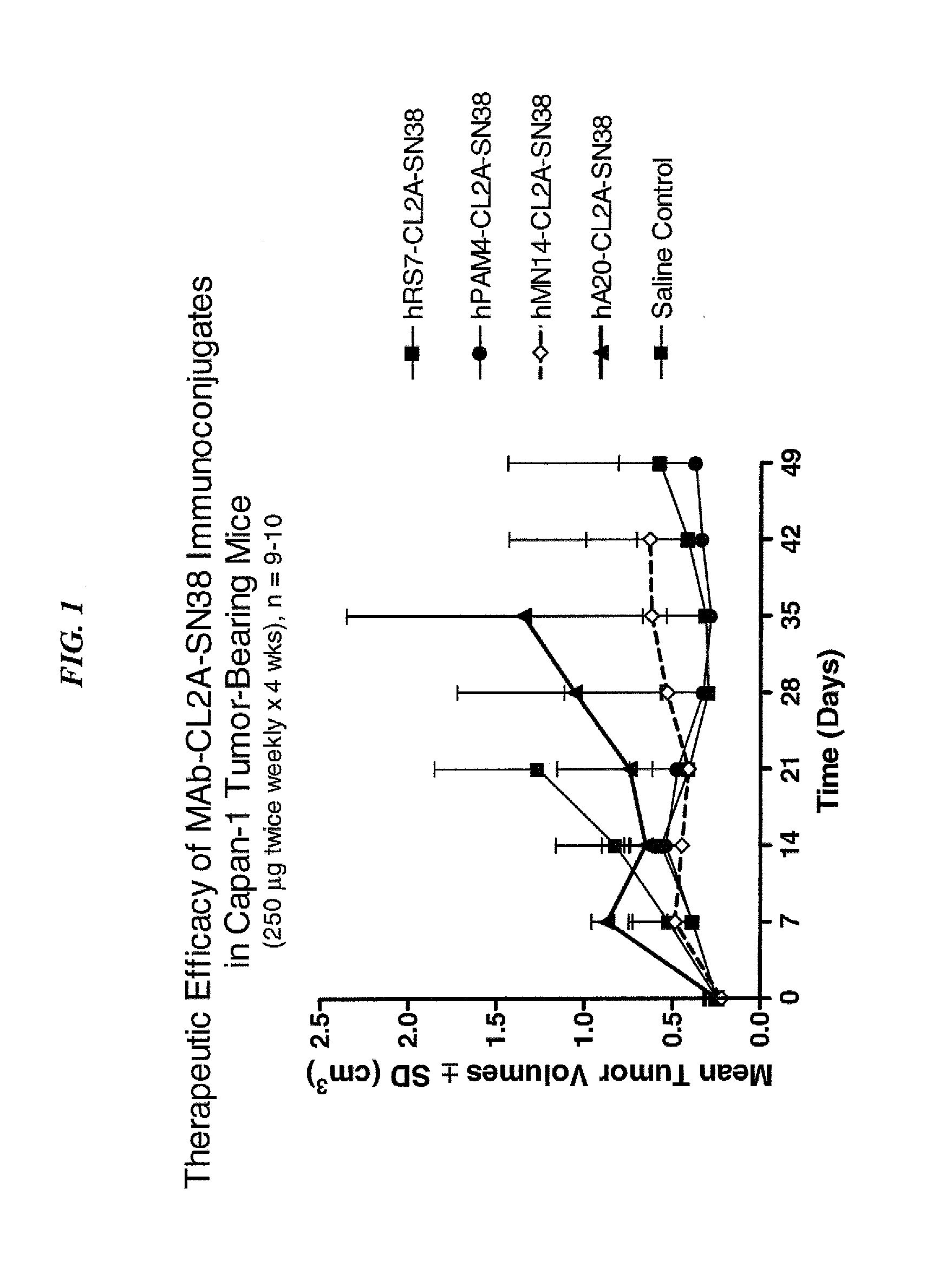
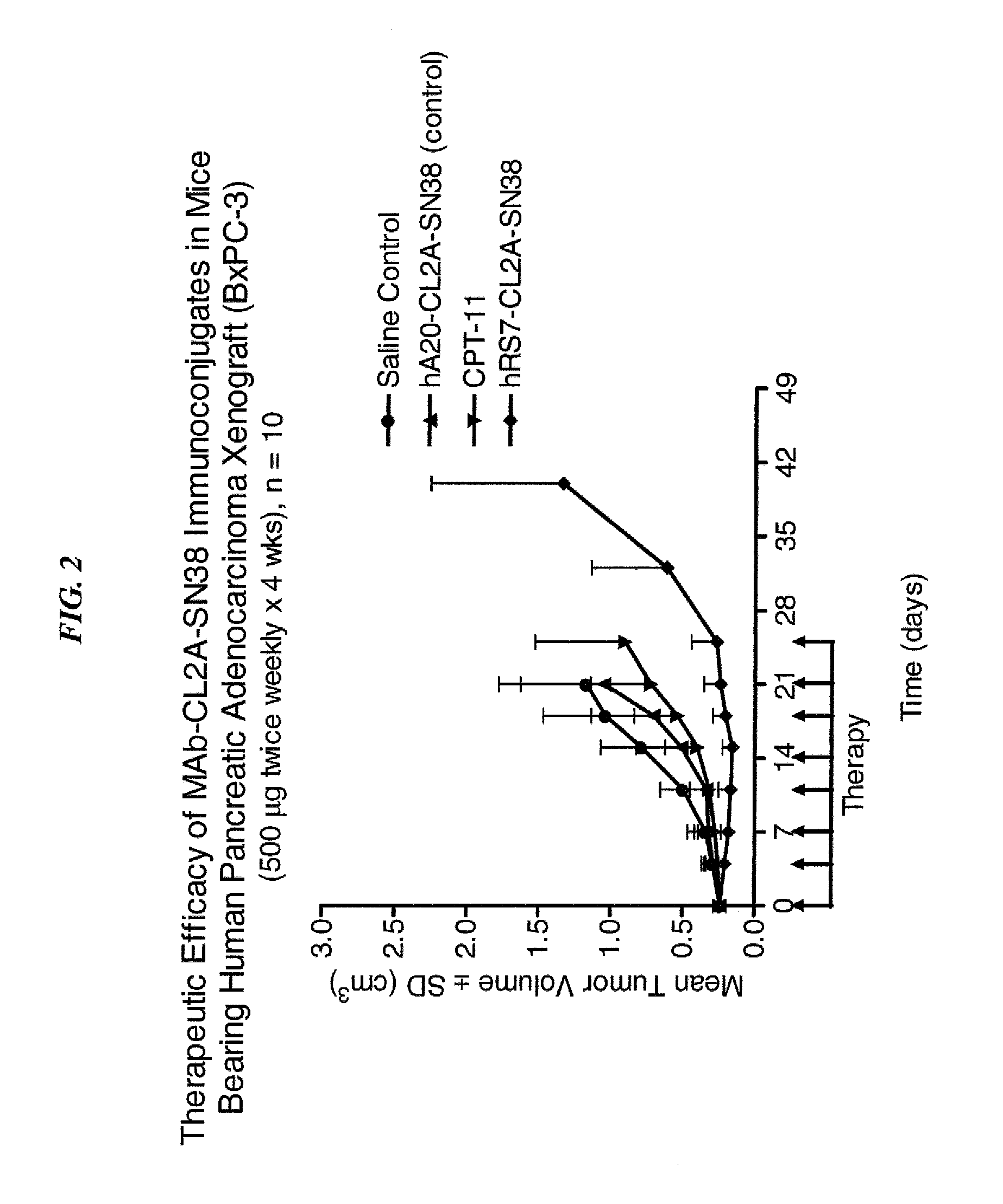
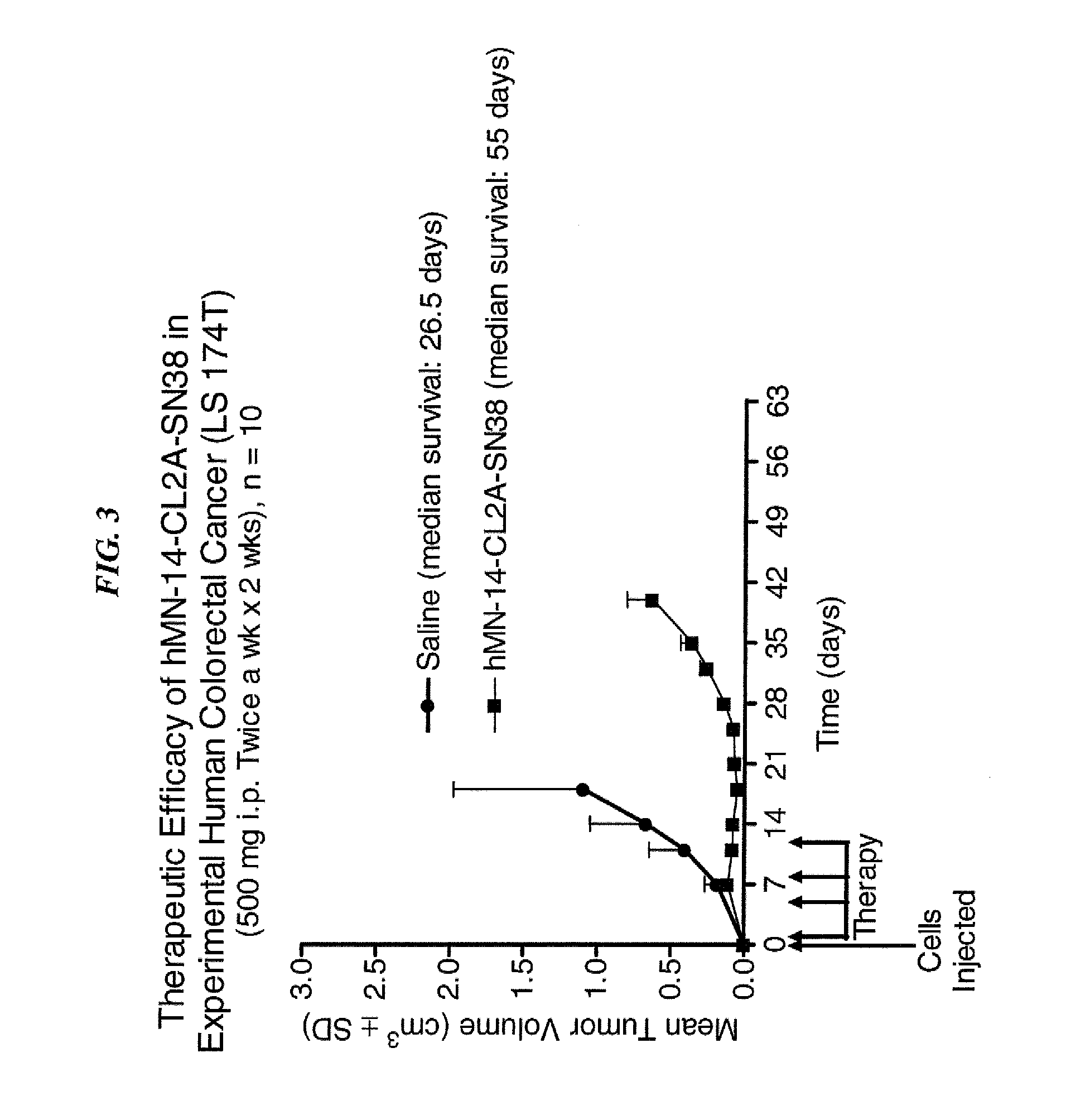
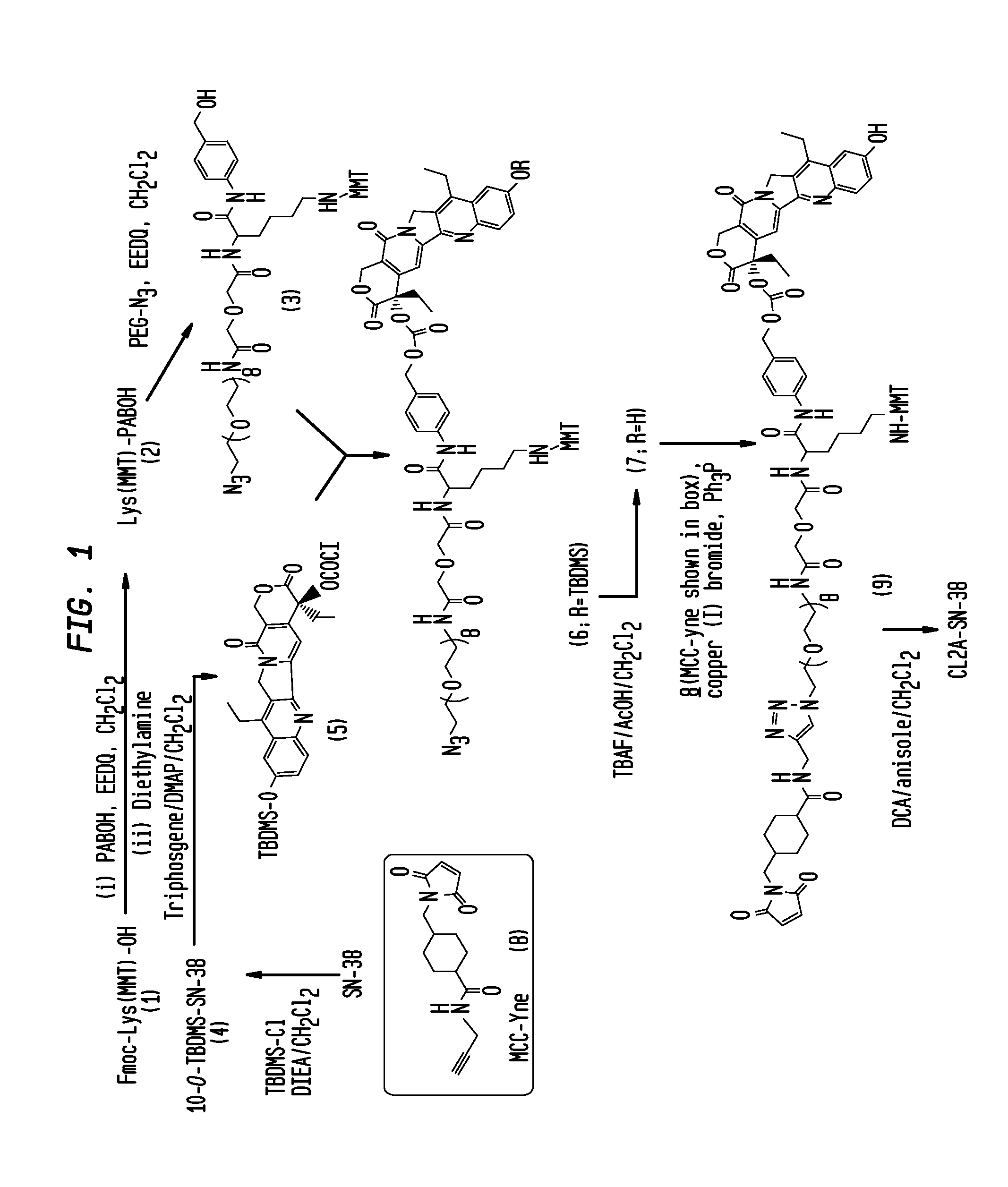
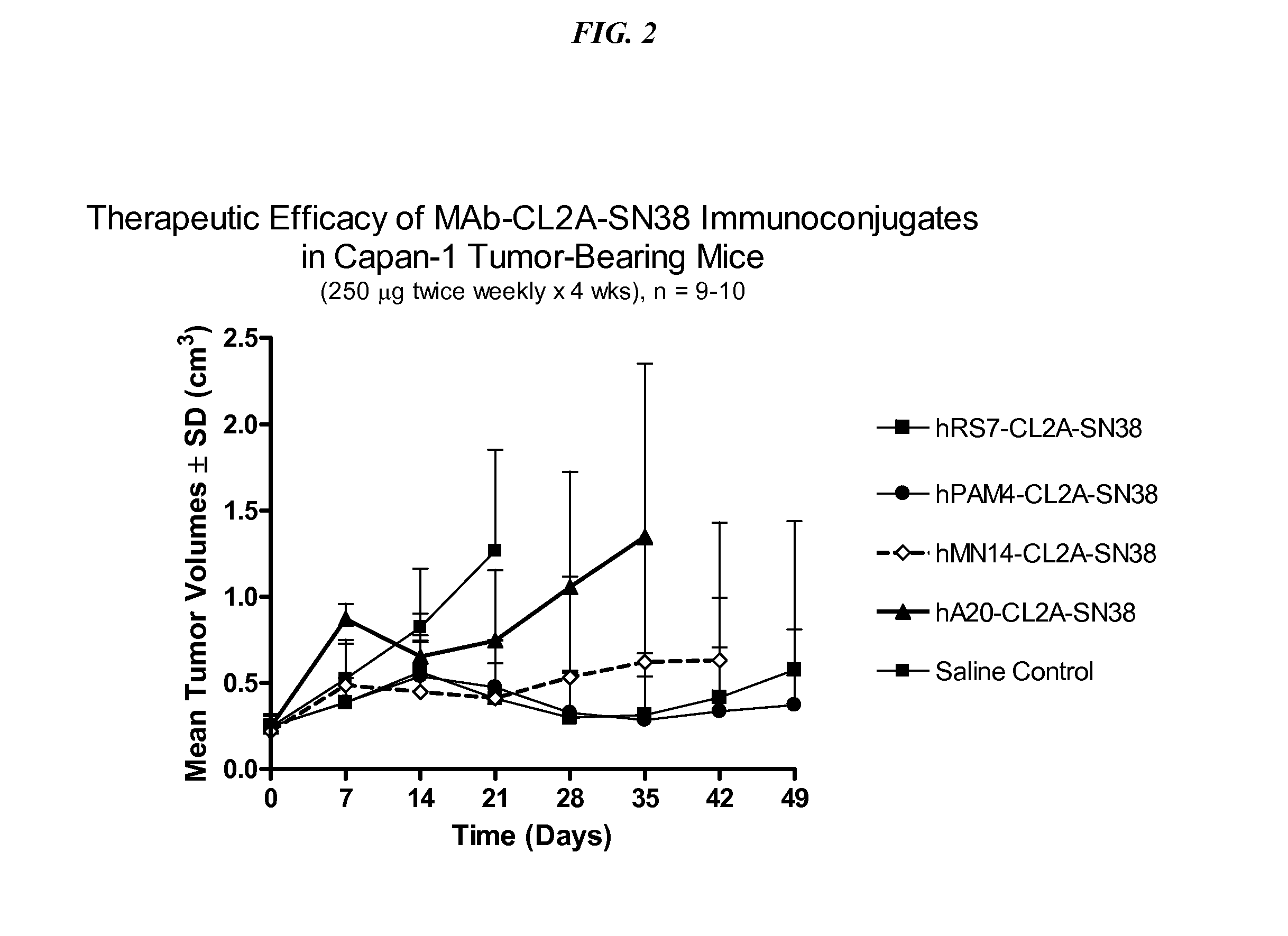
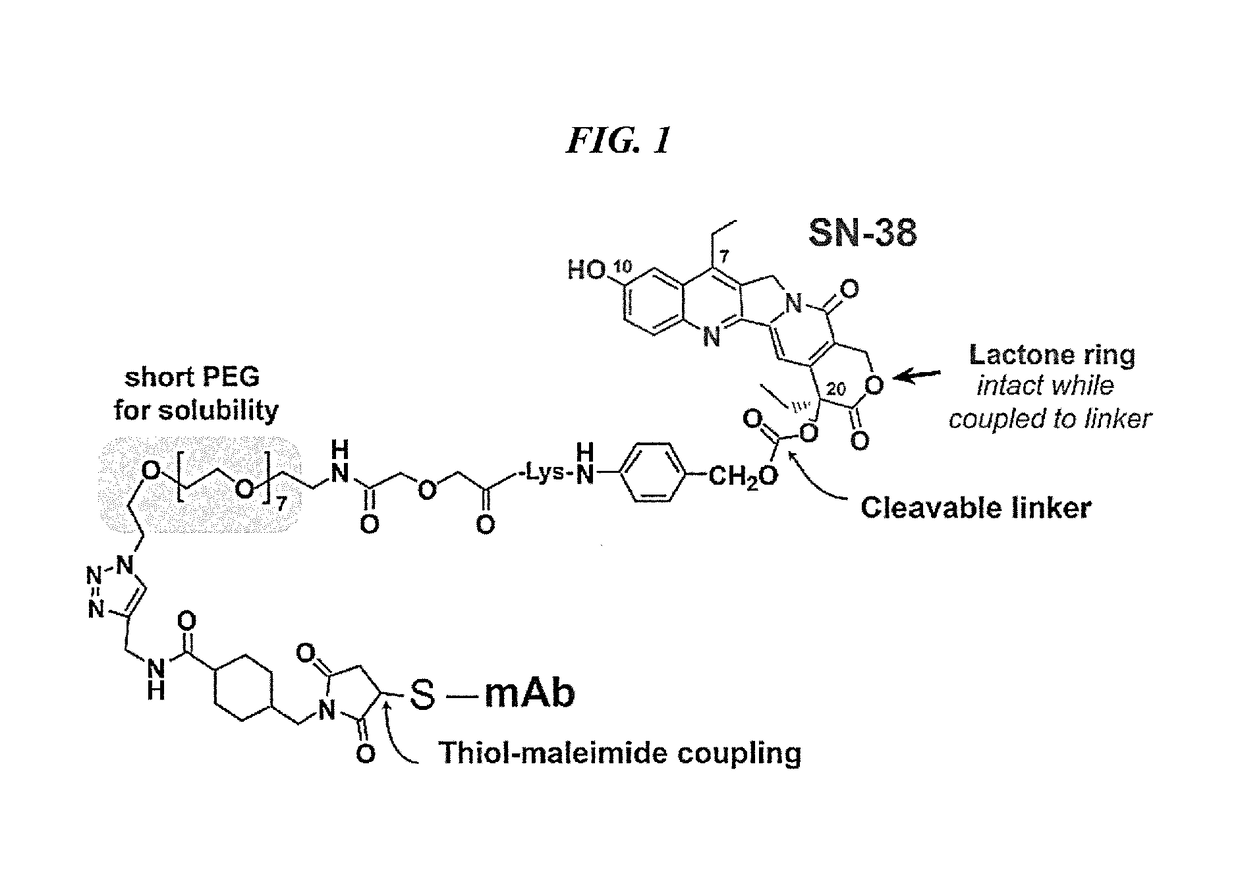
Antibody-sn-38 immunoconjugates with a cl2a linker
ActiveUS20140227180A1Improve drug bioavailabilityGood treatment effectOrganic active ingredientsPeptide/protein ingredientsDiseaseSide effect
The present invention concerns improved methods and compositions for preparing SN-38 conjugates of proteins or peptides, preferably immunoconjugates of antibodies or antigen-binding antibody fragments. More preferably, the SN-38 is attached to the antibody or antibody fragment using a CL2A linker, with 1-12, more preferably 6 or less, most preferably 1-5 SN-38 moieties per antibody or antibody fragment. Most preferably, the immunoconjugate is prepared in large scale batches, with various modifications to the reaction scheme to optimize yield and recovery in large scale. Other embodiments concern optimized dosages and / or schedules of administration of immunoconjugate to maximize efficacy for disease treatment and minimize side effects of administration.
Owner:IMMUNOMEDICS INC
