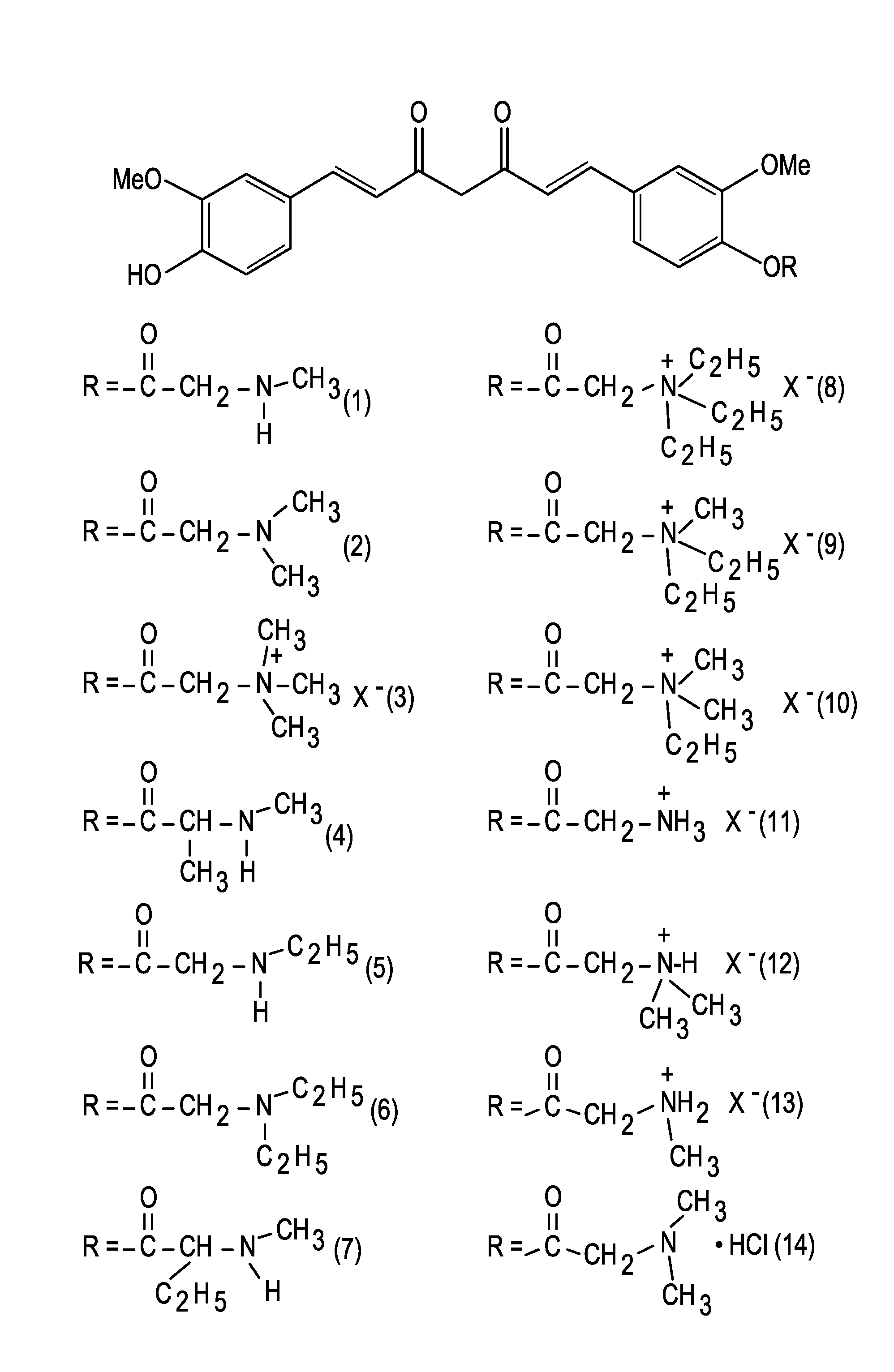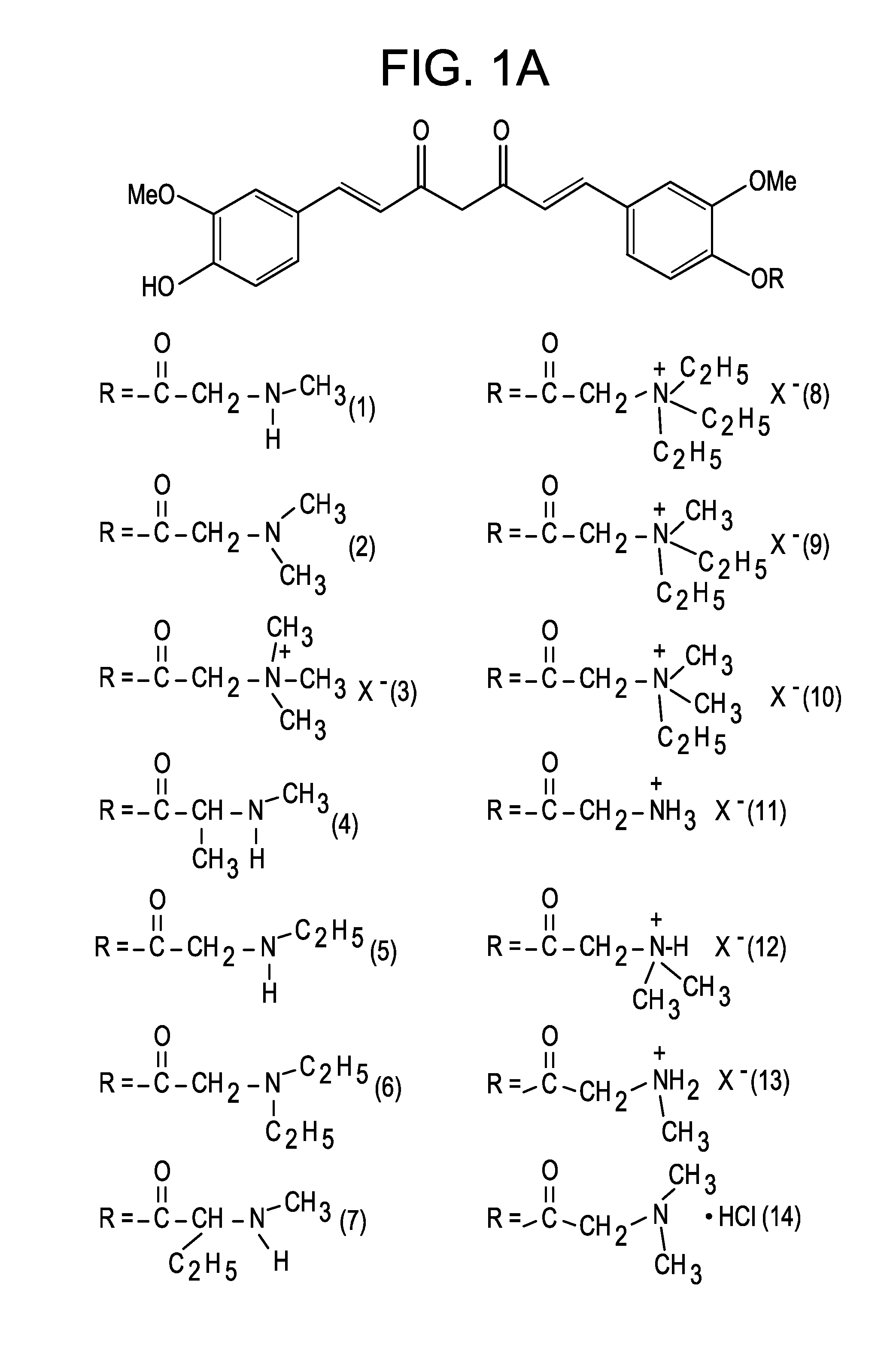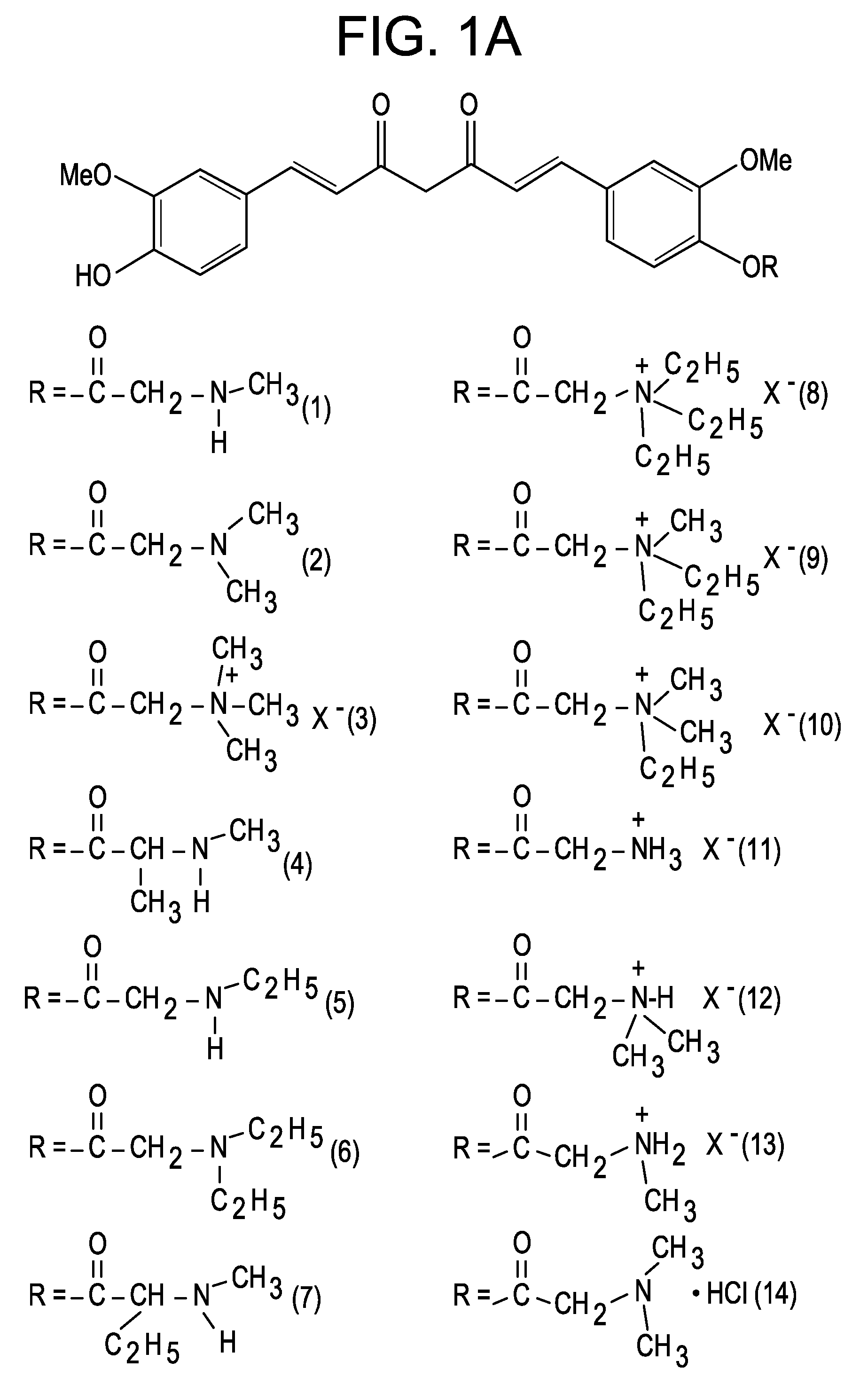Patents
Literature
135results about How to "Improve drug bioavailability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
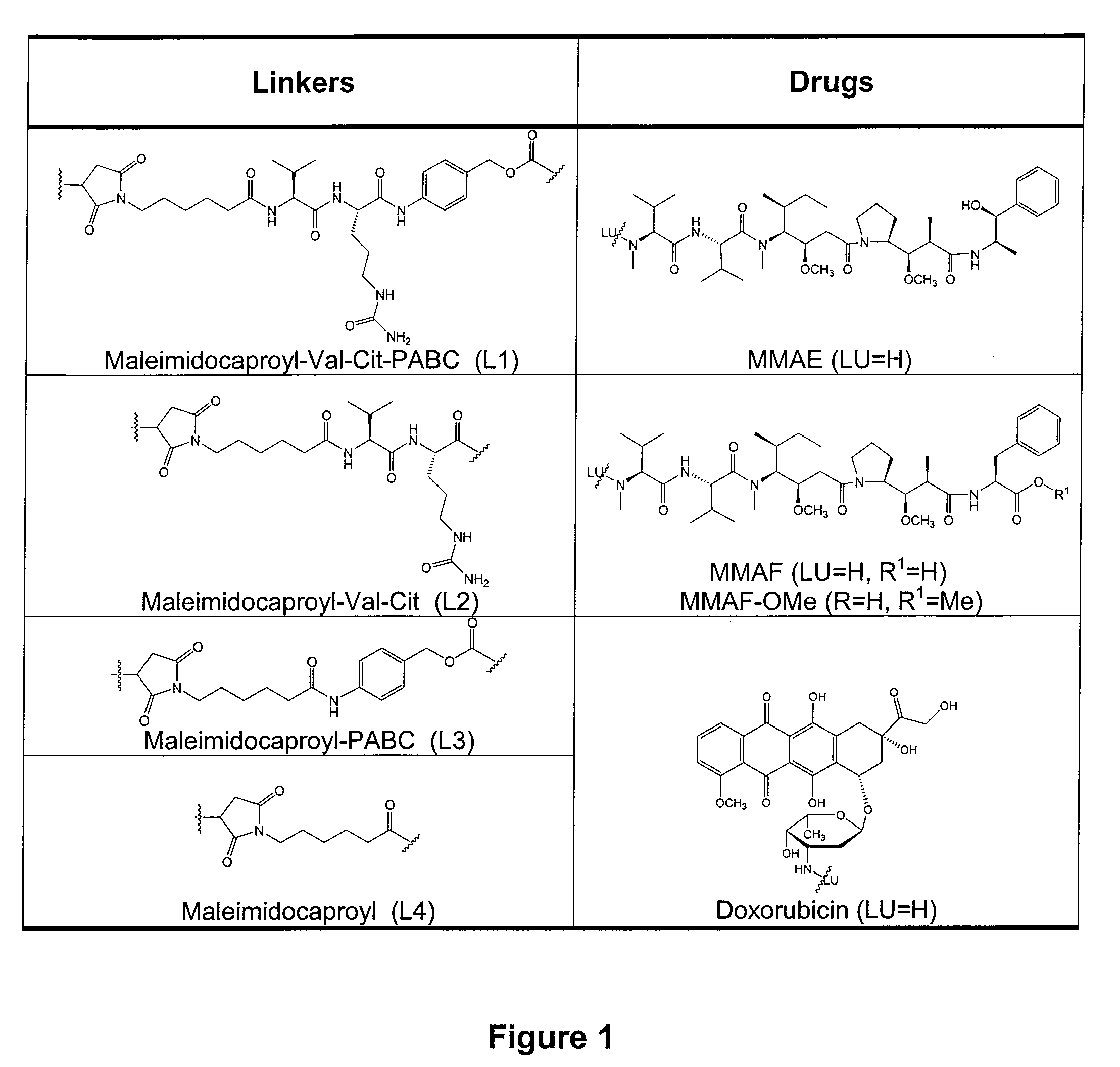
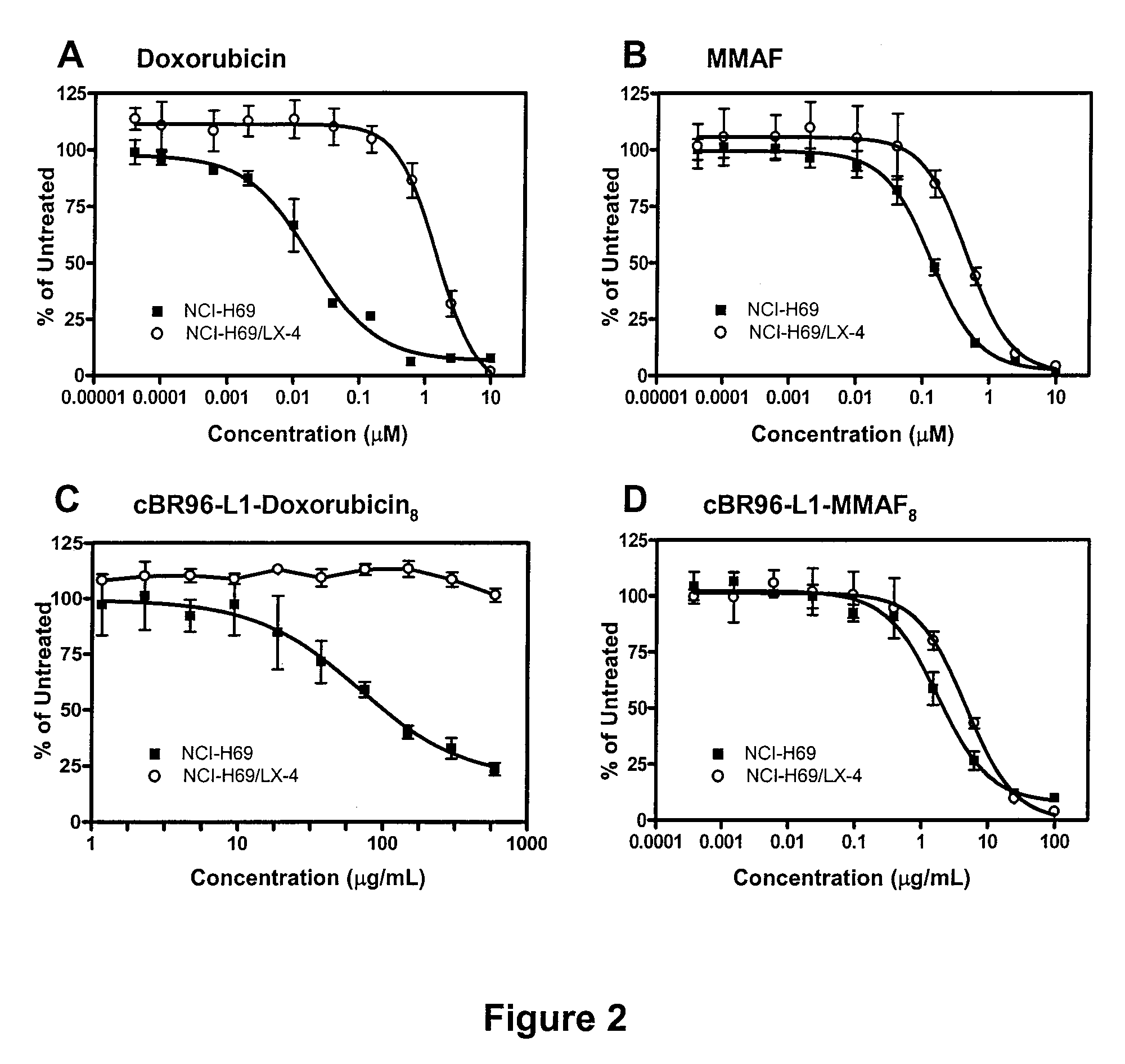
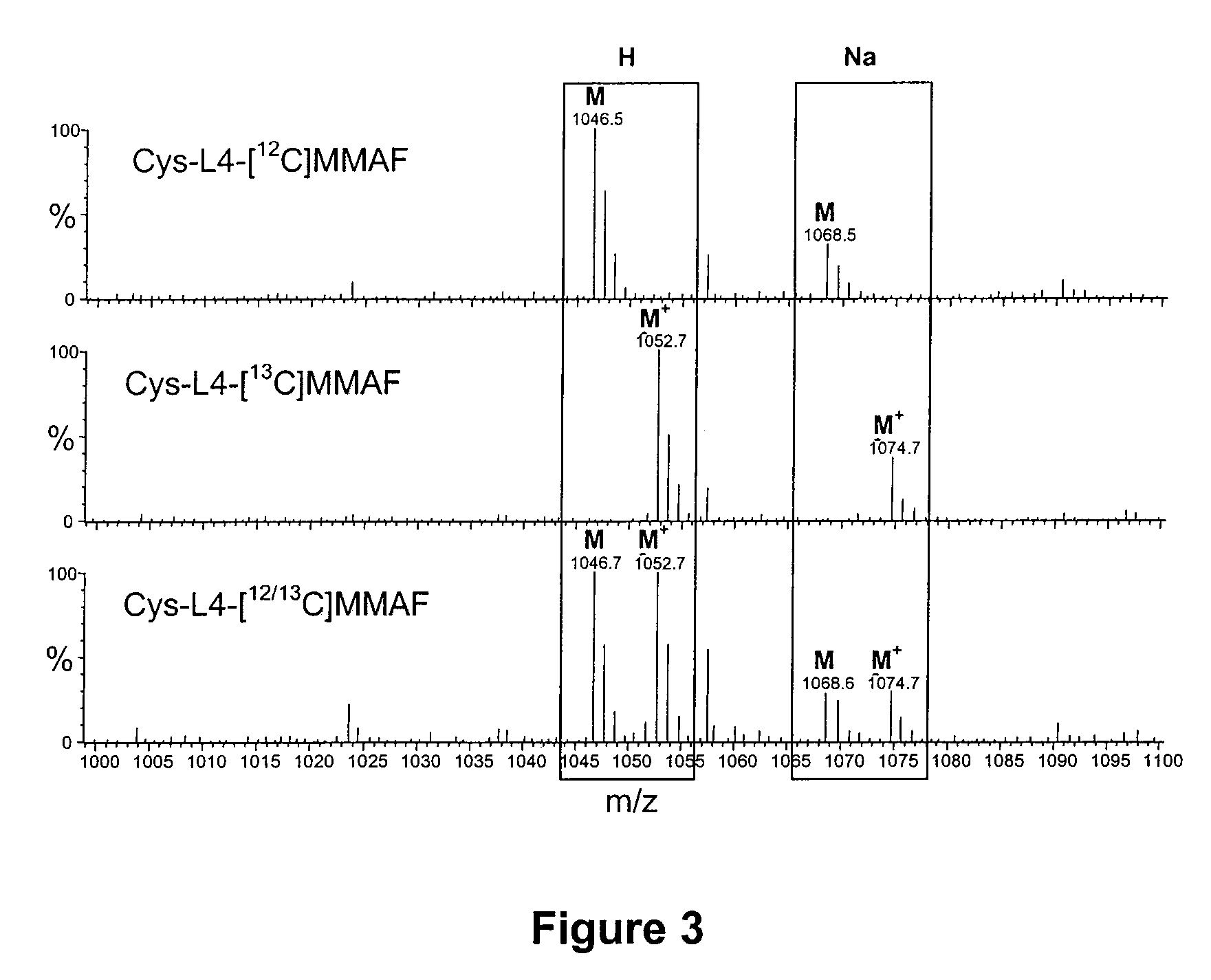
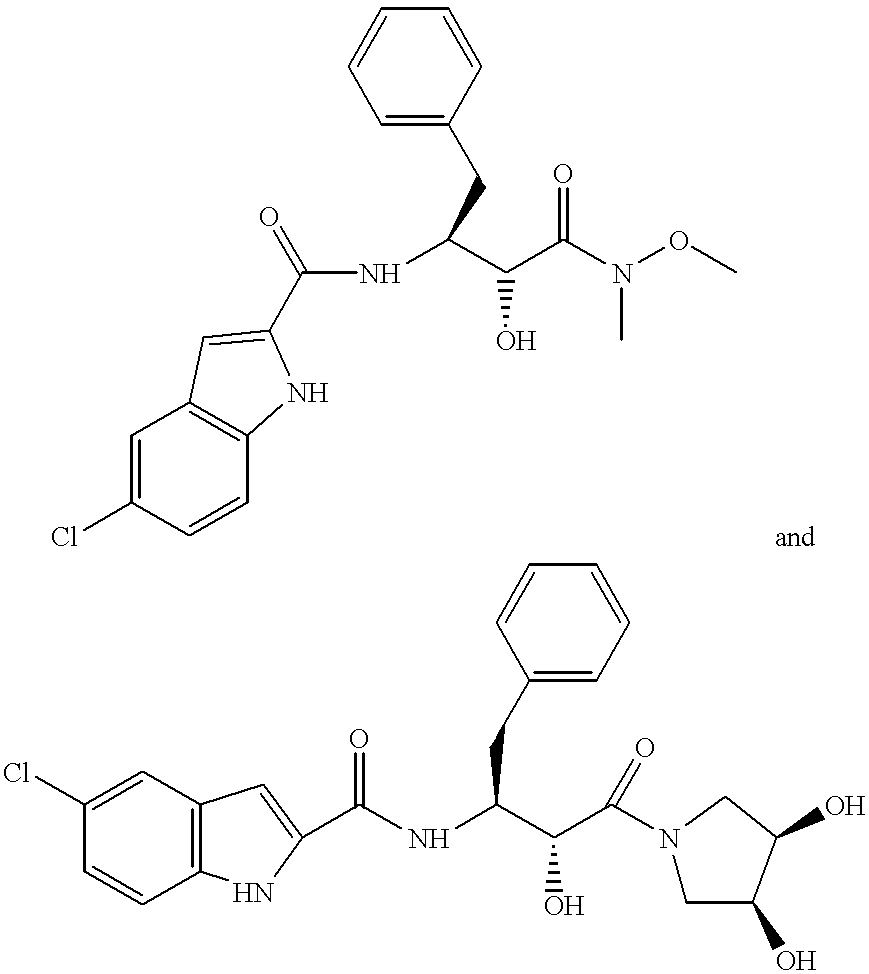
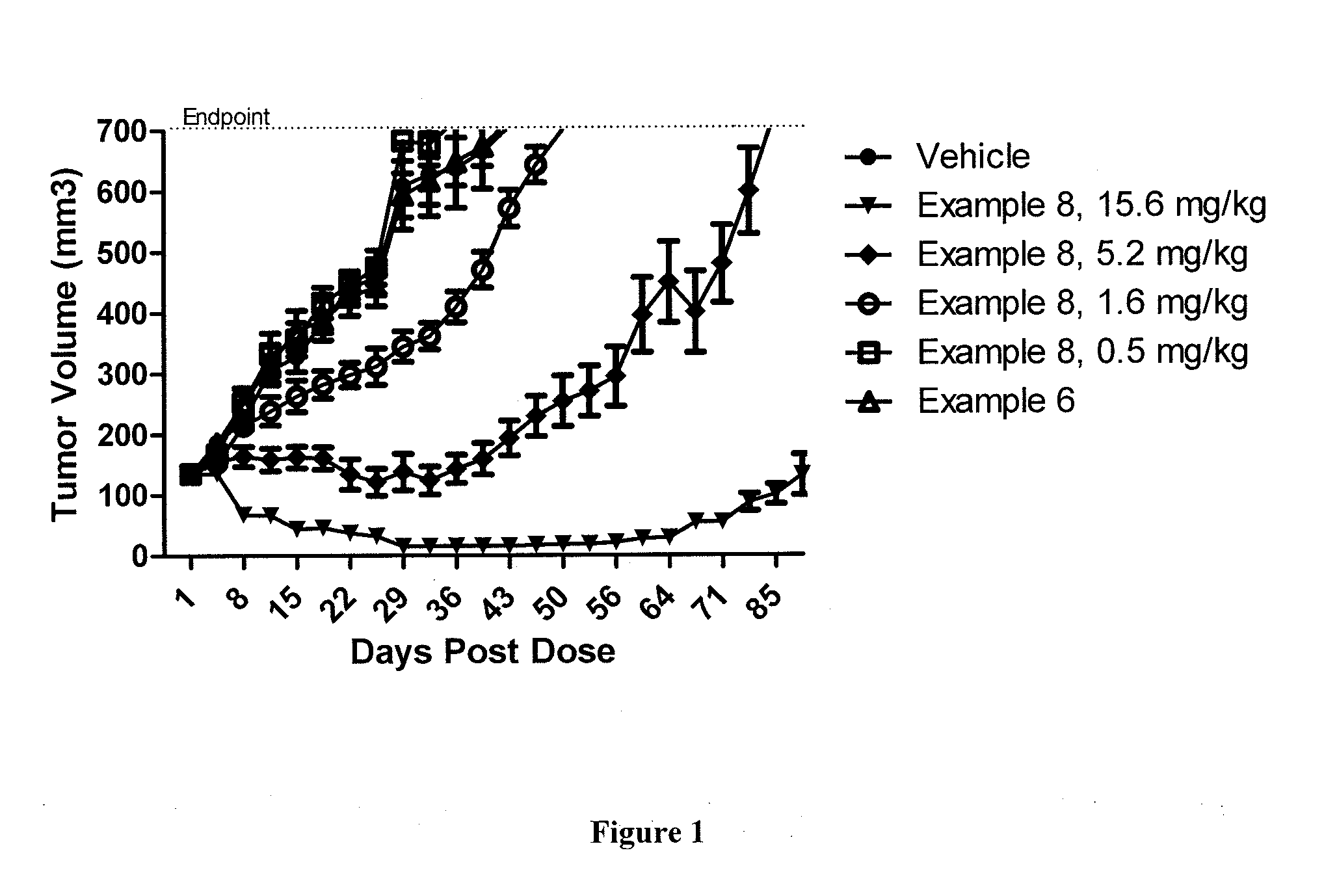
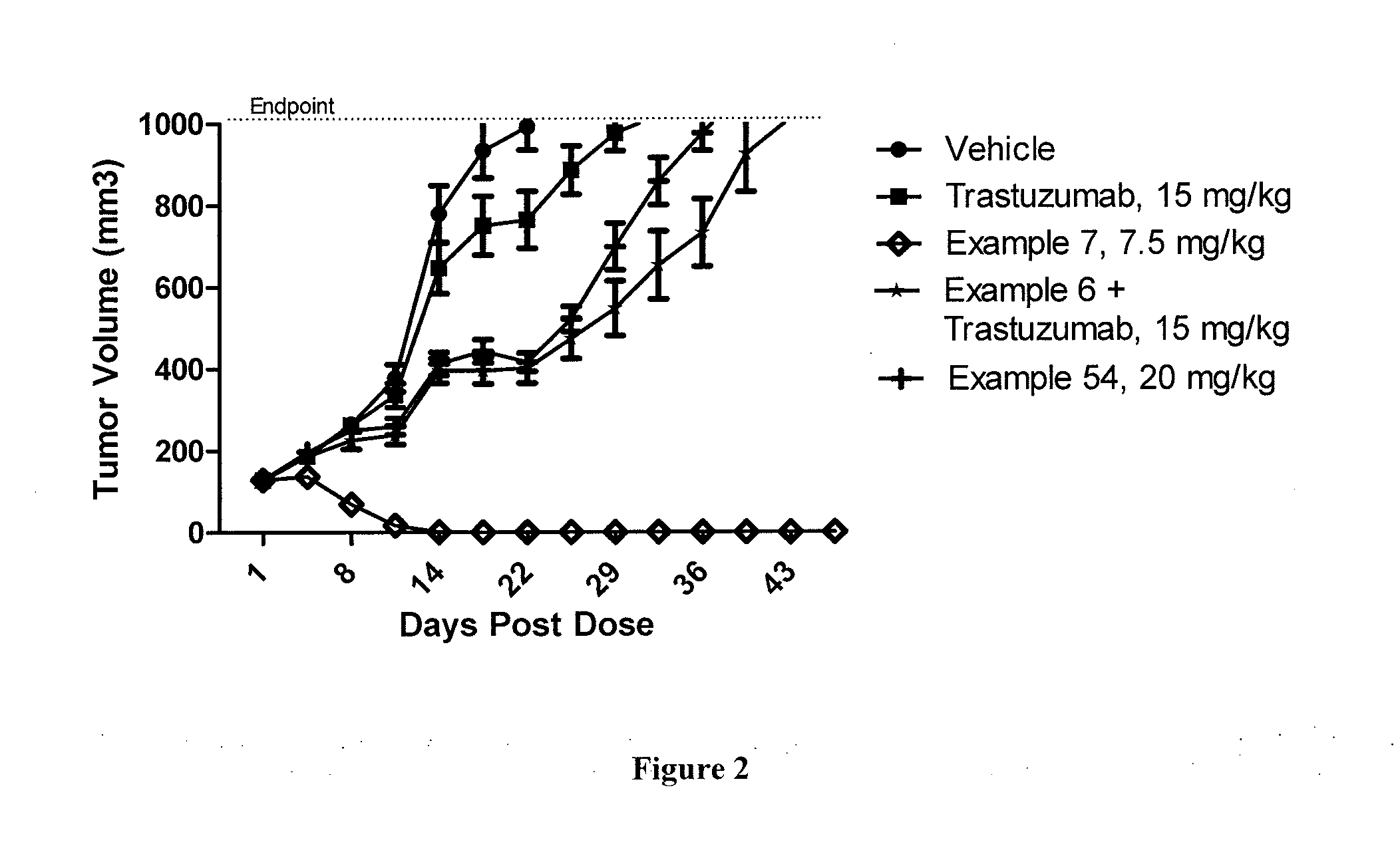
Antibody drug conjugate metabolites