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20140170063A1Overcome tumorImprove targetingHeavy metal active ingredientsOrganic active ingredientsCD20Lymphatic Spread
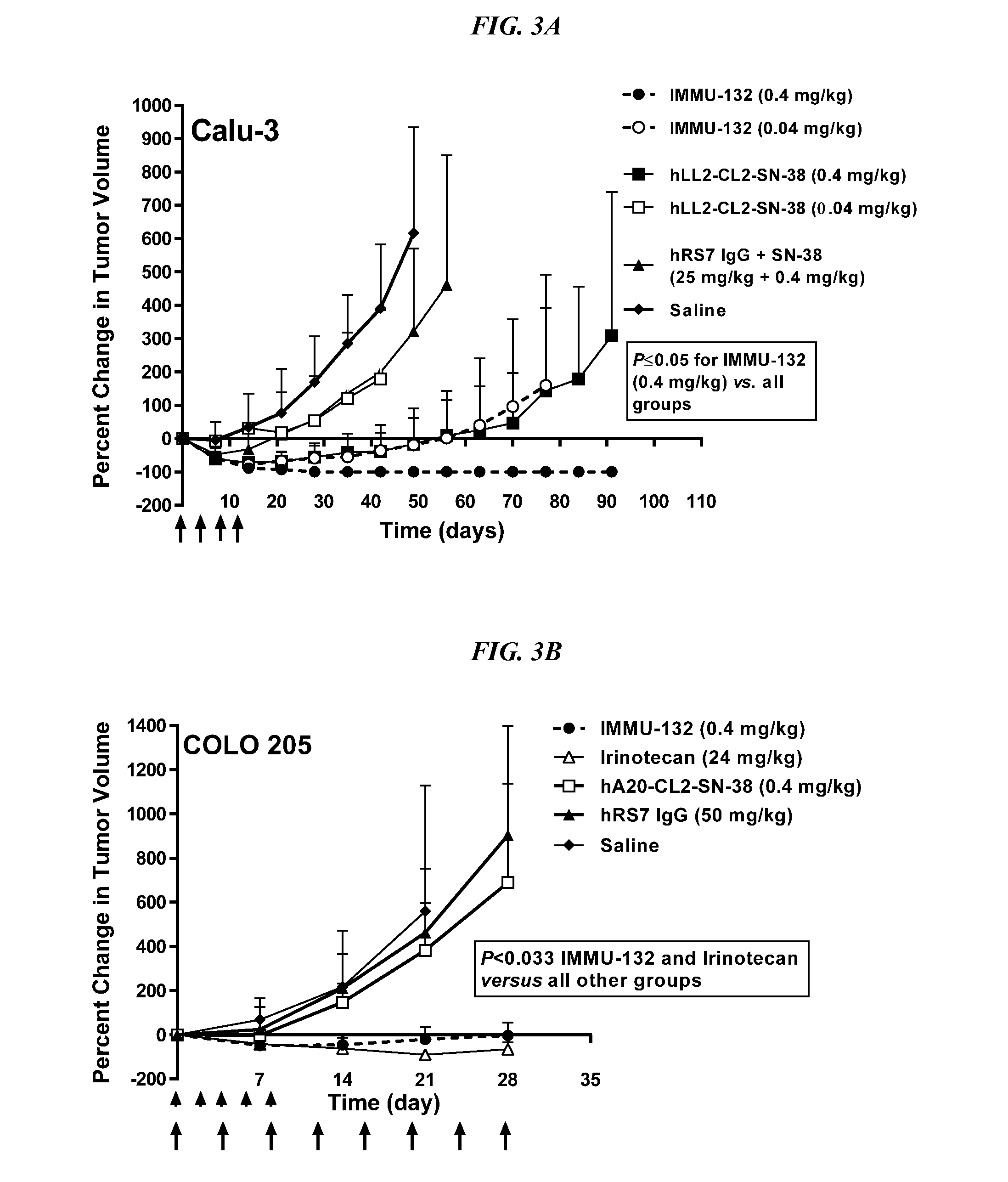
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 ROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20140219914A1Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsRadioactive preparation carriersCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Dosages of immunoconjugates of antibodies and SN-38 for improved efficacy and decreased toxicity
ActiveUS9028833B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsHeavy metal active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Camptothecin-binding moiety conjugates
InactiveUS8877901B2Reducing certain severe side effectsReceive treatment wellAntibacterial agentsNervous disorderAntibody fragmentsCamptothecin
Owner:IMMUNOMEDICS INC
Combining Anti-hla-dr or Anti-trop-2 antibodies with microtubule inhibitors, parp inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
ActiveUS20160296633A1Reducing certain severe side effectsReceive treatment wellHeavy metal active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadTreatment effect
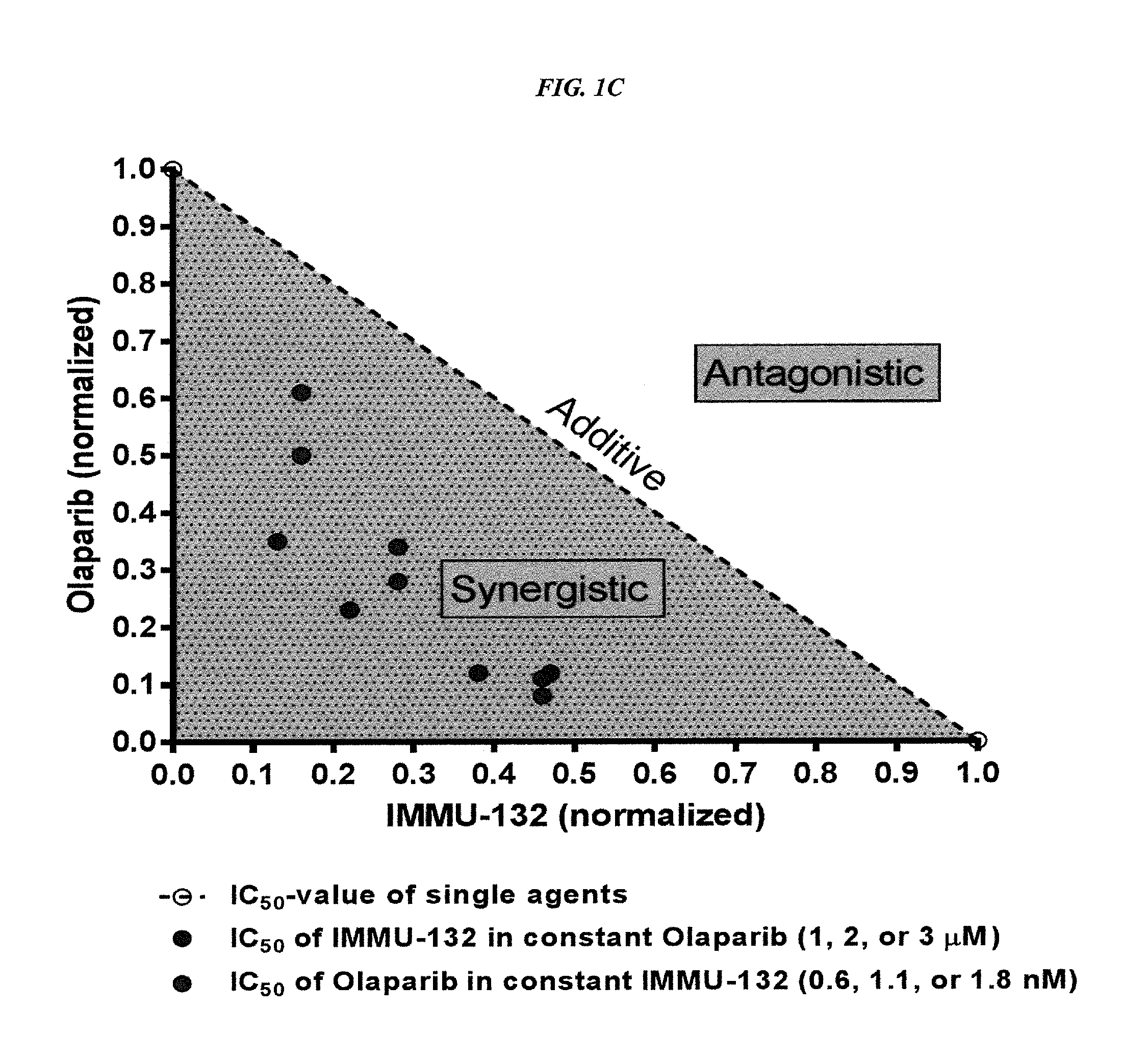
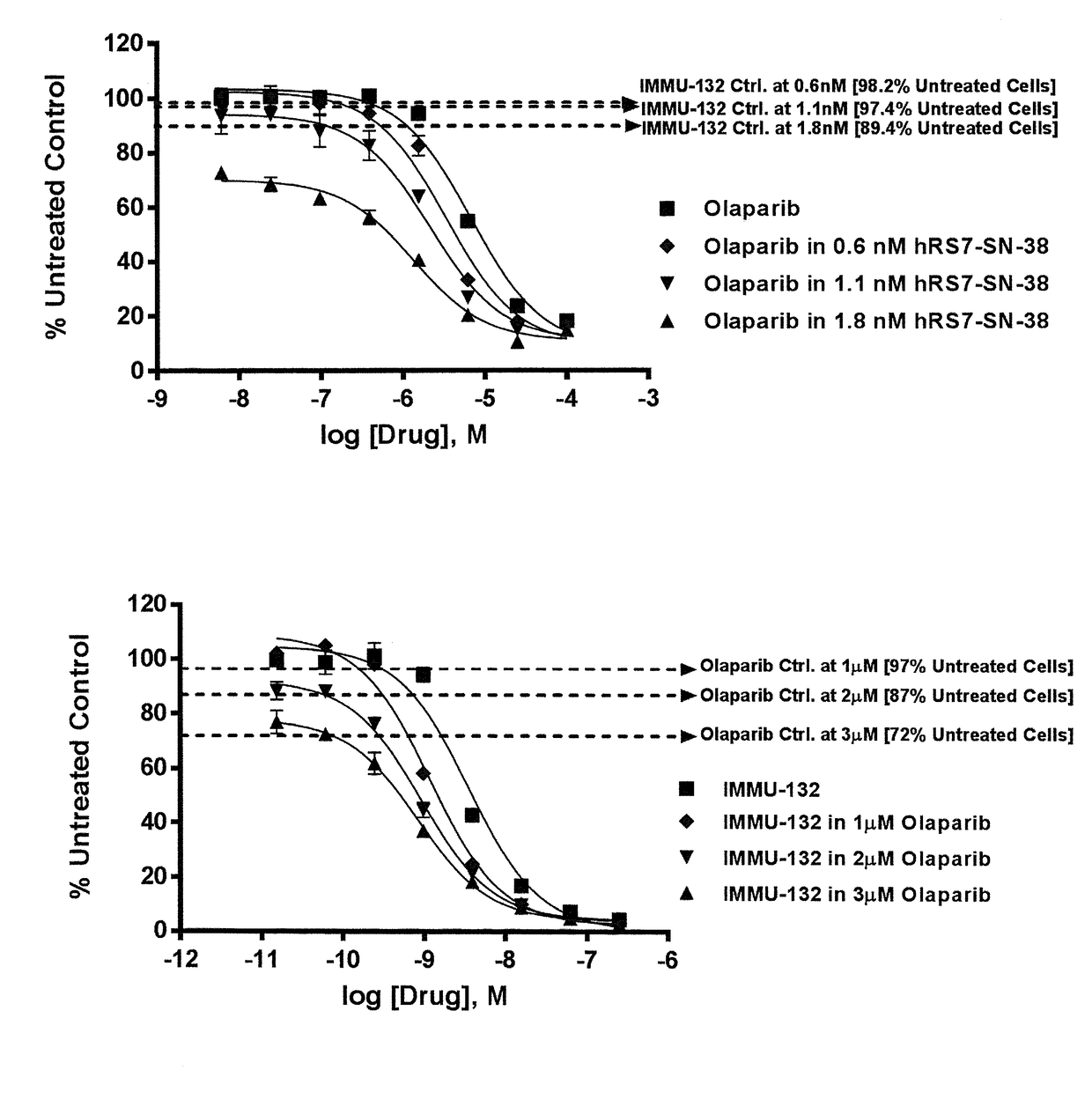
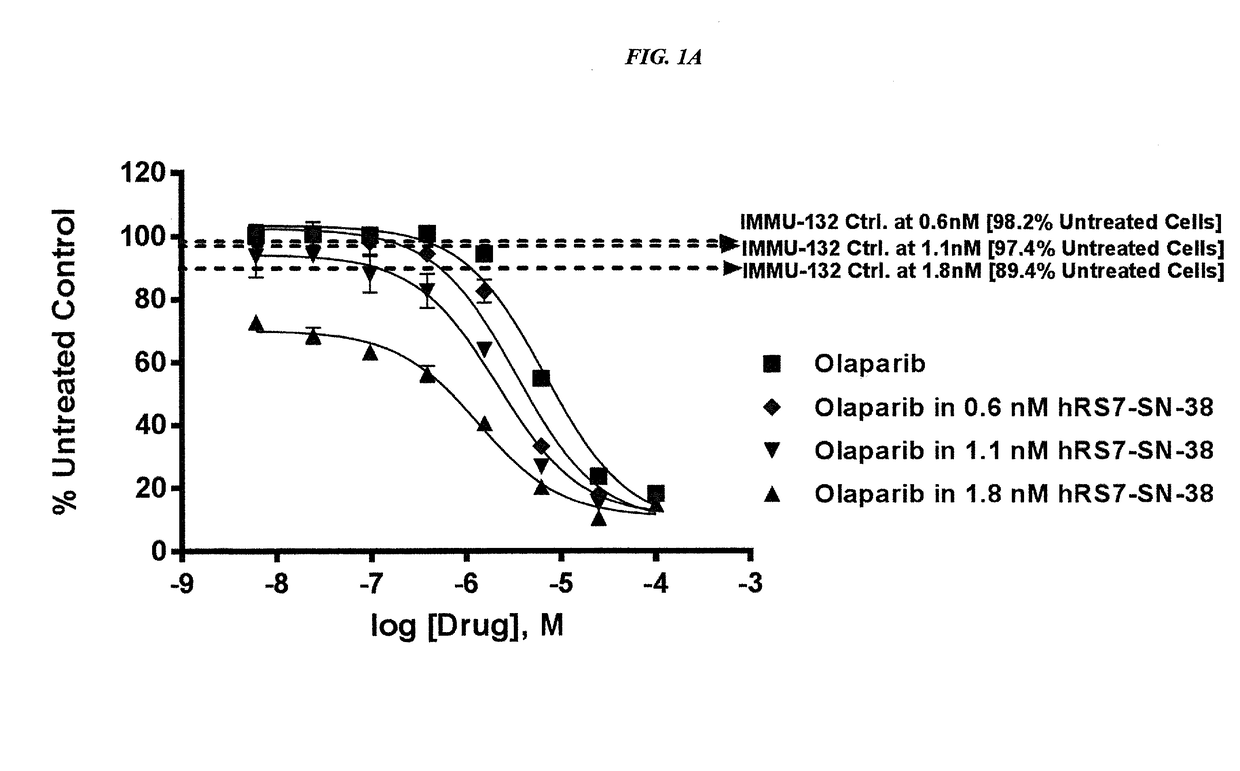
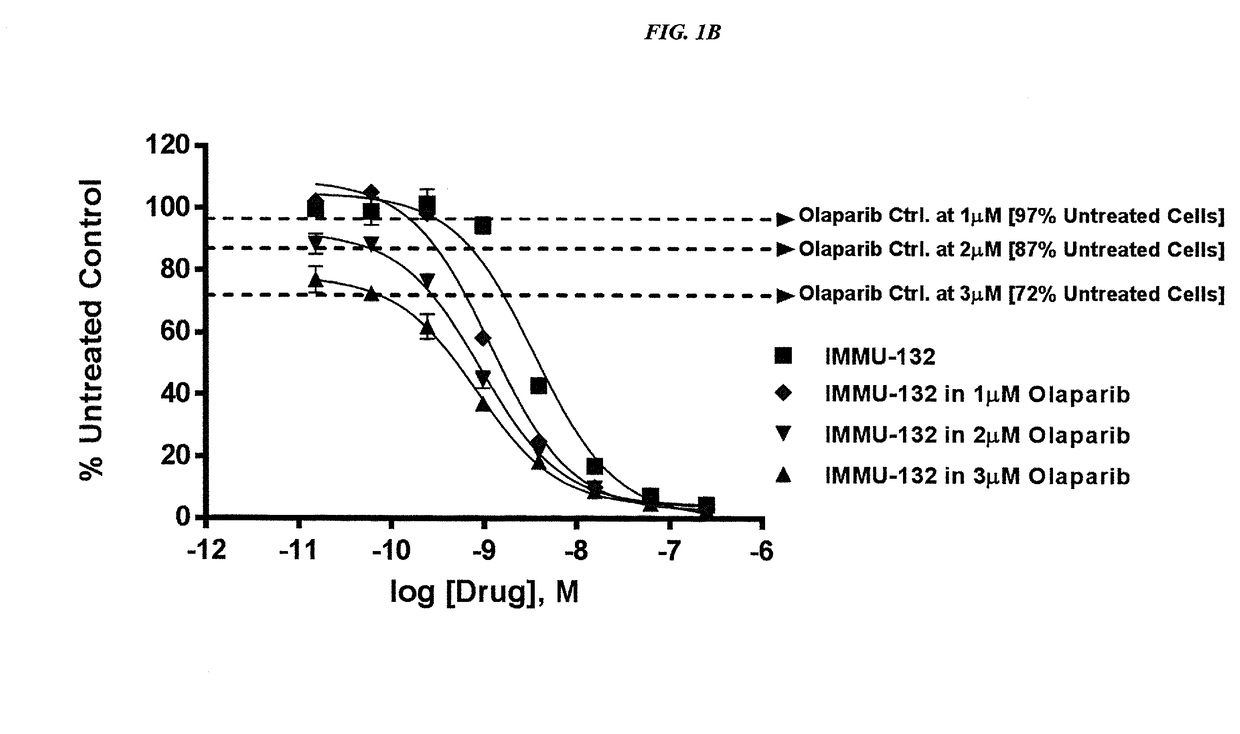
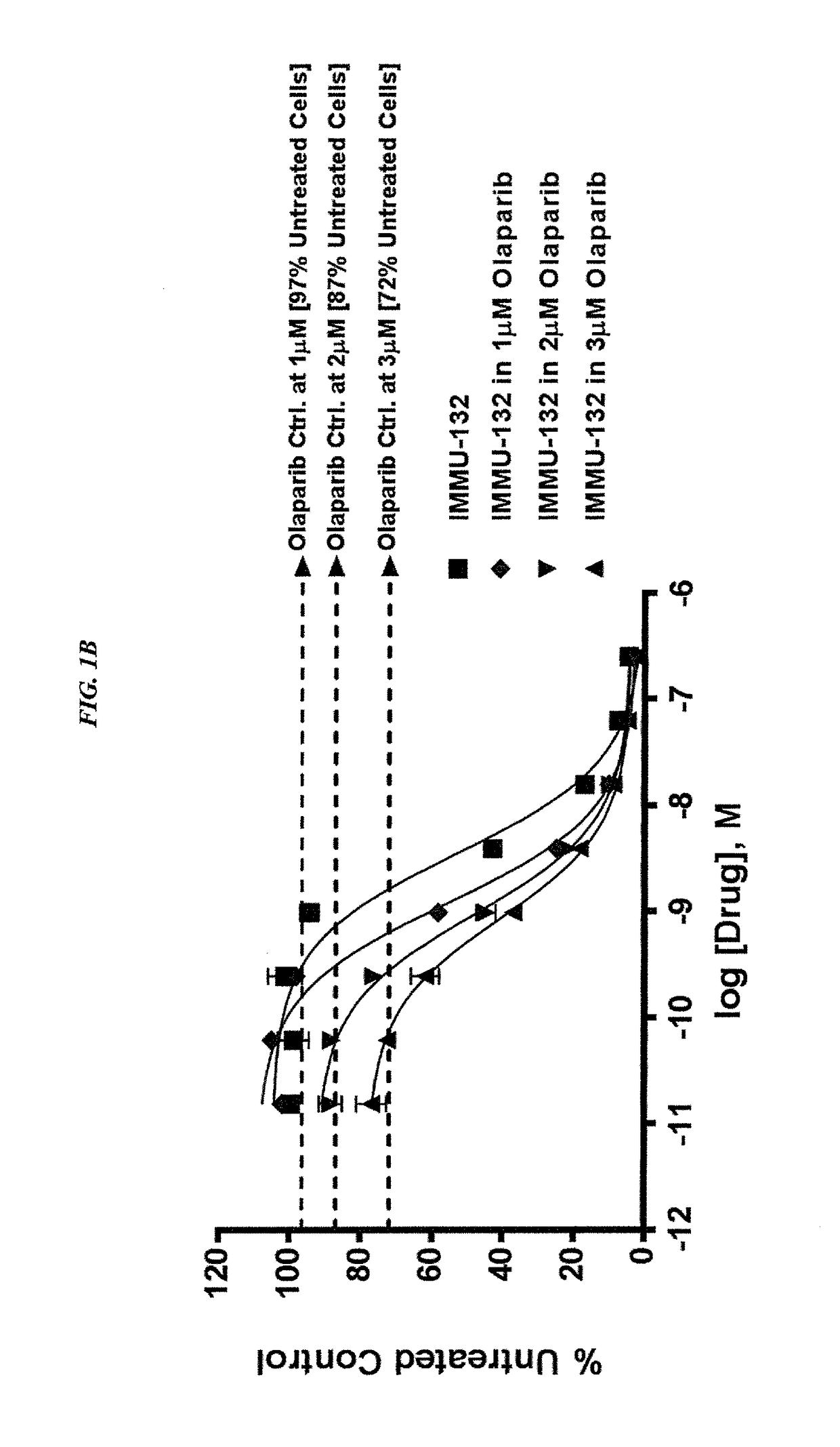
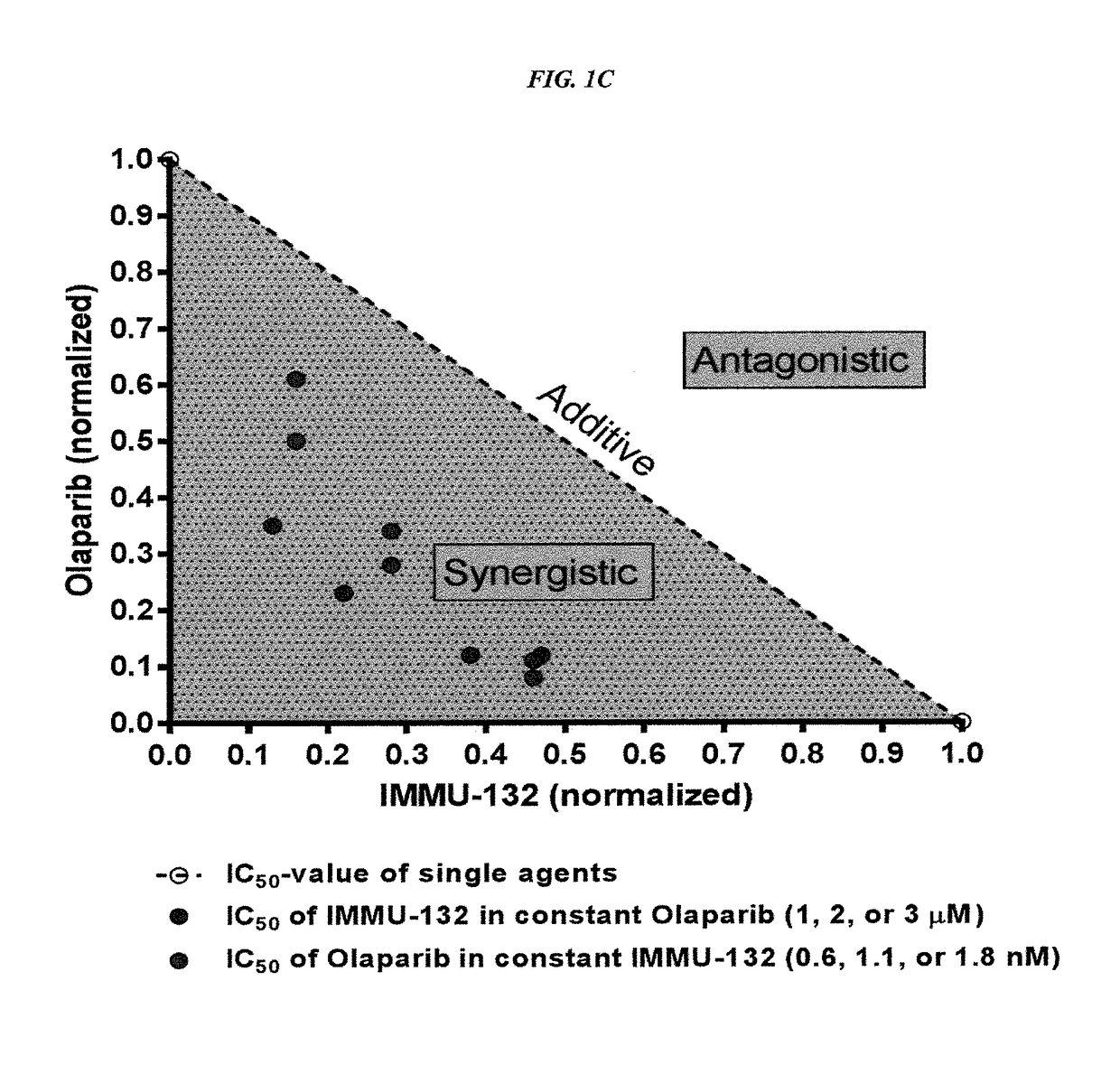
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
Combining Anti-hla-dr or Anti-trop-2 antibodies with microtubule inhibitors, parp inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
InactiveUS20170274093A1Reducing certain severe side effectsReceive treatment wellHeavy metal active ingredientsOrganic active ingredientsLymphatic SpreadCombined Modality Therapy
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
Humanized Anti-ceacam5 antibody and uses thereof
InactiveUS20150125386A1Overcome tumorImprove targetingImmunoglobulins against animals/humansRadioactive preparation carriersDrug conjugationWhole body
The present invention concerns compositions and methods of use of a humanized Class III anti-CEA antibody, comprising the heavy and light amino acid sequences SEQ ID NO:1 and SEQ ID NO:2. The antibody is effective to treat CEACAM5-expressing tumors, either alone or in combination with one or more therapeutic agents. Drug conjugated Class III anti-CEA antibodies, such as SN-38 or P2PDox