ActiveUS7750116B1Improve drug bioavailabilityImprove bioavailabilityPeptide/protein ingredientsImmunoglobulinsMetaboliteWilms' tumor
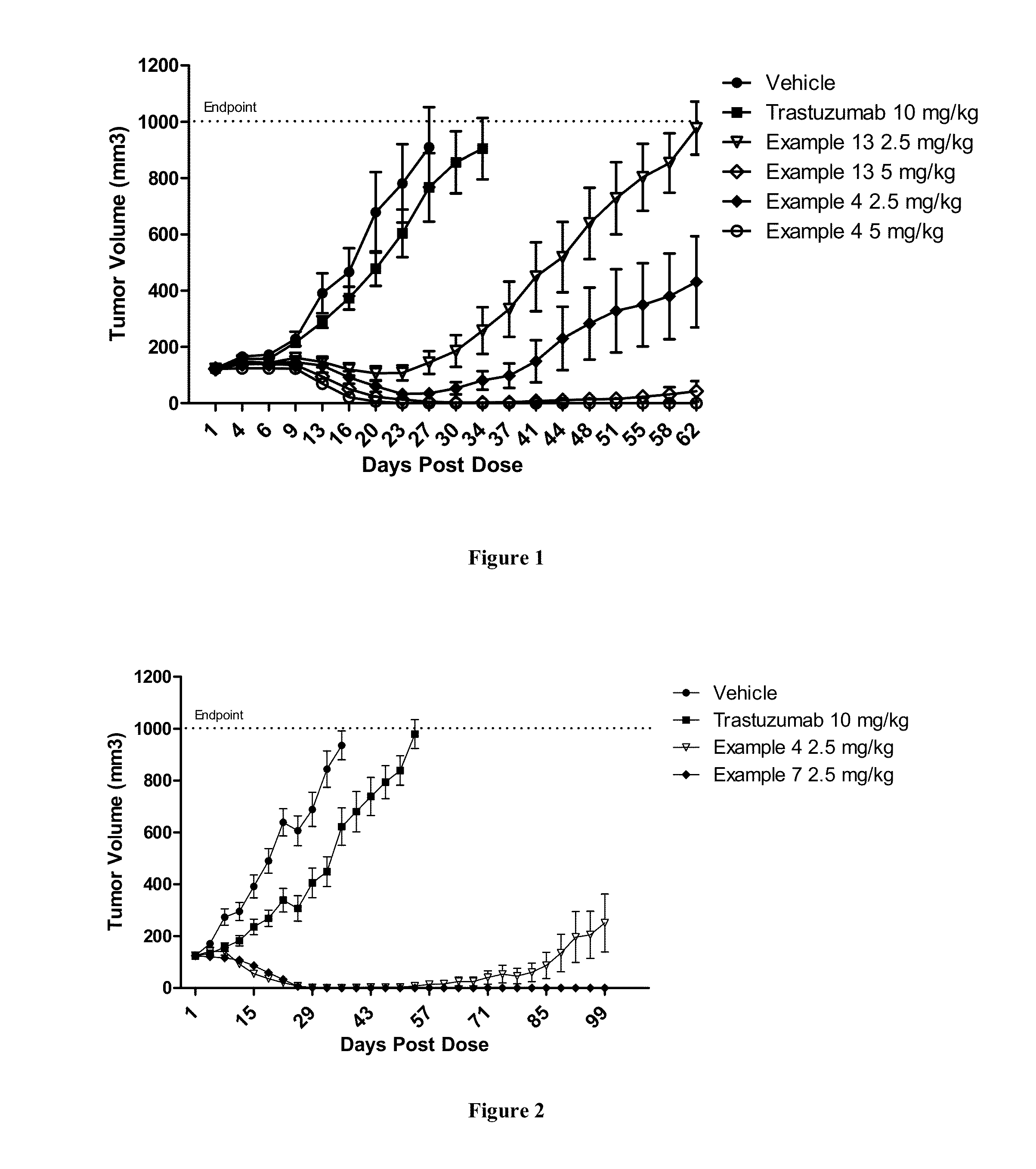
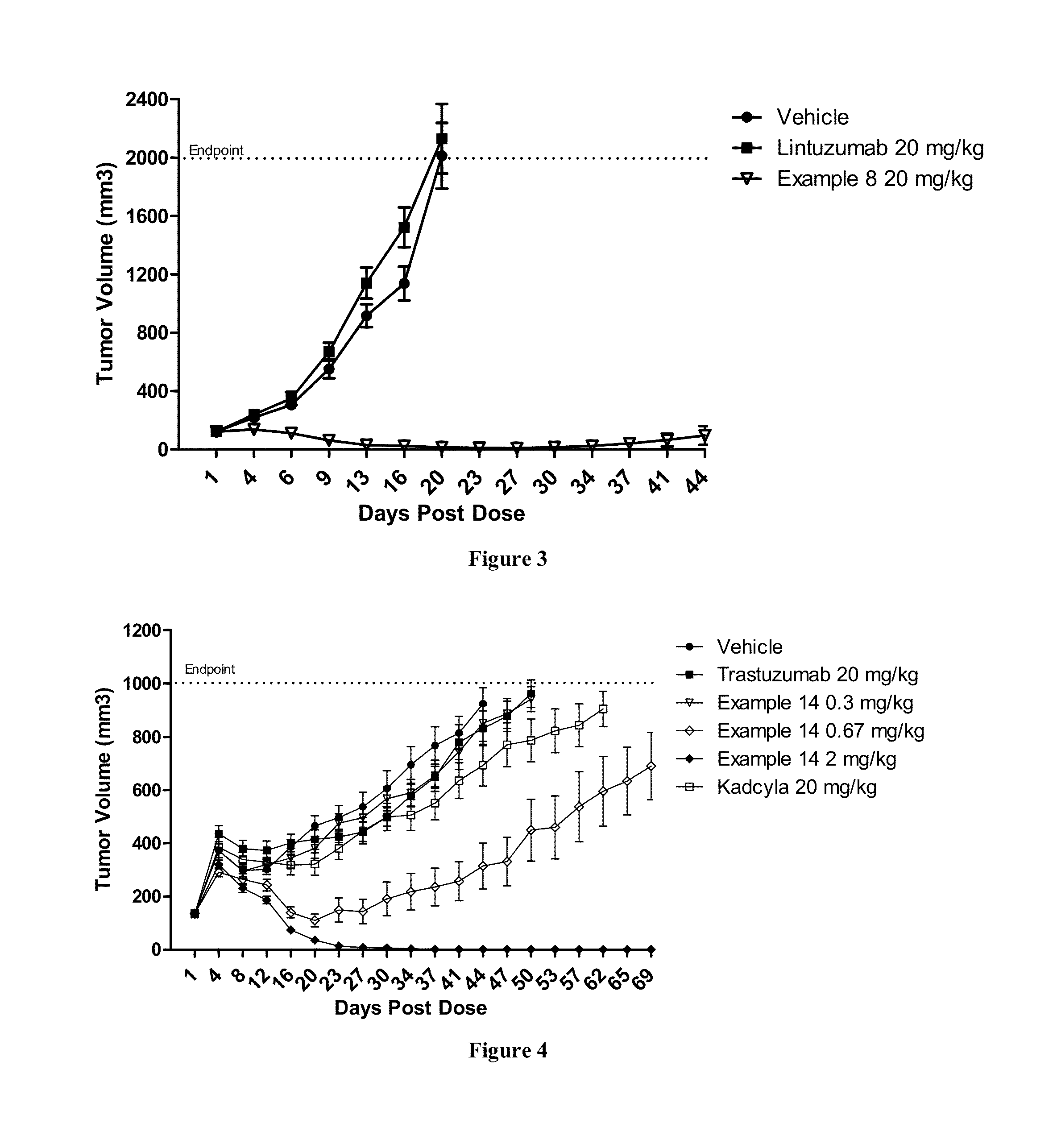
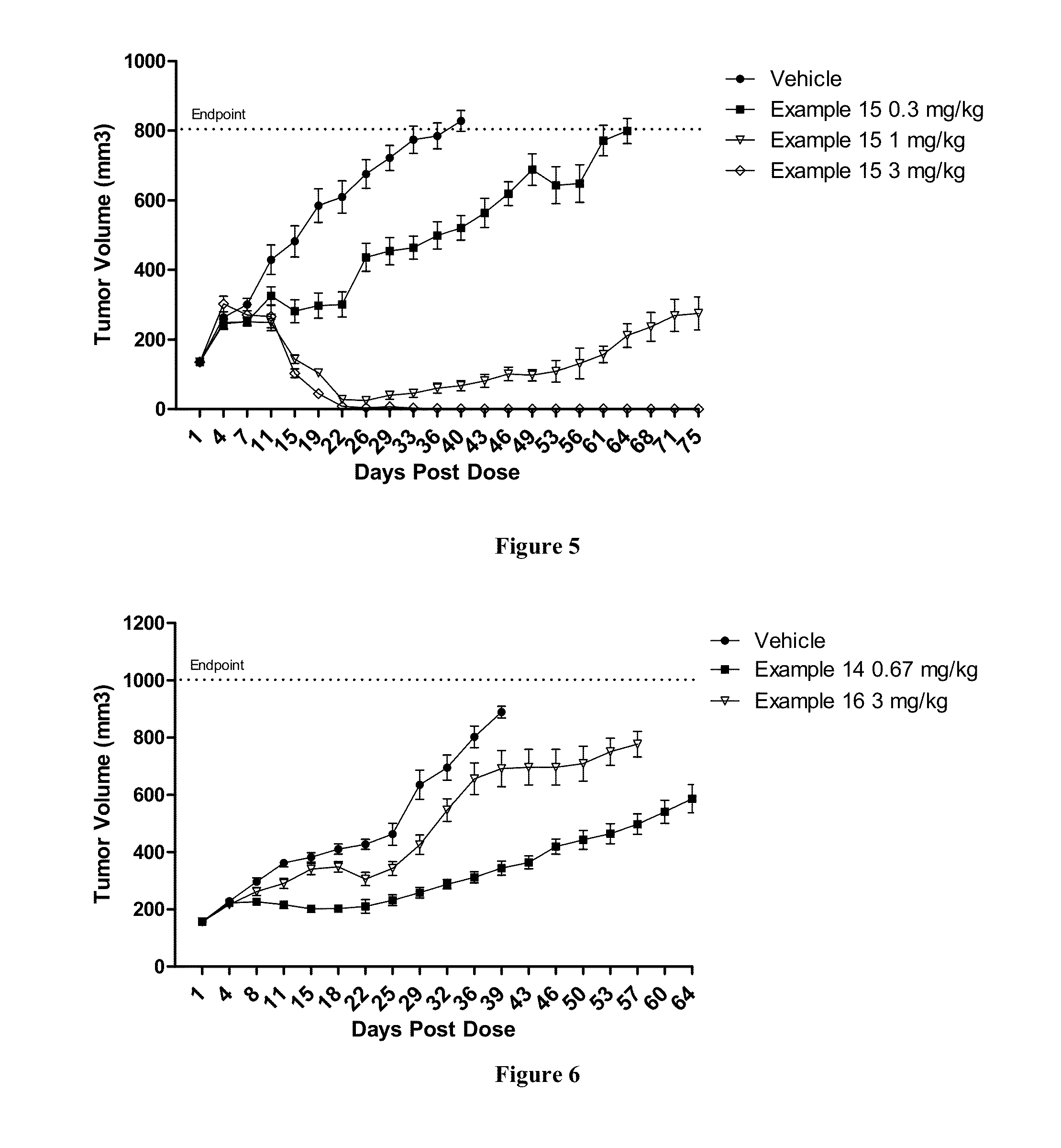
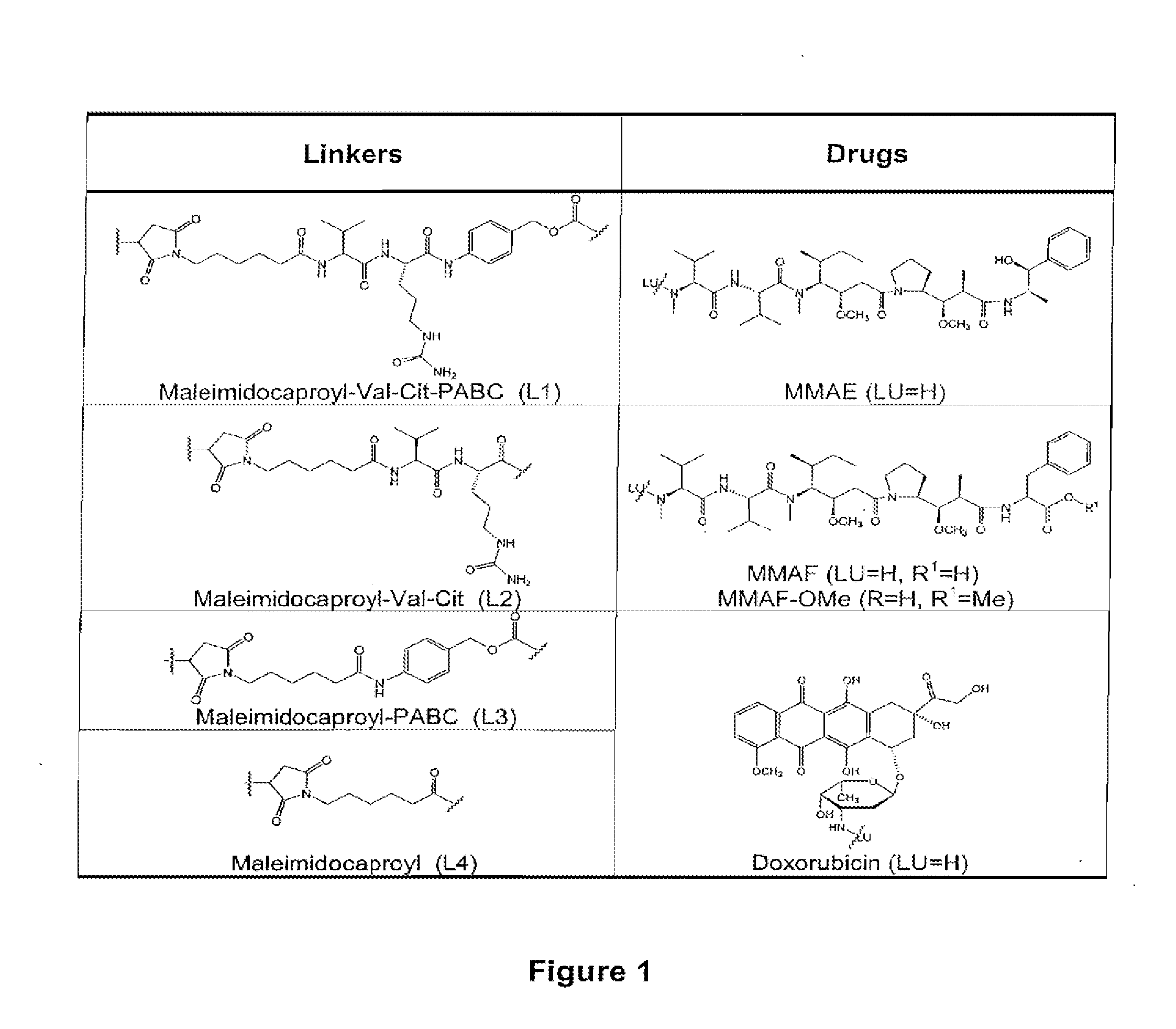
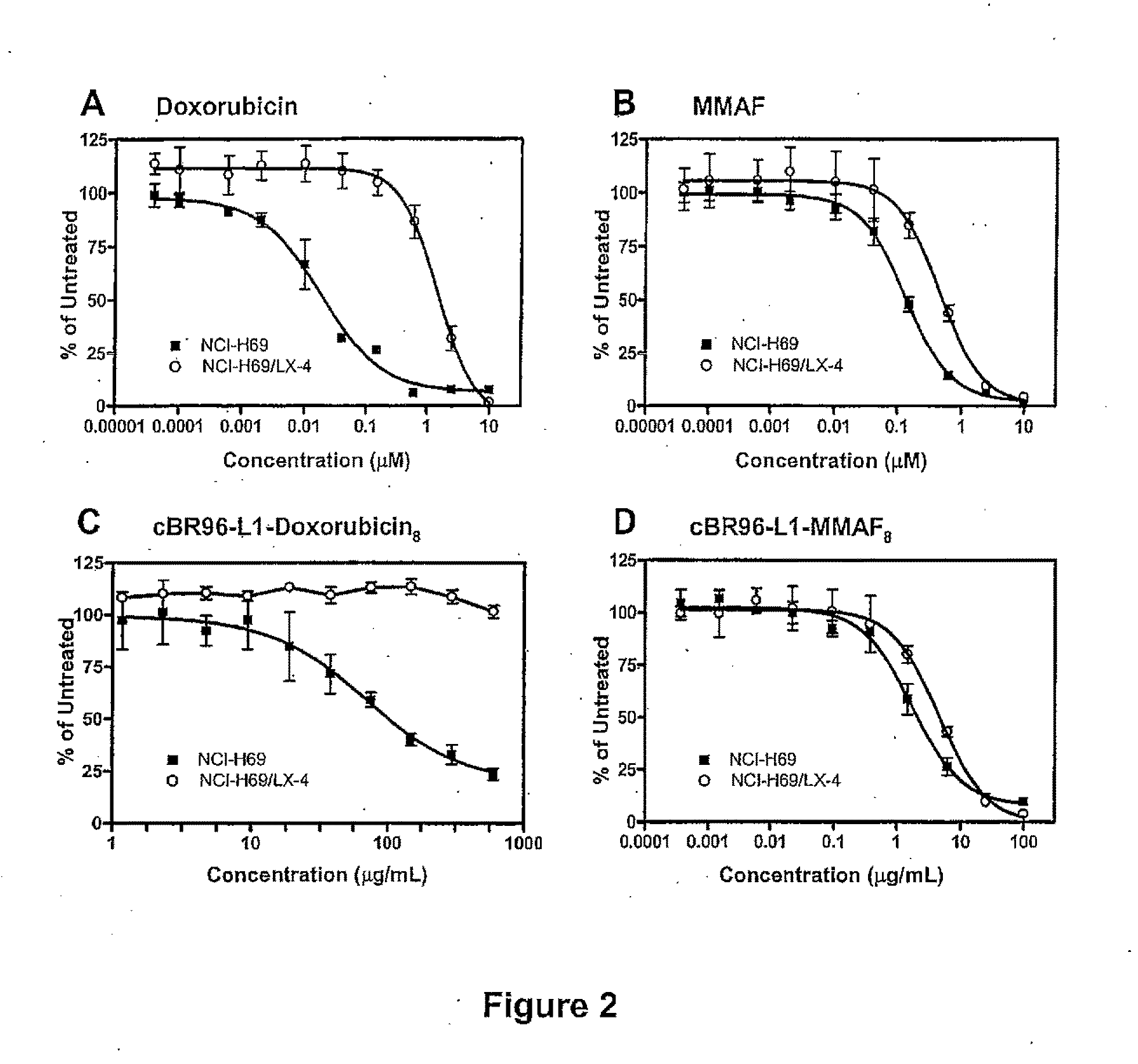
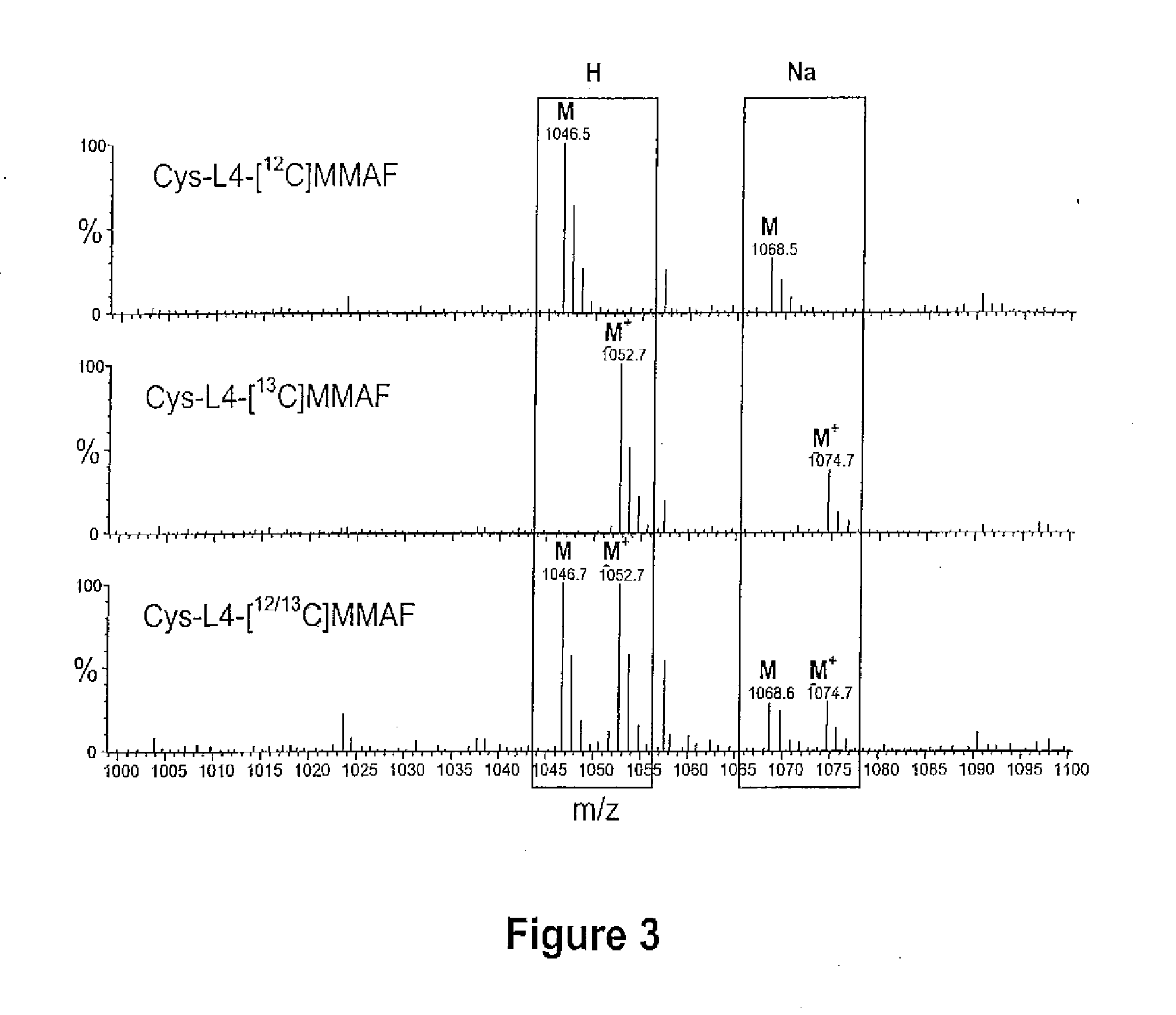
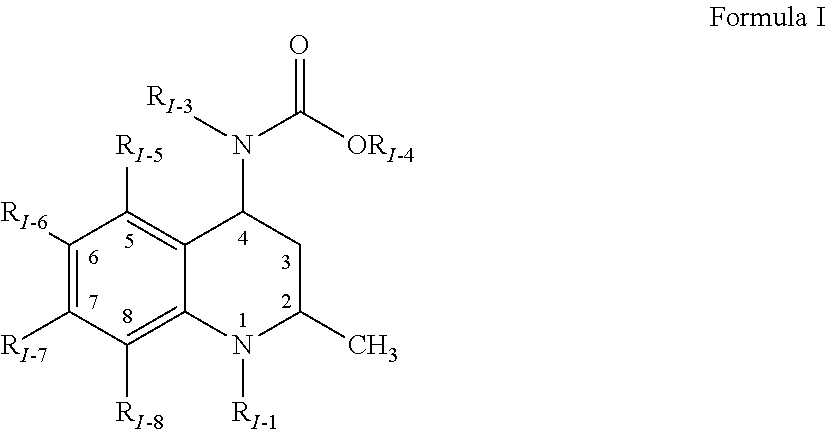
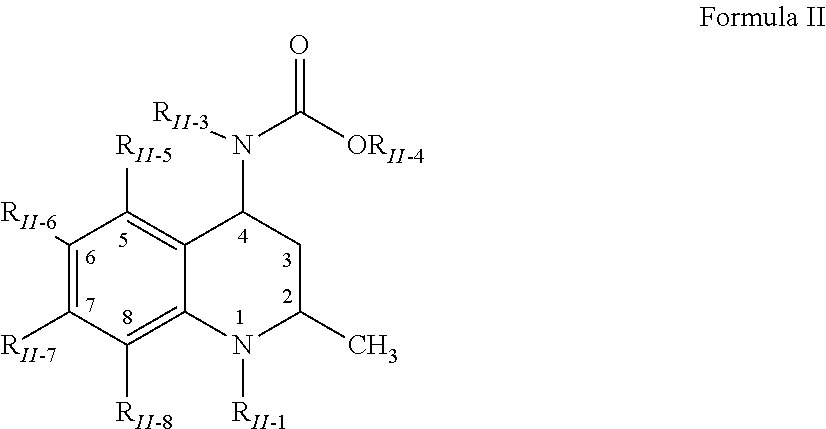
Methods of treating a refractory or drug resistant cancer, cell proliferative disorder and tumor cells are provided. Also provided are antibody drug conjugate metabolites.
Owner:SEAGEN INC
Porous drug matrices and methods of manufacture thereof
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
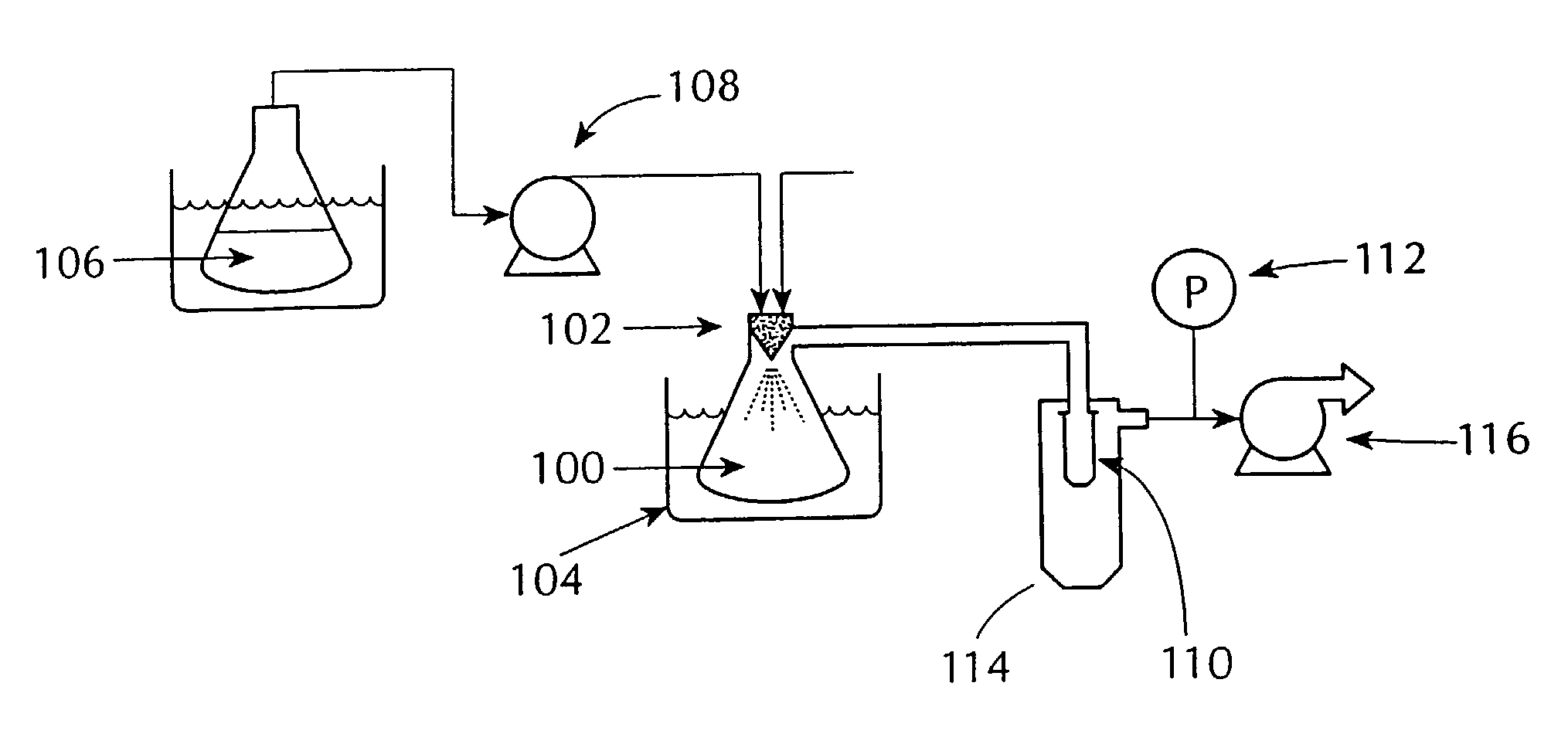
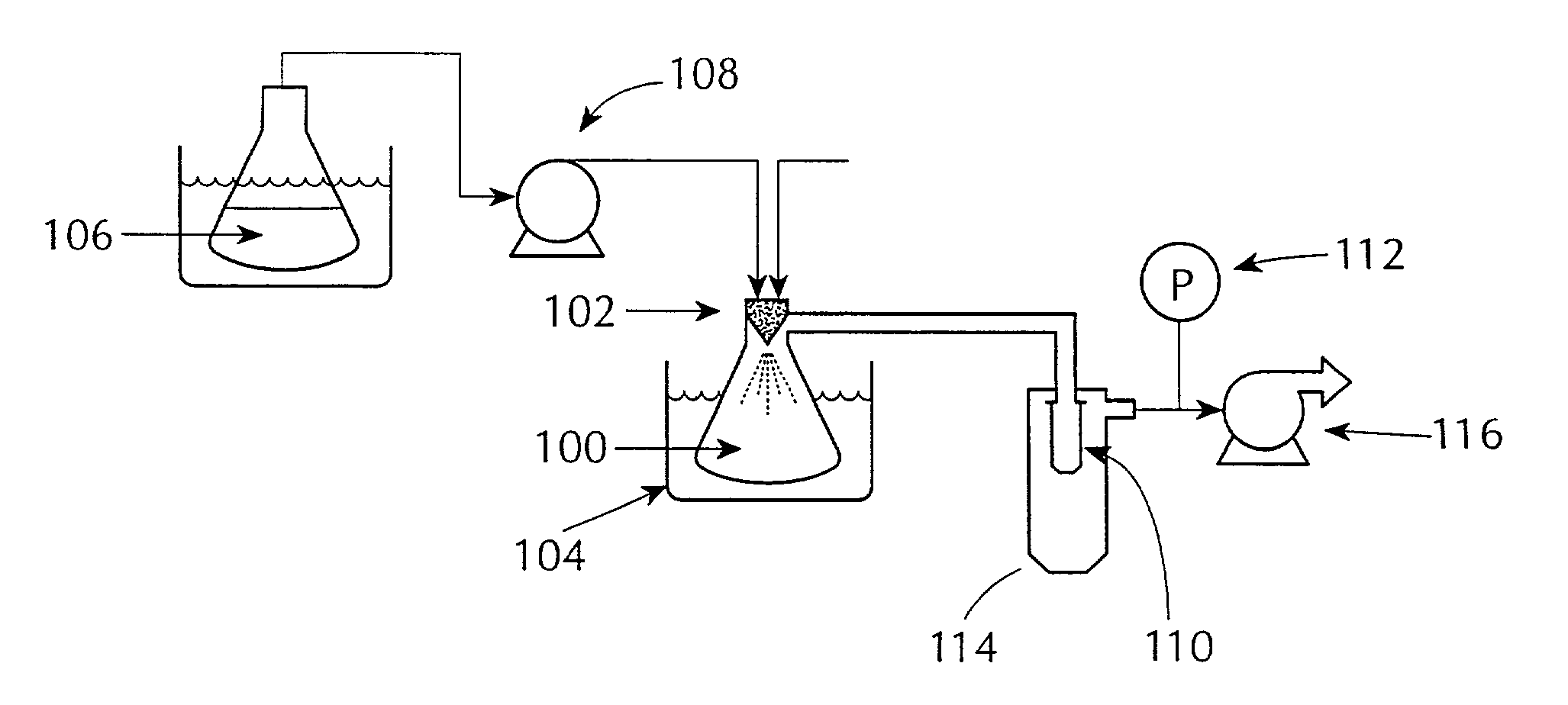
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Protein-Polymer-Drug Conjugates
ActiveUS20130101546A1Improve drug bioavailabilityImprove bioavailabilityPharmaceutical non-active ingredientsSynthetic polymeric active ingredientsDrug conjugationPharmaceutical drug
A drug conjugate is provided herein. The conjugate comprises a protein based recognition-molecule (PBRM) and a polymeric carrier substituted with one or more -LD-D, the protein based recognition-molecule being connected to the polymeric carrier by LP. Each occurrence of D is independently a therapeutic agent having a molecular weight≦5 kDa. LD and LP are linkers connecting the therapeutic agent and PBRM to the polymeric carrier respectively. Also disclosed are polymeric scaffolds useful for conjugating with a PBRM to form a polymer-drug-PBRM conjugate described herein, compositions comprising the conjugates, methods of their preparation, and methods of treating various disorders with the conjugates or their compositions.