immunoconjugates, are particularly efficacious. Surprisingly, the antibody-drug conjugates (ADCs) exhibit high anti-cancer efficacy, while exhibiting low levels of systemic toxicity that are readily treated with standard amelioration techniques. Antibodies and / or immunoconjugates comprising the amino acid sequences SEQ ID NO:1 and SEQ ID NO:2 are surprisingly efficacious for therapy of solid tumors, even when the tumor has proven resistant to standard anti-cancer therapies.
Owner:IMMUNOMEDICS INC
Combining anti-HLA-DR or anti-Trop-2 antibodies with microtubule inhibitors, PARP inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
ActiveUS9707302B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsHeavy metal active ingredientsLymphatic SpreadCombined Modality Therapy
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
Delivery system for cytotoxic drugs by bispecific antibody pretargeting
InactiveUS8435539B2Reducing certain severe side effectsReceive treatment wellBiocidePeptide/protein ingredientsDiseaseBinding site
The present invention relates to methods and compositions for pretargeting delivery of therapeutic agents. In preferred embodiments, the pretargeting method comprises: a) administering a bispecific antibody with a first binding site for a disease-associated antigen and a hapten on a targetable construct; b) administering a targetable construct comprising at least one therapeutic agent. In preferred embodiments, the bispecific antibody is made by the dock-and-lock (DNL) technique. In a more preferred embodiment, the targetable construct comprises one or more SN-38 moieties.
Owner:IMMUNOMEDICS INC
Antibody-SN-38 immunoconjugates with a CL2A linker
ActiveUS9107960B2Improve drug bioavailabilityGood treatment effectOrganic active ingredientsHeavy metal active ingredientsDiseaseSide effect
The present invention concerns improved methods and compositions for preparing SN-38 conjugates of proteins or peptides, preferably immunoconjugates of antibodies or antigen-binding antibody fragments. More preferably, the SN-38 is attached to the antibody or antibody fragment using a CL2A linker, with 1-12, more preferably 6 or less, most preferably 1-5 SN-38 moieties per antibody or antibody fragment. Most preferably, the immunoconjugate is prepared in large scale batches, with various modifications to the reaction scheme to optimize yield and recovery in large scale. Other embodiments concern optimized dosages and / or schedules of administration of immunoconjugate to maximize efficacy for disease treatment and minimize side effects of administration.
Owner:IMMUNOMEDICS INC
Biomarkers for antibody-drug conjugate monotherapy or combination therapy
PendingUS20210093730A1Predict resistancePredict sensitivityMicrobiological testing/measurementOrganic non-active ingredientsDiseaseAnticarcinogen
The present invention relates to biomarkers of use in cancer therapy, wherein the therapy comprises treatment with anti-Trop-2, anti-CEACAM5 or anti-HLA-DR ADCs (antibody-drug conjugates), alone or in combination with and one or more anti-cancer agents, such as a DDR inhibitor, an ABCG2 inhibitor, a microtubule inhibitor, a checkpoint inhibitor, a PI3K inhibitor, an AKT inhibitor, a CDK 4 inhibitor, a CDK 5 inhibior, a tyrosine kinase inhibitor or a platinum-based chemotherapeutic agent. Preferably, the combination therapy has a synergistic effect on inhibiting tumor growth. The biomarkers are of use to predict efficacy and / or toxicity of ADC therapy, determine tumor response to treatment, identify minimal residual disease or relapse, determine prognosis, stratify patients for initial therapy or to optimize treatment for the patient, based on the specific biomarkers detected.