Owner:MERSANA THERAPEUTICS INC
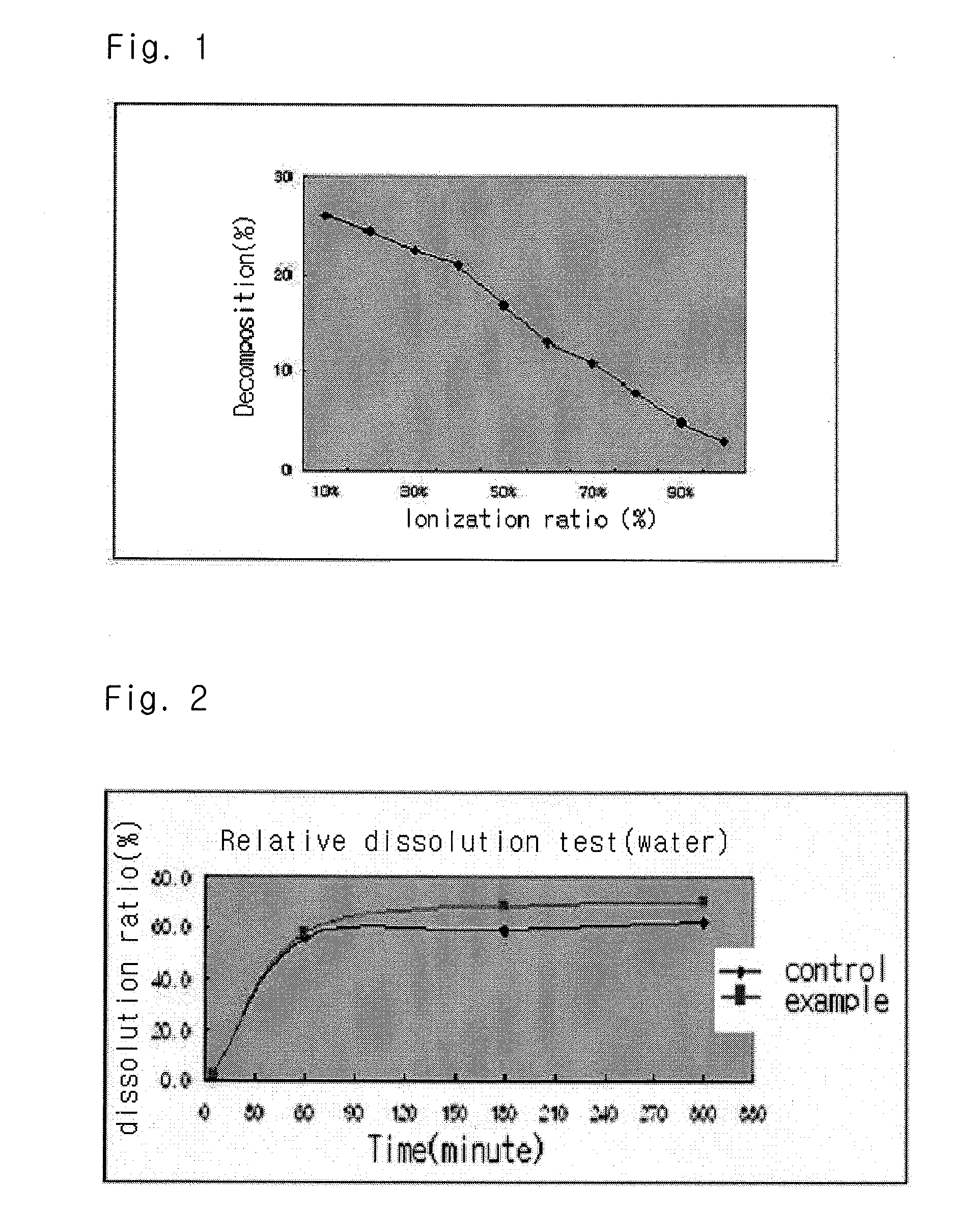
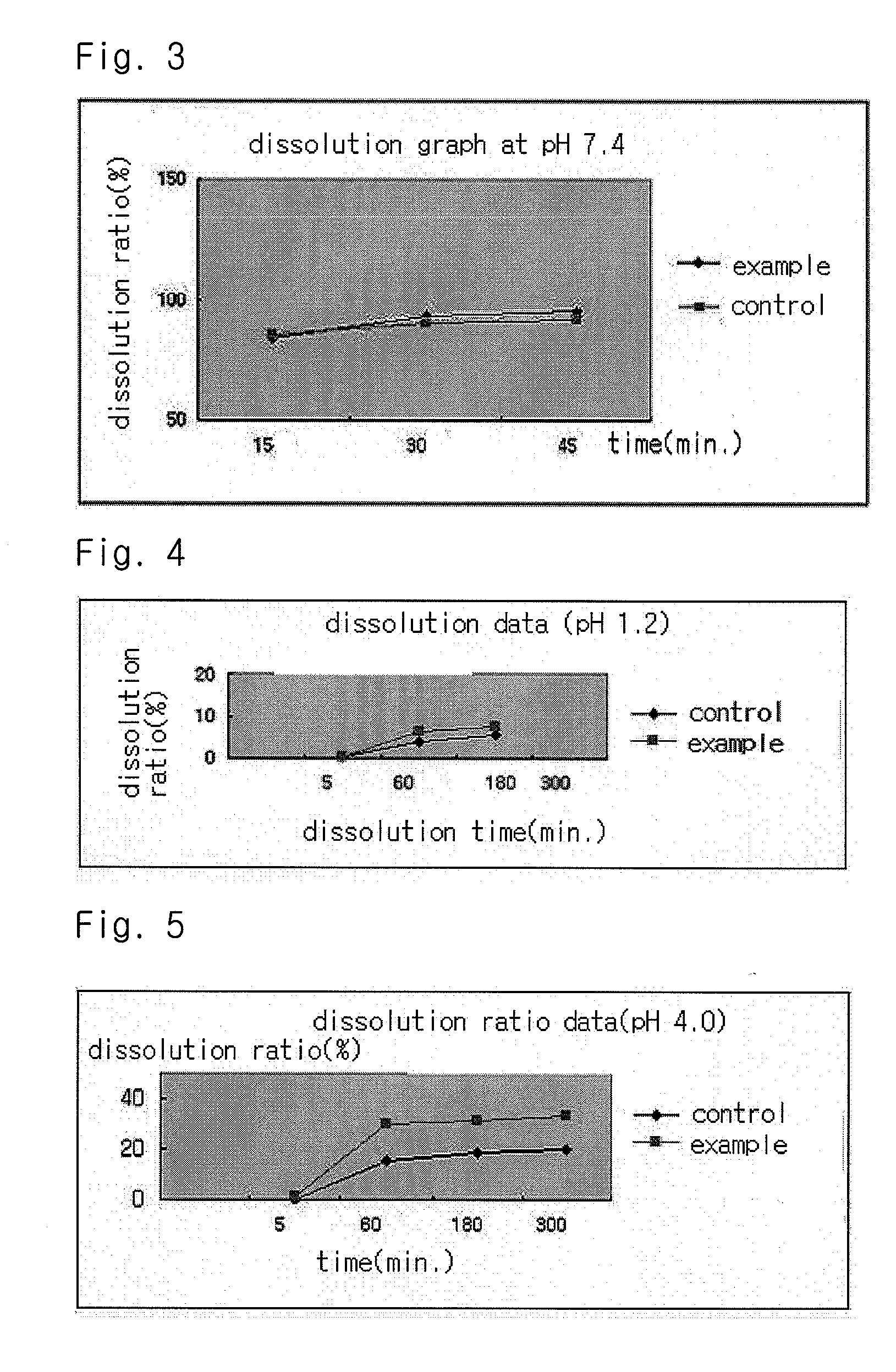
Solvent system of hardly soluble drug with improved dissolution rate
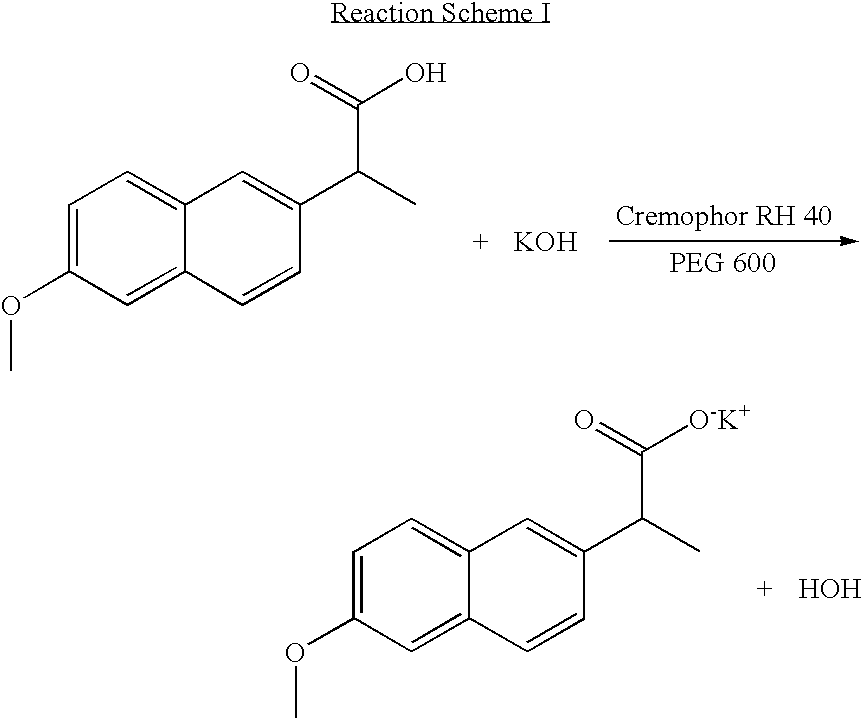
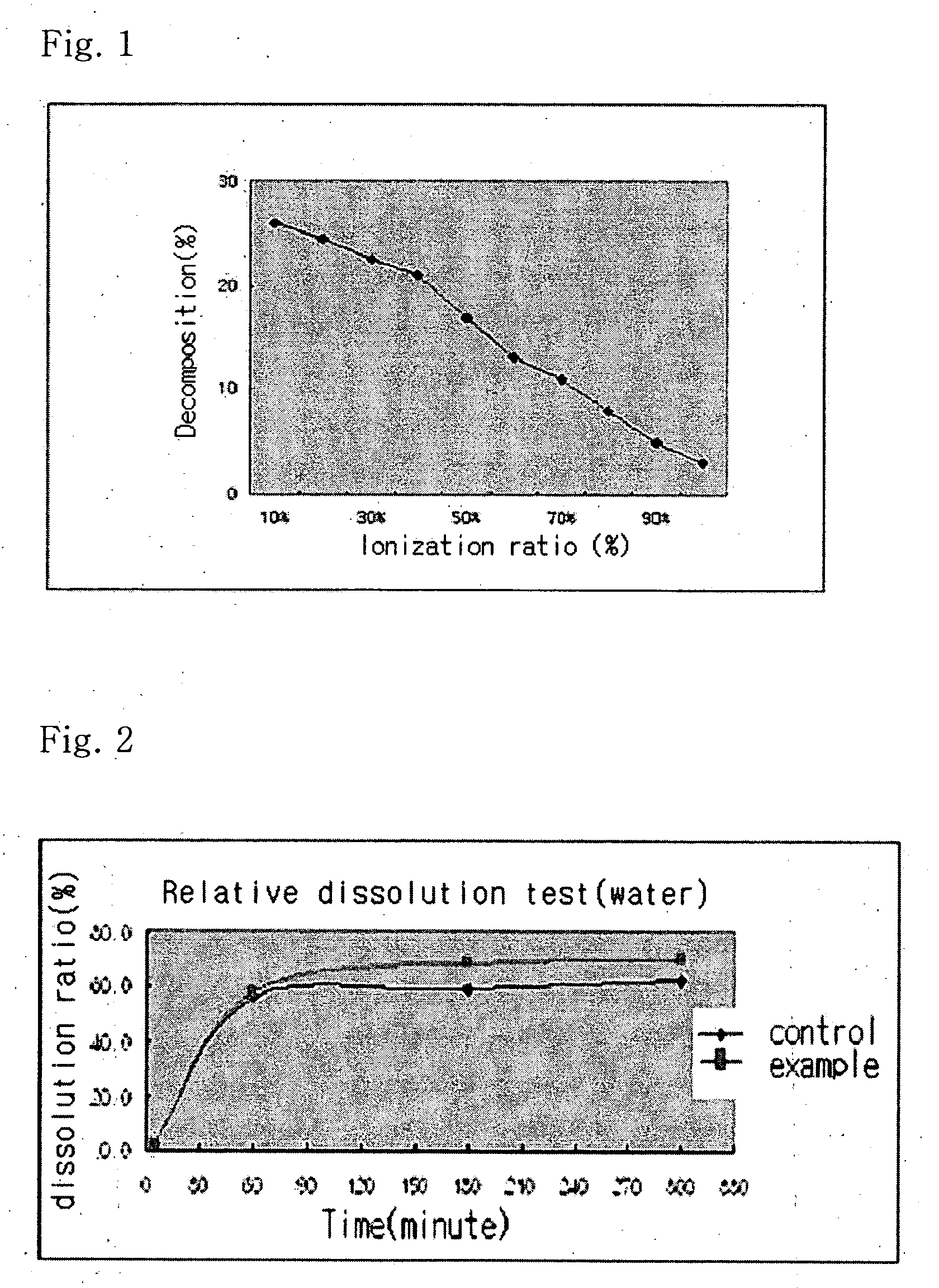
InactiveUS20040157928A1Good disintegrationPromote dissolutionBiocideAntipyreticDissolutionIonization
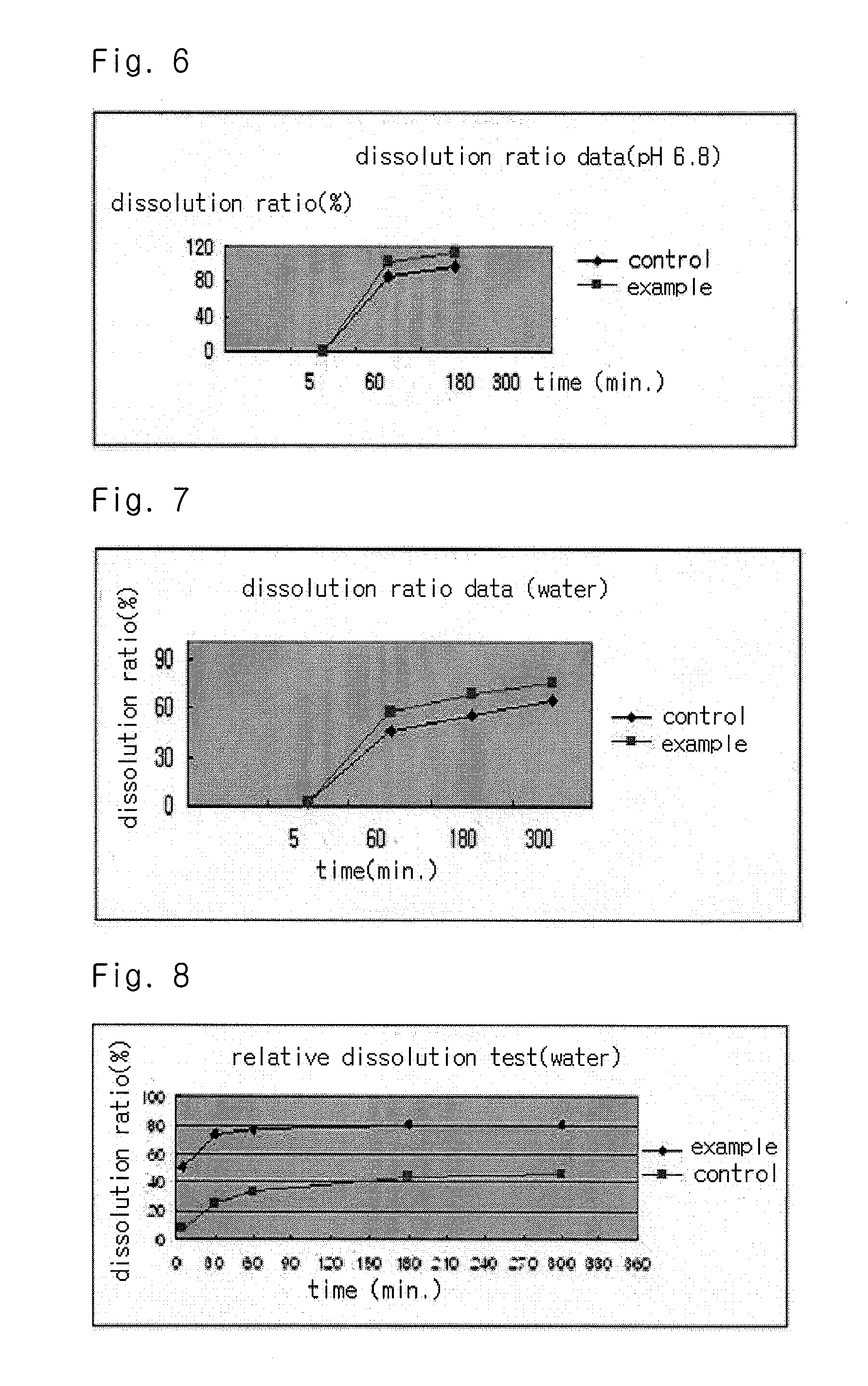
The present invention relates to a solvent system with improved disintegration degree and dissolution ratio of a hardly soluble drug by highly concentrating the drug through partial ionization, and by establishing optimal conditions for enhancing bioavailability of the drug, such as the co-relation between the acid drug and the accompanied components, ionization degree of a solvent system, use of an appropriate cation acceptance, water content, selection of optimal mixing ratio of the respective components and use of specific surfactants, and to a pharmaceutical preparation comprising the same. The solvent system of the invention has advantages in that it can enhance bioavailability by improving the disintegration degree and dissolution ratio of a hardly soluble drug and also provide a capsule with a sufficiently small volume to permit easy swallowing.
Owner:R & P KOREA
Compositions for Drug Administration
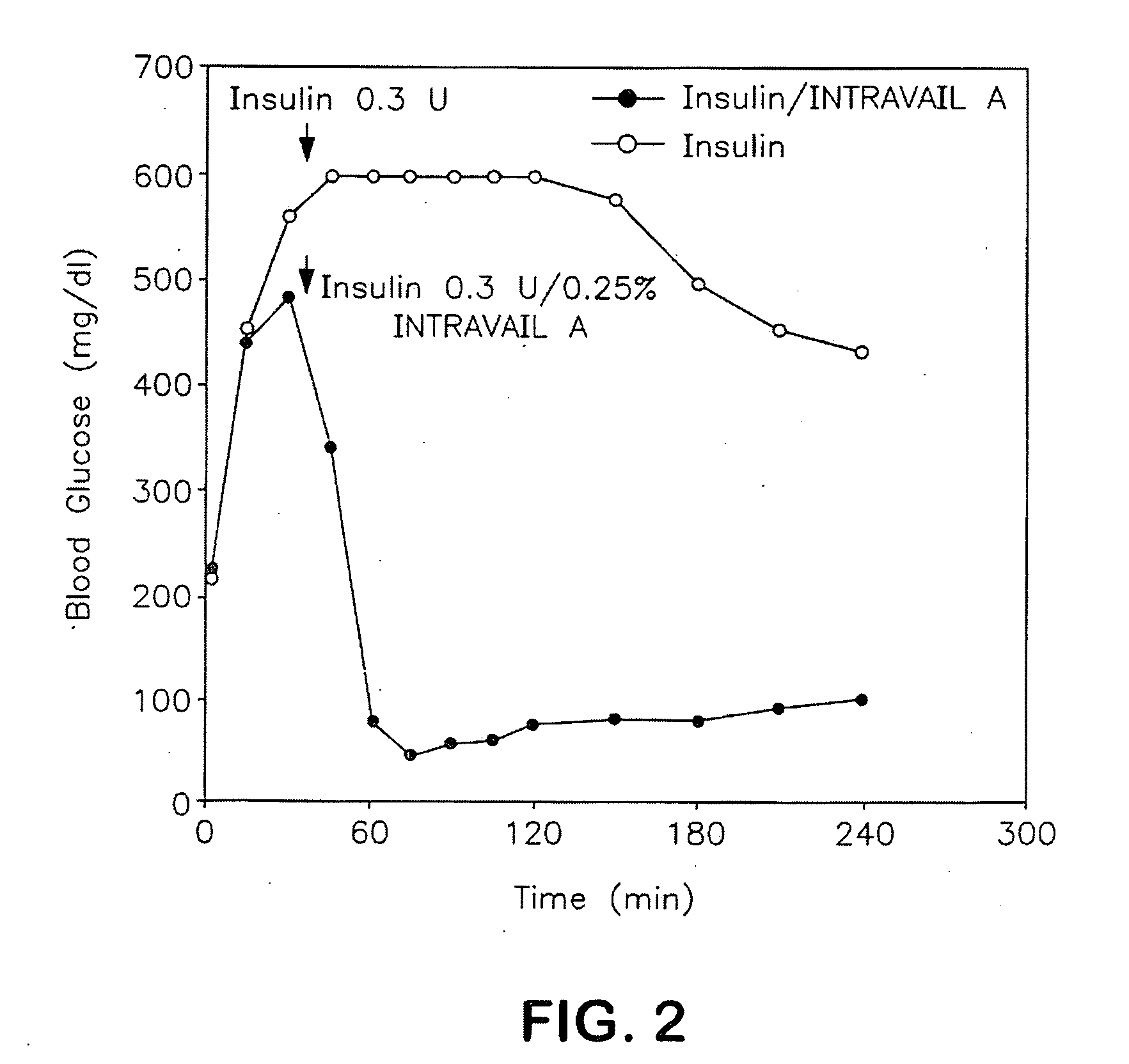
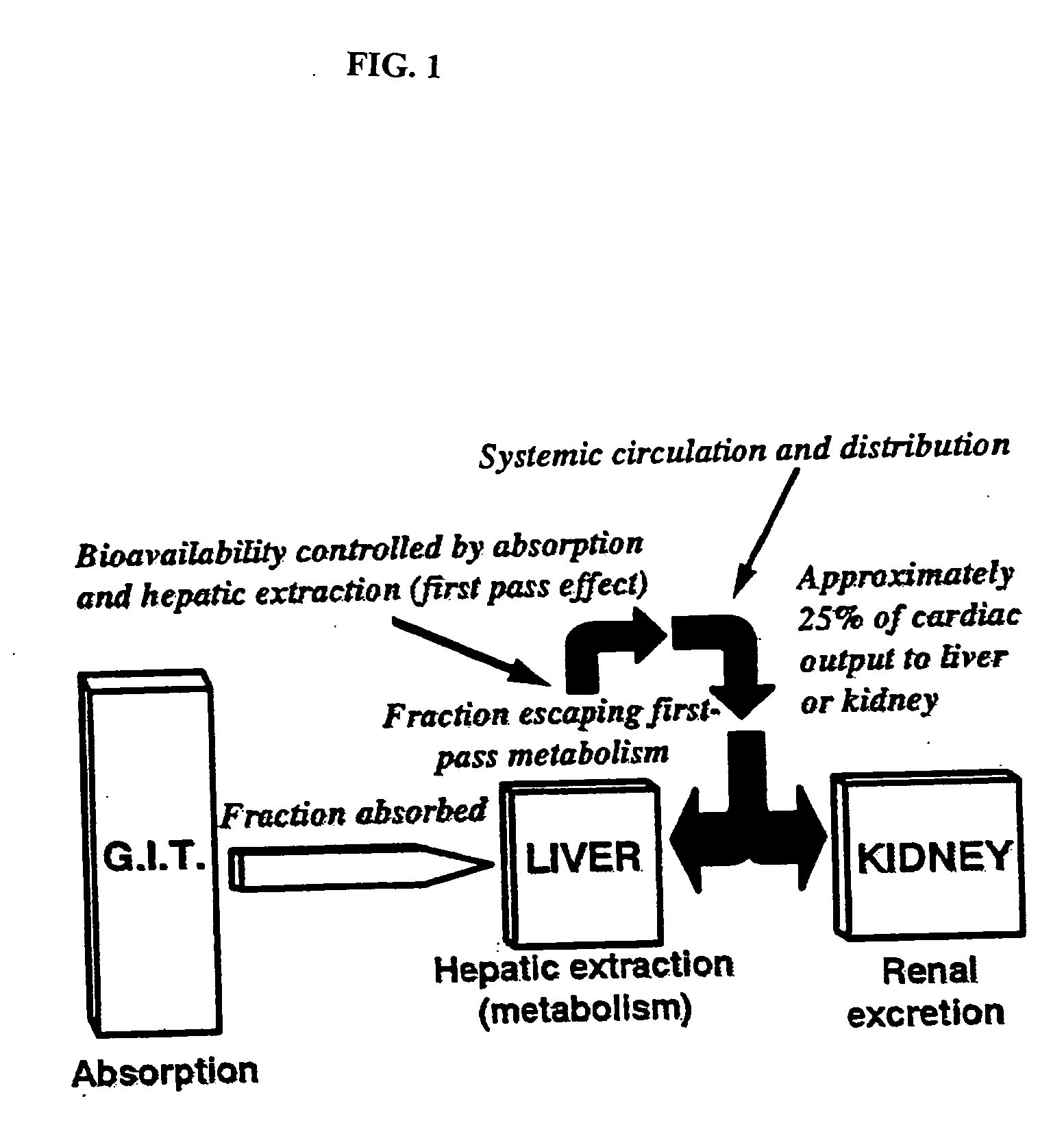
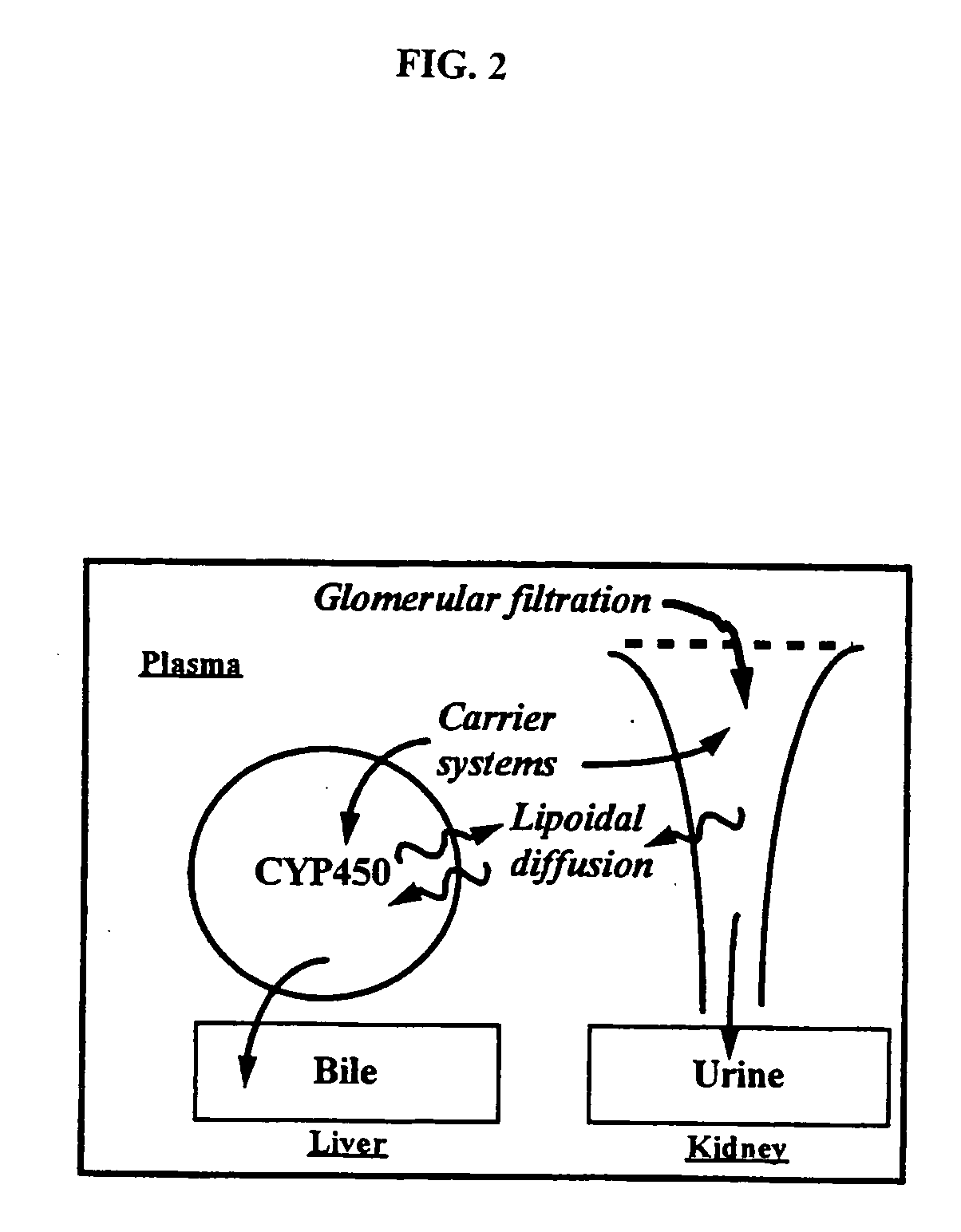
InactiveUS20090047347A1Improve absorption and bioavailabilityToxic effectsPowder deliveryBiocideDrug metabolismHepatic first pass effect
The present invention provides compositions and methods and for speeding the onset of drug action and reducing the first-pass effect drug metabolism in fast-dispersing drug formulations.
Owner:AEGIS THERAPEUTICS LLC
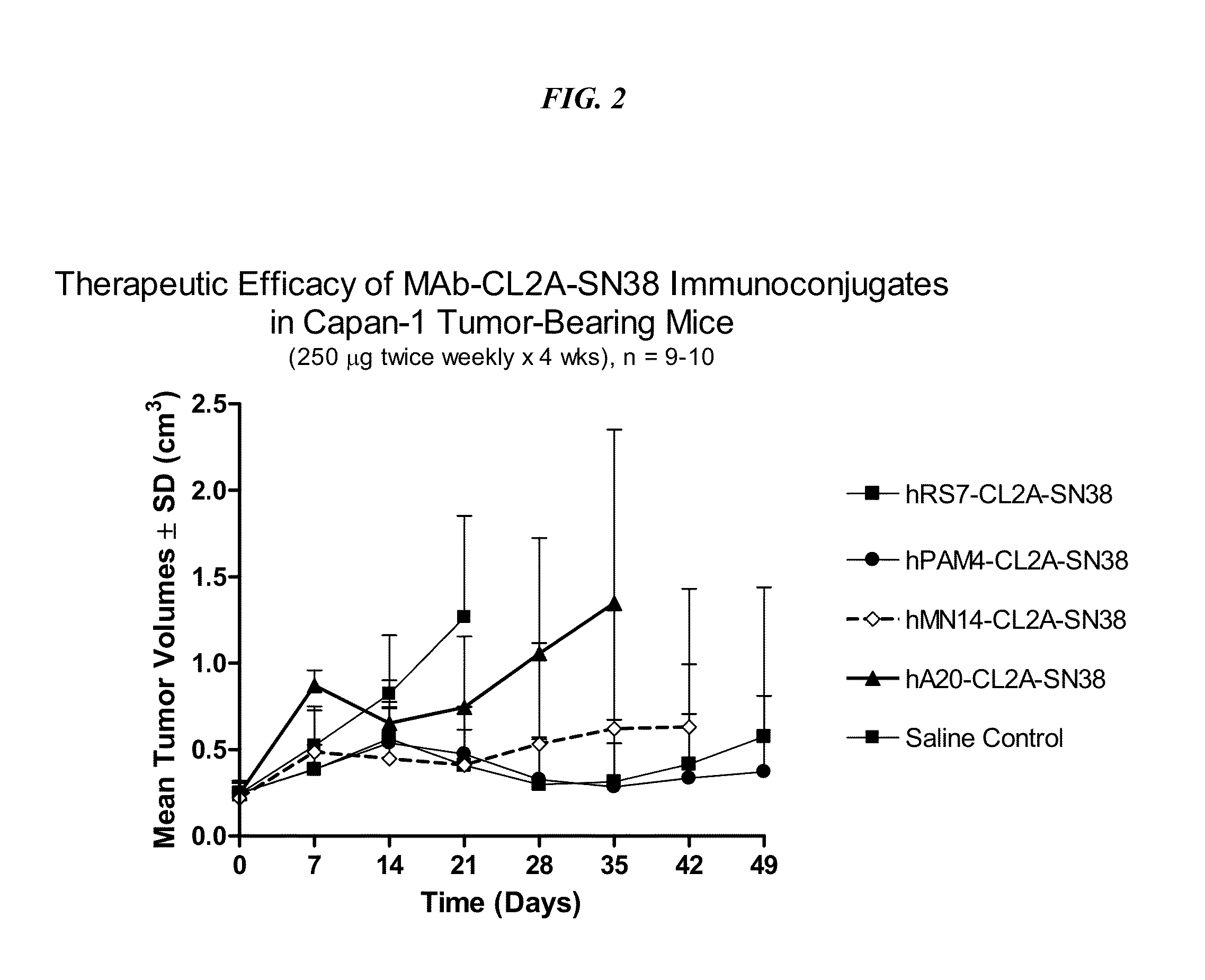
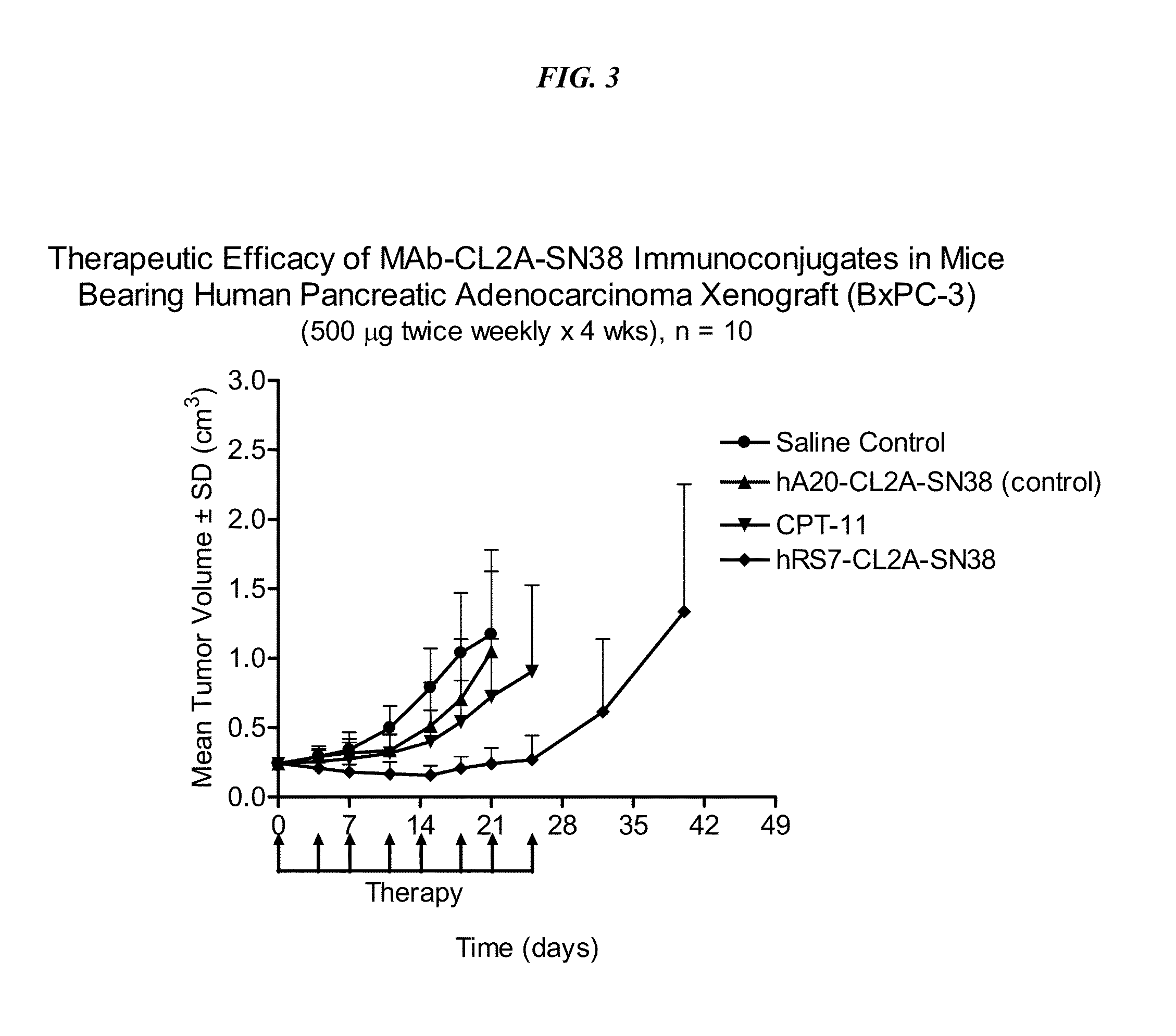
Antibody-sn-38 immunoconjugates with a cl2a linker
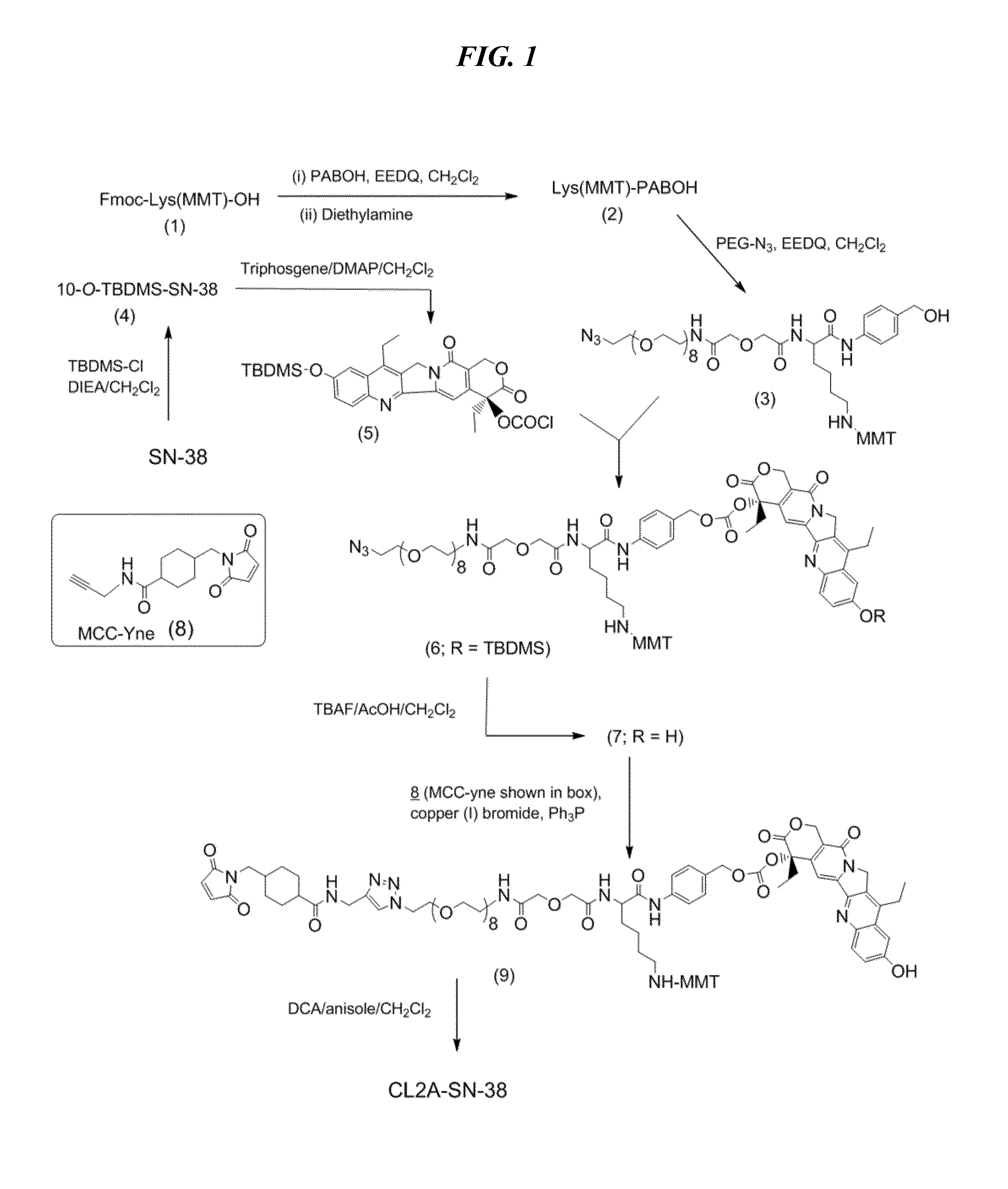
ActiveUS20140227180A1Improve drug bioavailabilityGood treatment effectOrganic active ingredientsPeptide/protein ingredientsDiseaseSide effect
The present invention concerns improved methods and compositions for preparing SN-38 conjugates of proteins or peptides, preferably immunoconjugates of antibodies or antigen-binding antibody fragments. More preferably, the SN-38 is attached to the antibody or antibody fragment using a CL2A linker, with 1-12, more preferably 6 or less, most preferably 1-5 