Owner:IMMUNOMEDICS INC
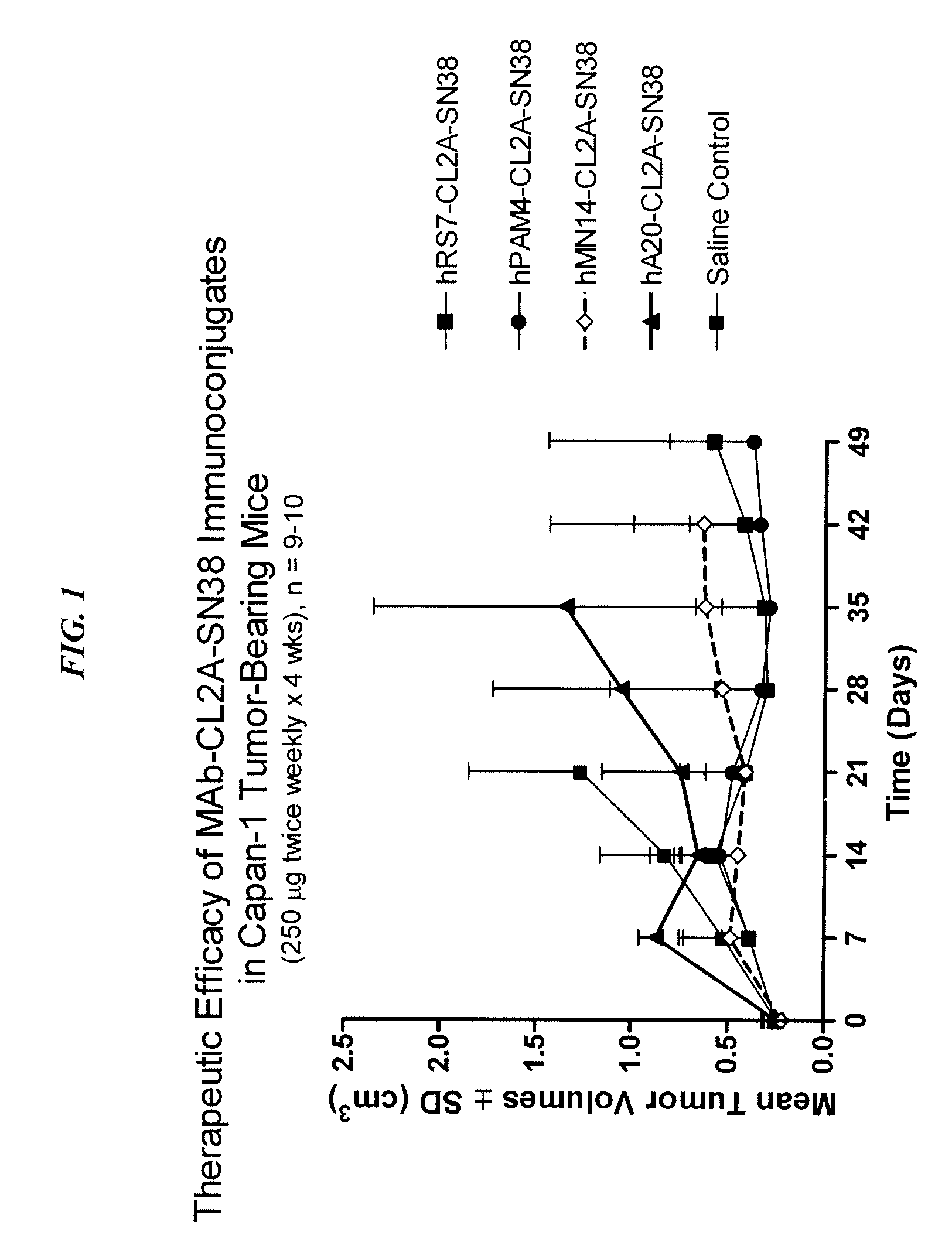
Therapy for metastatic urothelial cancer with the antibody-drug conjugate, sacituzumab govitecan (immu-132)
ActiveUS20180110772A1Reduce effectReduce therapyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigen bindingUrothelial cancer
The present invention relates to therapeutic ADCs comprising SN-38 attached to an anti-Trop-2 antibody or antigen-binding antibody fragment. The ADC may be administered at a dosage of between 4 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, most preferably 8 to 10 mg / kg. When administered at specified dosages and schedules, the ADC can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the ADC is administered in combination with one or more other therapeutic agents, such as a PARP inhibitor, a microtubule inhibitor, a Bruton kinase inhibitor or a PI3K inhibitor. Most preferably, the ADC is of use for treating a Trop-2 expressing cancer, such as metastatic urothelial cancer.
Owner:IMMUNOMEDICS INC
Efficacy of Anti-trop-2-sn-38 antibody drug conjugates for therapy of tumors relapsed/refractory to checkpoint inhibitors
ActiveUS20170313781A1Improve targetingLow toxicityOrganic active ingredientsDigestive systemAntiendomysial antibodiesEfficacy
The present invention relates to therapeutic ADCs comprising SN-38 attached to an anti-Trop-2 antibody or antigen-binding antibody fragment, more particularly sacituzumab govitecan. The ADC is administered to a subject with a Trop-2 positive cancer that is resistant to or relapsed from prior treatment with a checkpoint inhibitor. The therapy is effective to treat cancers that are resistant to checkpoint inhibitors.
Owner:IMMUNOMEDICS INC
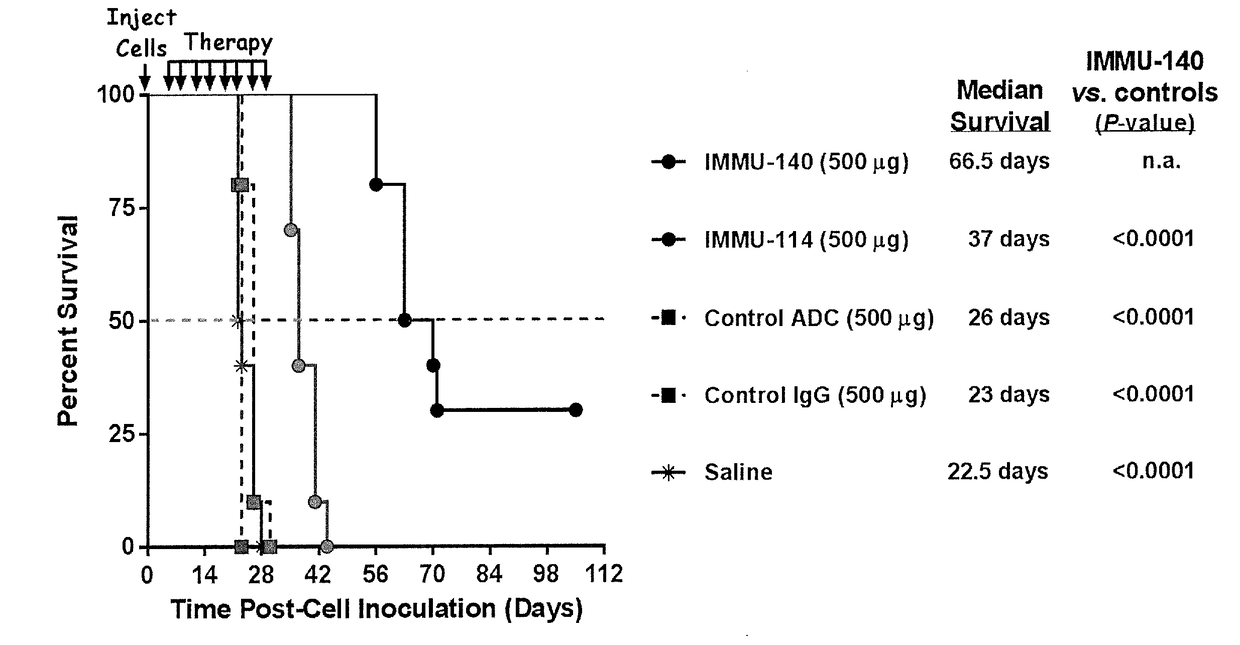
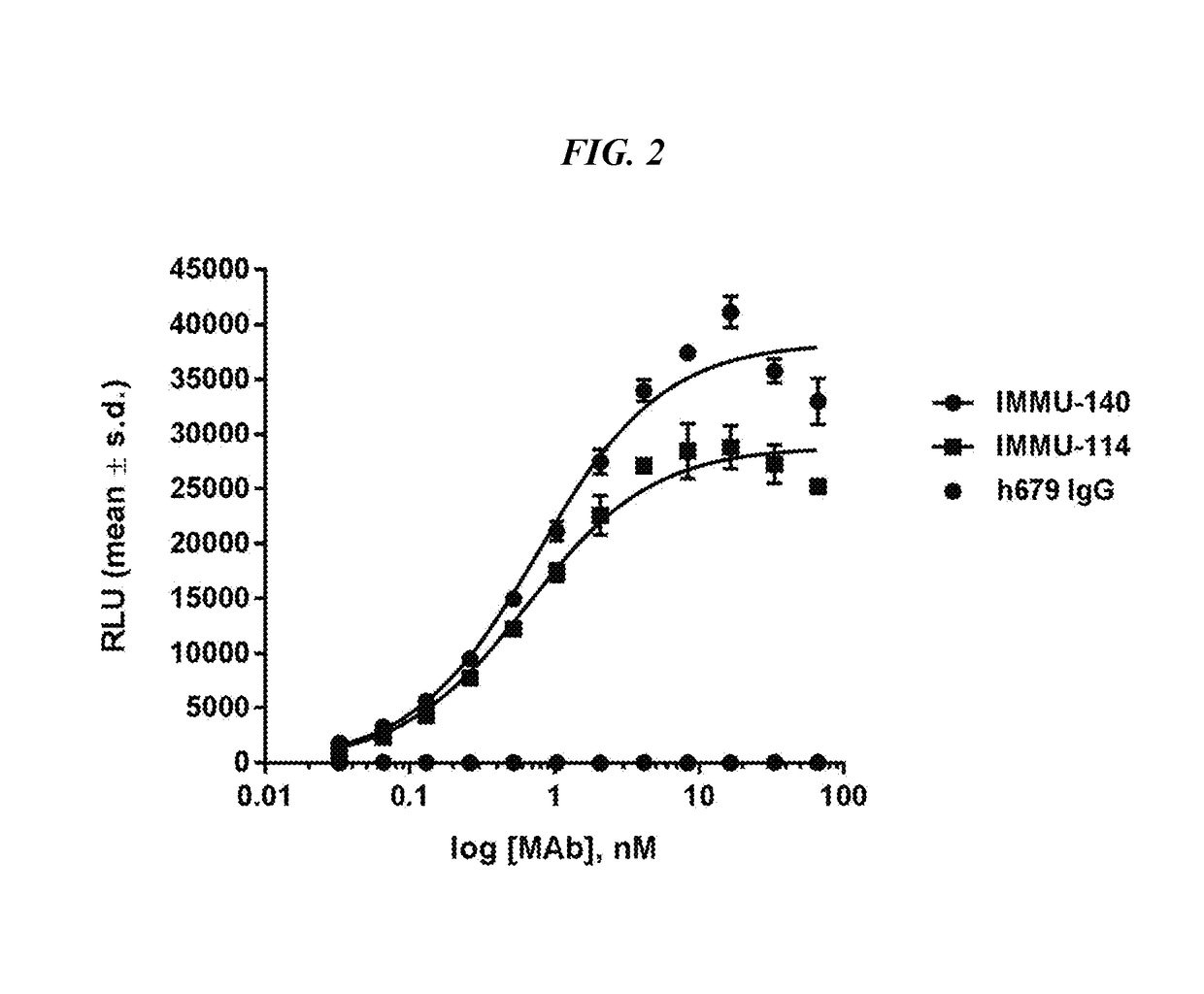
Efficacy of anti-HLA-DR antiboddy drug conjugate IMMU-140 (hL243-CL2A-SN-38) in HLA-DR positive cancers
ActiveUS10206918B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadAntibody fragments
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an anti-HLA-DR antibody or antigen-binding antibody fragment. The immunoconjugate may be administered at a dosage of between 3 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8, 10 or 12 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. The methods and compositions are particularly useful for treating AML, ALL or multiple myeloma.
Owner:IMMUNOMEDICS INC
Efficacy of anti-trop-2-SN-38 antibody drug conjugates for therapy of tumors relapsed/refractory to checkpoint inhibitors
ActiveUS10266605B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsDigestive systemAntibody fragmentsRefractory
Owner:IMMUNOMEDICS INC
Neoadjuvant use of antibody-drug conjugates
InactiveUS20160095939A1Enhance therapyImprove efficacyOrganic active ingredientsIn-vivo radioactive preparationsStandard treatmentAntibody
Owner:IMMUNOMEDICS INC
Antibody-sn-38 immunoconjugates with a cl2a linker
InactiveUS20150306243A1Improve drug bioavailabilityGood treatment effectOrganic active ingredientsPeptide/protein ingredientsDiseaseSide effect
The present invention concerns improved methods and compositions for preparing SN-38 conjugates of proteins or peptides, preferably immunoconjugates of antibodies or antigen-binding antibody fragments. More preferably, the SN-38 is attached to the antibody or antibody fragment using a CL2A linker, with 1-12, more preferably 6 or less, most preferably 1-5 SN-38 moieties per antibody or antibody fragment. Most preferably, the immunoconjugate is prepared in large scale batches, with various modifications to the reaction scheme to optimize yield and recovery in large scale. Other embodiments concern optimized dosages and / or schedules of administration of immunoconjugate to maximize efficacy for disease treatment and minimize side effects of administration.
Owner:IMMUNOMEDICS INC
Delivery system for cytotoxic drugs by bispecific antibody pretargeting
InactiveUS8652484B2Reducing certain severe side effectsReceive treatment wellPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseBinding site
The present invention relates to methods and compositions for pretargeting delivery of therapeutic agents. In preferred embodiments, the pretargeting method comprises: a) administering a bispecific antibody with a first binding site for a disease-associated antigen and a hapten on a targetable construct; b) administering a targetable construct comprising at least one therapeutic agent. In preferred embodiments, the bispecific antibody is made by the dock-and-lock (DNL) technique. In a more preferred embodiment, the targetable construct comprises one or more SN-38 moieties.
Owner:IMMUNOMEDICS INC
Therapy for metastatic urothelial cancer with the antibody-drug conjugate, sacituzumab govitecan (IMMU-132)
ActiveUS10413539B2Reducing certain severe side effectsReceive treatment wellImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsLymphatic SpreadAntibody fragments
The present invention relates to therapeutic ADCs comprising SN-38 attached to an anti-Trop-2 antibody or antigen-binding antibody fragment. The ADC may be administered at a dosage of between 4 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, most preferably 8 to 10 mg / kg. When administered at specified dosages and schedules, the ADC can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the ADC is administered in combination with one or more other therapeutic agents, such as a PARP inhibitor, a microtubule inhibitor, a Bruton kinase inhibitor or a PI3K inhibitor. Most preferably, the ADC is of use for treating a Trop-2 expressing cancer, such as metastatic urothelial cancer.
Owner:IMMUNOMEDICS INC
Tumor therapy by bispecific antibody pretargeting
InactiveUS20160287732A1Reducing certain severe side effectsReceive treatment wellAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellBinding site
The present invention relates to methods and compositions for pretargeting delivery of alpha-emitting radionuclides, such as 213Bi or 225AC to a target cell or tissue, such as a cancer cell or a tumor. In preferred embodiments, the pretargeting method comprises: a) administering a bispecific antibody comprising at least one binding site for a tumor-associated antigen (TAA) and at least one binding site for a hapten; and b) administering a hapten-conjugated targetable construct that is labeled with an alpha-emitting radionuclide. More preferably, the bispecific antibody is rapidly internalized into the target cell, along with the radionuclide. In most preferred embodiments, the bispecific antibody is made as a dock-and-lock (DNL) complex.
Owner:IMMUNOMEDICS INC
Delivery System for Cytotoxic Drugs by Bispecific Antibody Pretargeting
InactiveUS20110076233A1Receive treatment wellReducing certain severe side effectsBiocidePeptide/protein ingredientsDiseaseBinding site
The present invention relates to methods and compositions for pretargeting delivery of therapeutic agents. In preferred embodiments, the pretargeting method comprises: a) administering a bispecific antibody with a first binding site for a disease-associated antigen and a hapten on a targetable construct; b) administering a targetable construct comprising at least one therapeutic agent. In preferred embodiments, the bispecific antibody is made by the dock-and-lock (DNL) technique. In a more preferred embodiment, the targetable construct comprises one or more SN-38 moieties.
Owner:IMMUNOMEDICS INC
EFFICACY OF ANTI-HLA-DR ANTIBODY DRUG CONJUGATE IMMU-140 (hL243-CL2A-SN-38) IN HLA-DR POSITIVE CANCERS
ActiveUS20170360770A1Overcome tumorImprove targetingOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadAntibody fragments
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an anti-HLA-DR antibody or antigen-binding antibody fragment. The immunoconjugate may be administered at a dosage of between 3 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8, 10 or 12 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. The methods and compositions are particularly useful for treating AML, ALL or multiple myeloma.
Owner:IMMUNOMEDICS INC
Combination of ABCG2 inhibitors with sacituzumab govitecan (IMMU-132) overcomes resistance to SN-38 in Trop-2 expressing cancers
ActiveUS10954305B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsRadioactive preparation carriersAntiendomysial antibodiesAntigen binding
The present invention relates to therapeutic ADCs comprising a drug attached to an anti-cancer antibody or antigen-binding antibody fragment. Preferably the drug is SN-38. More preferably the antibody or fragment thereof binds to Trop-2 and the therapy is used to treat a Trop-2 positive cancer. Most preferably the antibody is hRS7. The ADC is administered to a subject with a cancer in combination with an ABCG2 inhibitor. The combination therapy is effective to treat cancers that are resistant to drug alone and / or to ADC alone.
Owner:IMMUNOMEDICS INC
Therapy for metastatic urothelial cancer with the antibody-drug conjugate, sacituzumab govitecan (immu-132)
PendingUS20210393617A1Receive treatment wellUnexpected synergistic effectInorganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesAntigen binding
The present invention relates to therapeutic ADCs comprising SN-38 attached to an anti-Trop-2 antibody or antigen-binding antibody fragment. The ADC may be administered at a dosage of between 4 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, most preferably 8 to 10 mg / kg. When administered at specified dosages and schedules, the ADC can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the ADC is administered in combination with one or more other therapeutic agents, such as a PARP inhibitor, a microtubule inhibitor, a Bruton kinase inhibitor or a PI3K inhibitor. Most preferably, the ADC is of use for treating a Trop-2 expressing cancer, such as metastatic urothelial cancer.
Owner:IMMUNOMEDICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com