SN-38 moieties per antibody or antibody fragment. Most preferably, the immunoconjugate is prepared in large scale batches, with various modifications to the reaction scheme to optimize yield and recovery in large scale. Other embodiments concern optimized dosages and / or schedules of administration of immunoconjugate to maximize efficacy for disease treatment and minimize side effects of administration.
Owner:IMMUNOMEDICS INC
Solvent system of hardly soluble drug with improved dissolution rate
InactiveUS20090318558A1Good disintegrationPromote dissolutionBiocideAntipyreticActive agentDissolution
The present invention relates to a solvent system with improved disintegration degree and dissolution ratio of a hardly soluble drug by highly concentrating the drug through partial ionization, and by establishing optimal conditions for enhancing bioavailability of the drug, such as the co-relation between the acid drug and the accompanied components, ionization degree of a solvent system, use of an appropriate cation acceptor, water content, selection of optimal mixing ratio of the respective components and use of specific surfactants, and to a pharmaceutical preparation comprising the same. The solvent system of the invention has advantages in that it can enhance bioavailability by improving the disintegration degree and dissolution ratio of a hardly soluble drug and also provide a capsule with a sufficiently small volume to permit easy swallowing.
Owner:R & P KOREA
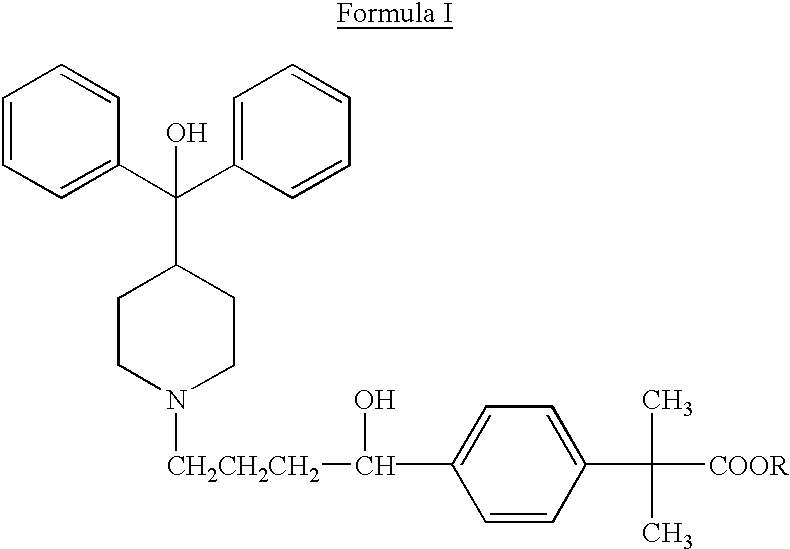
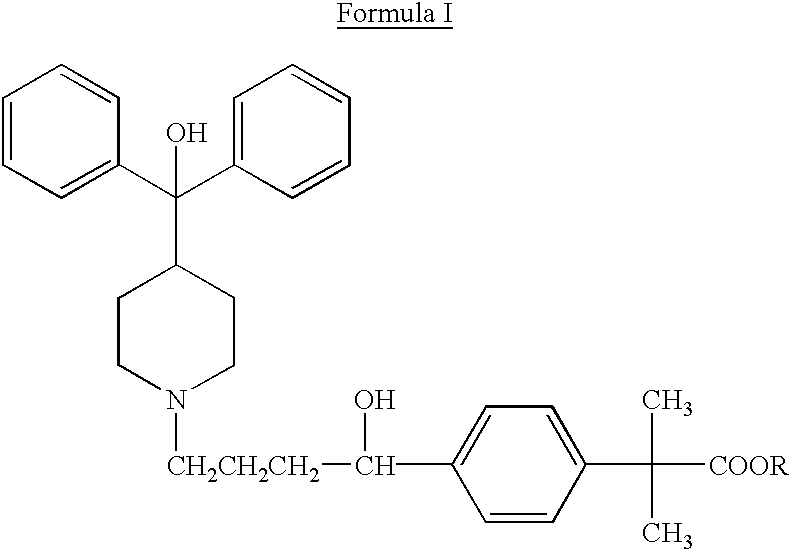
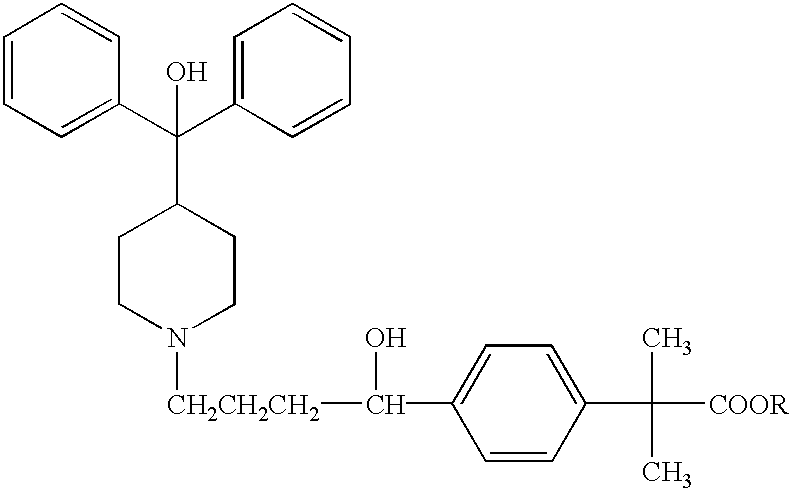
Method of enhancing bioavailability of fexofenadine and its derivatives
The present invention relates to a method of enhancing the bioavailability of a piperidinoalkanol antihistamine in a patient which comprises co-administering to said patient an effective antihistaminic amount of said piperidinoalkanol and an effective p-glycoprotein inhibiting amount of a p-glycoprotein inhibitor.
Owner:AVENTISUB II INC +1
Compositions for Drug Administration
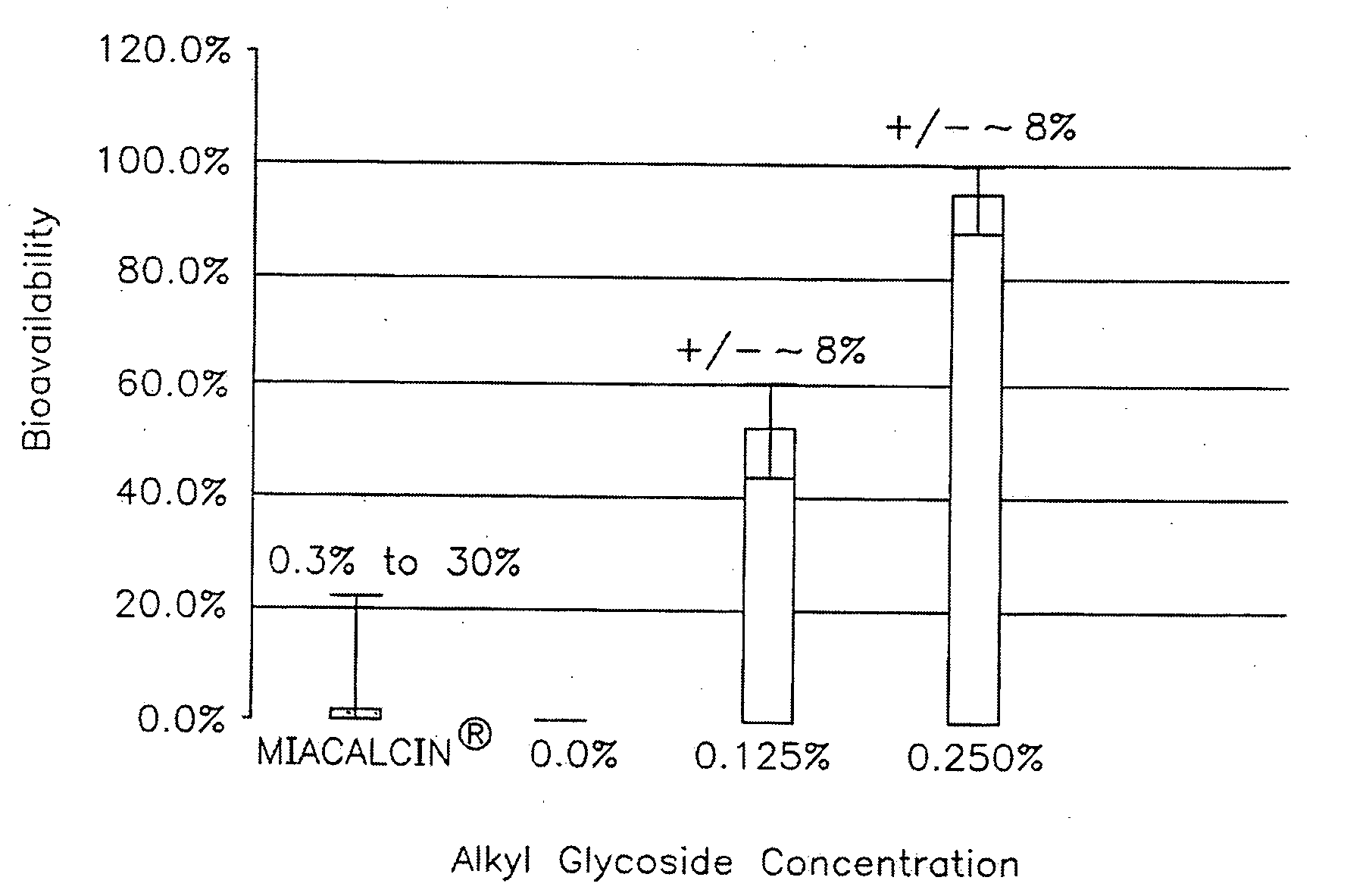
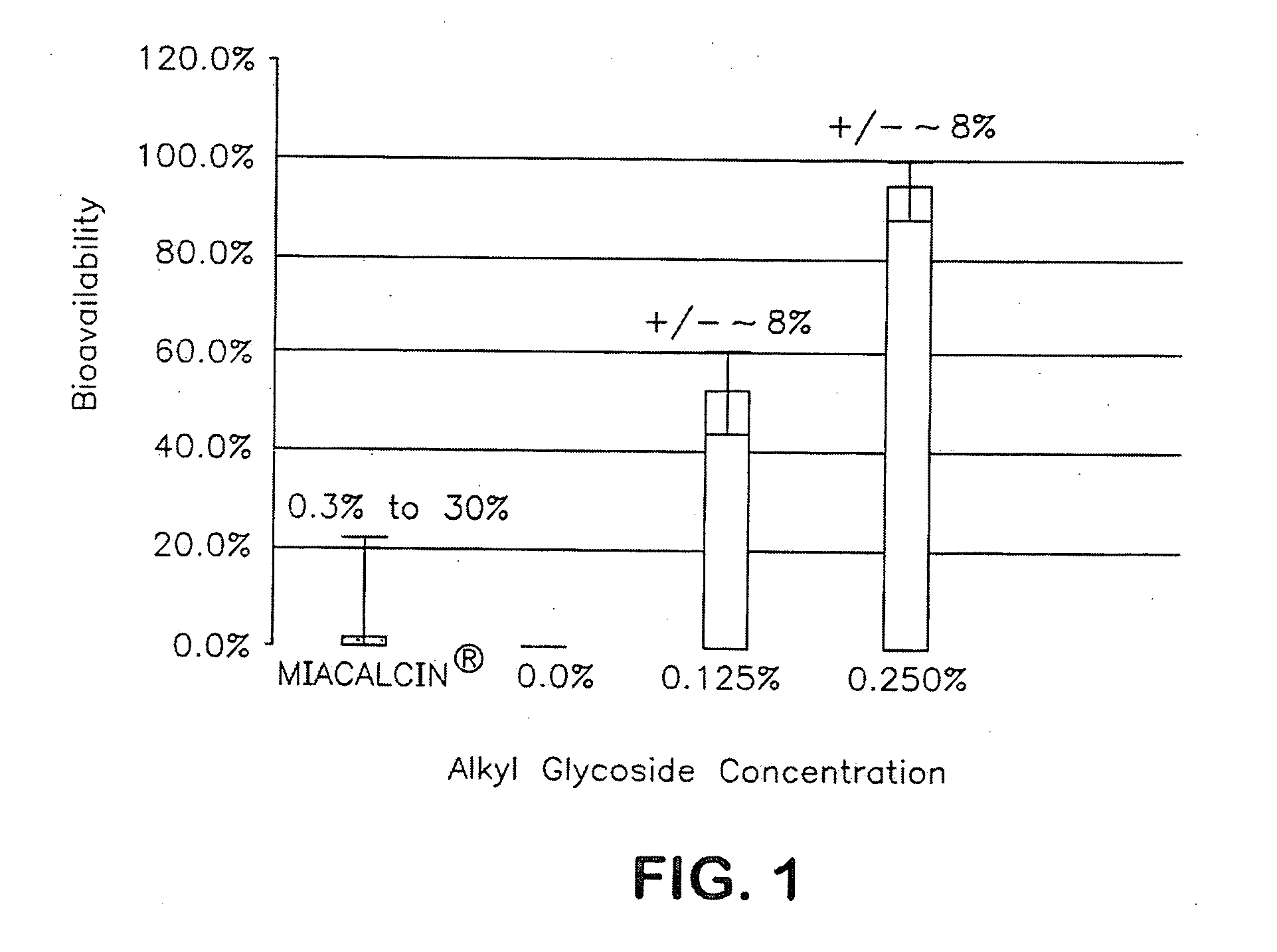
ActiveUS20090163447A1Promote absorptionImprove bioavailabilityBiocideNervous disorderMedicineChain length
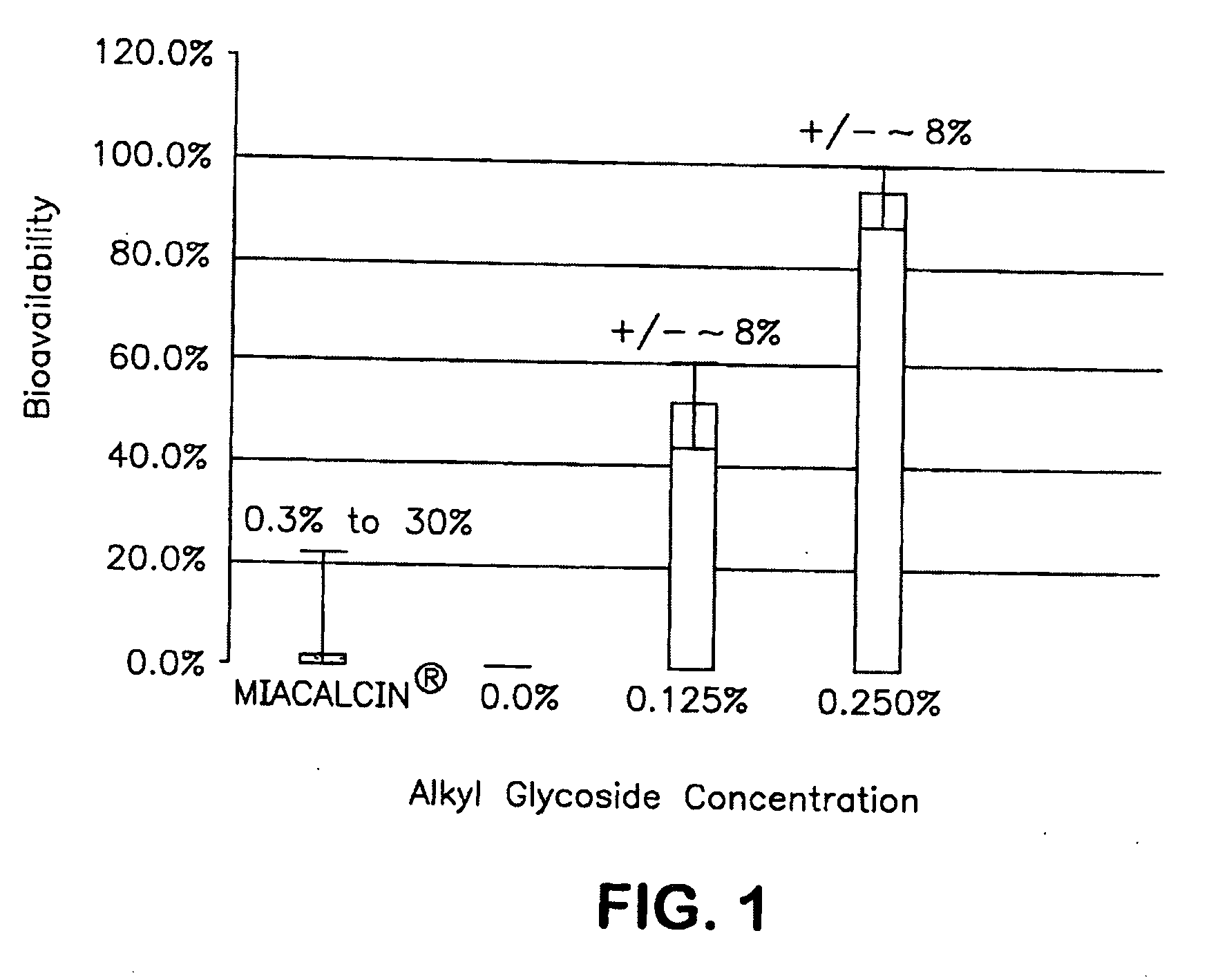
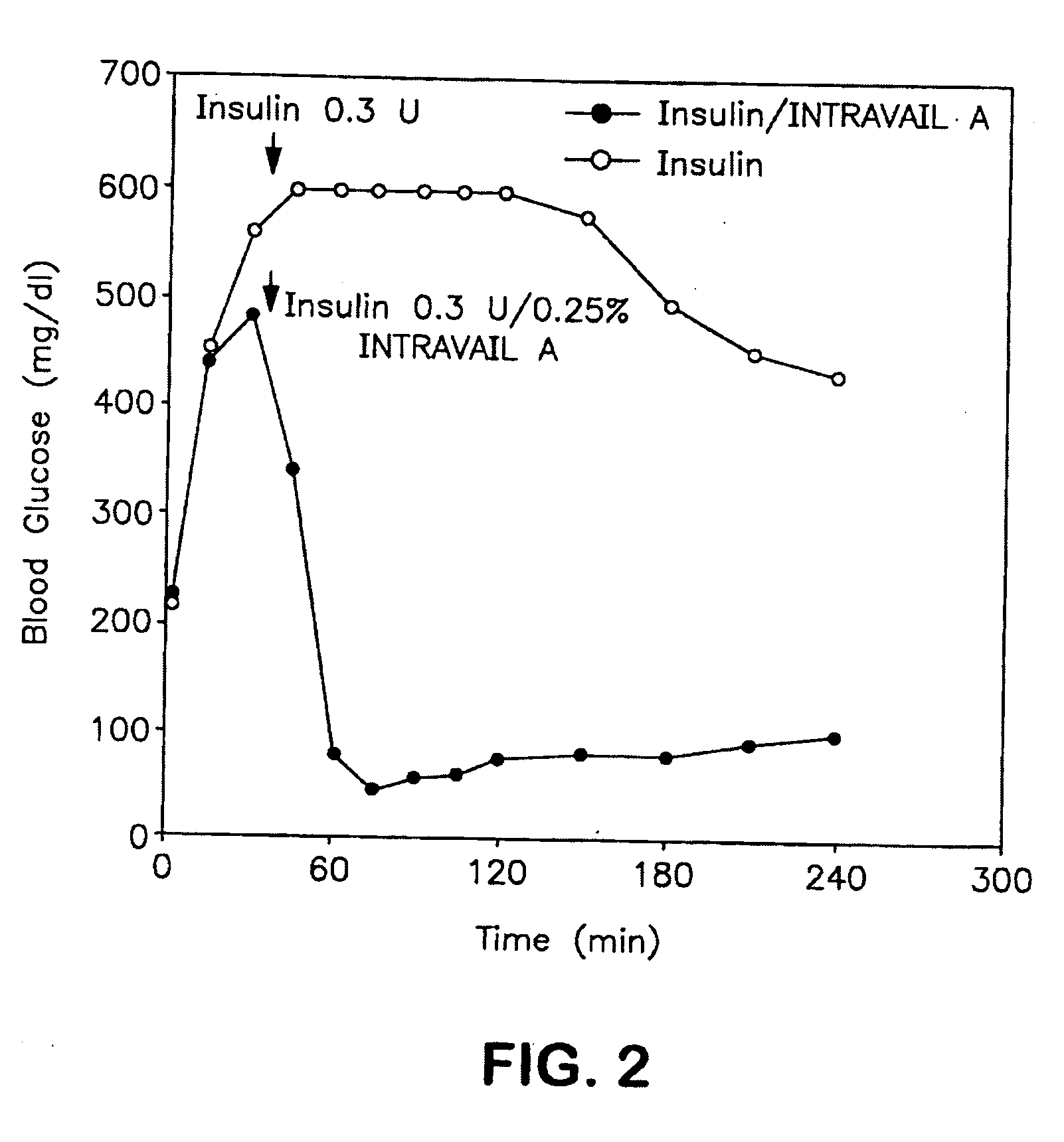
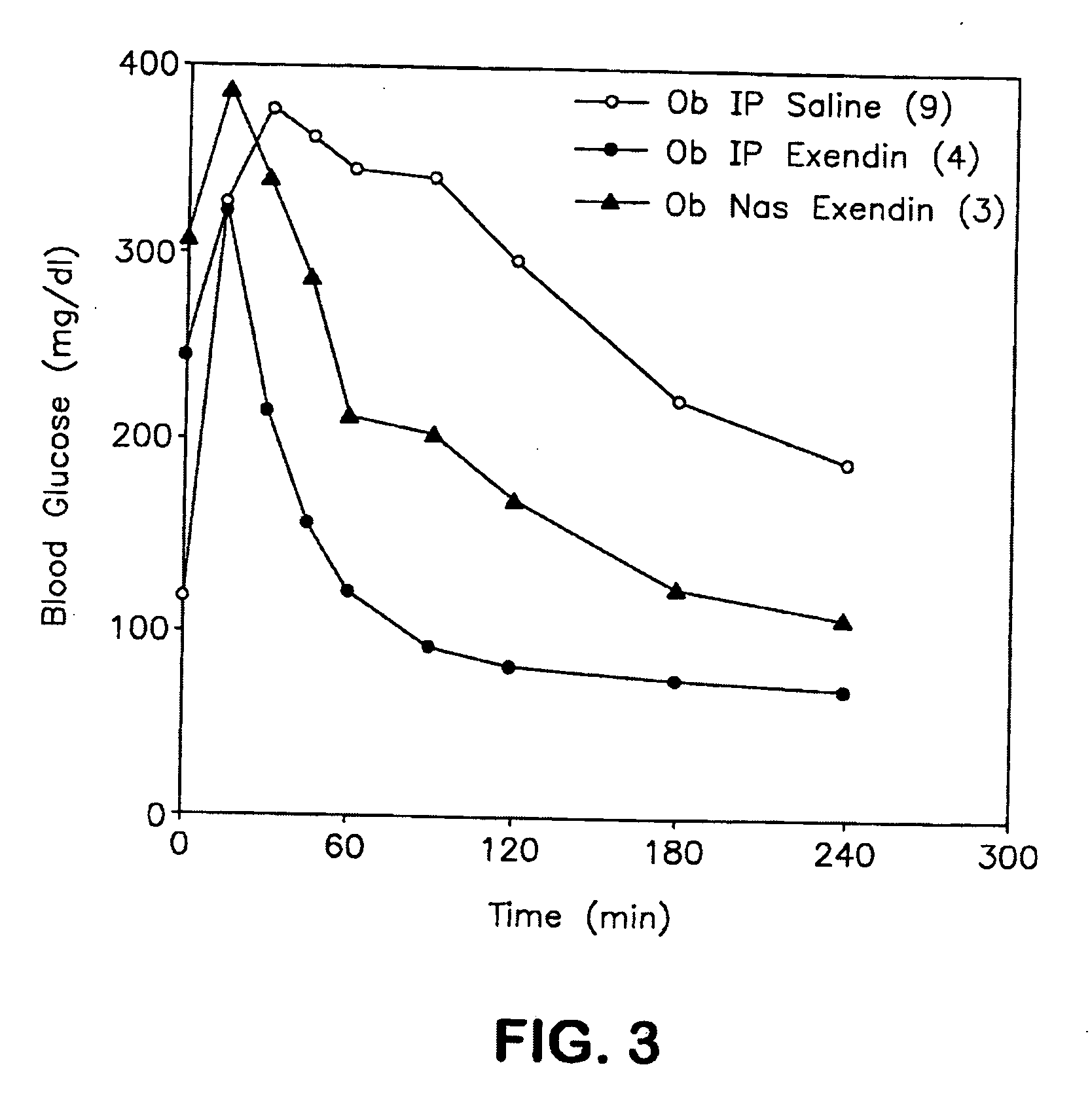
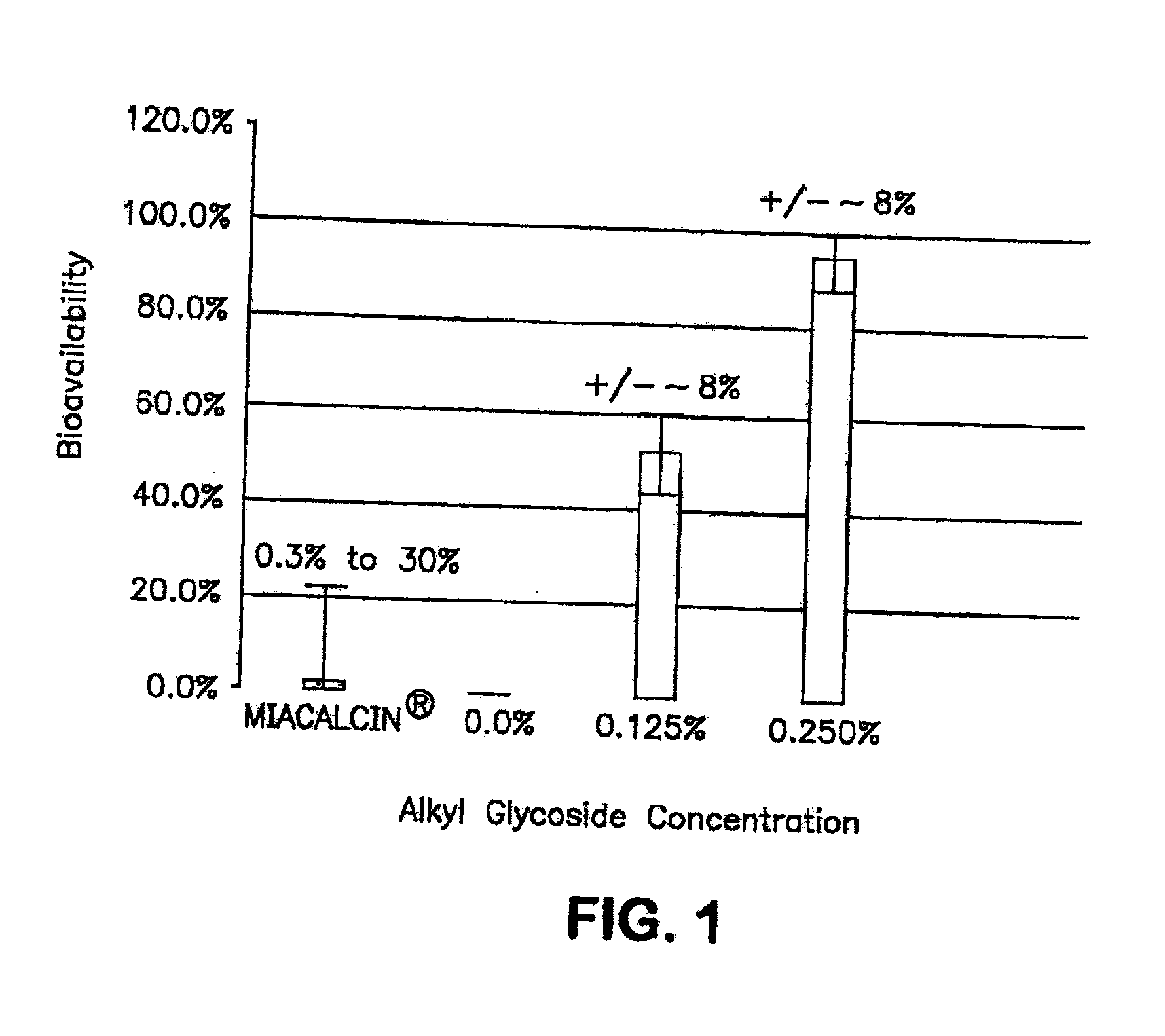
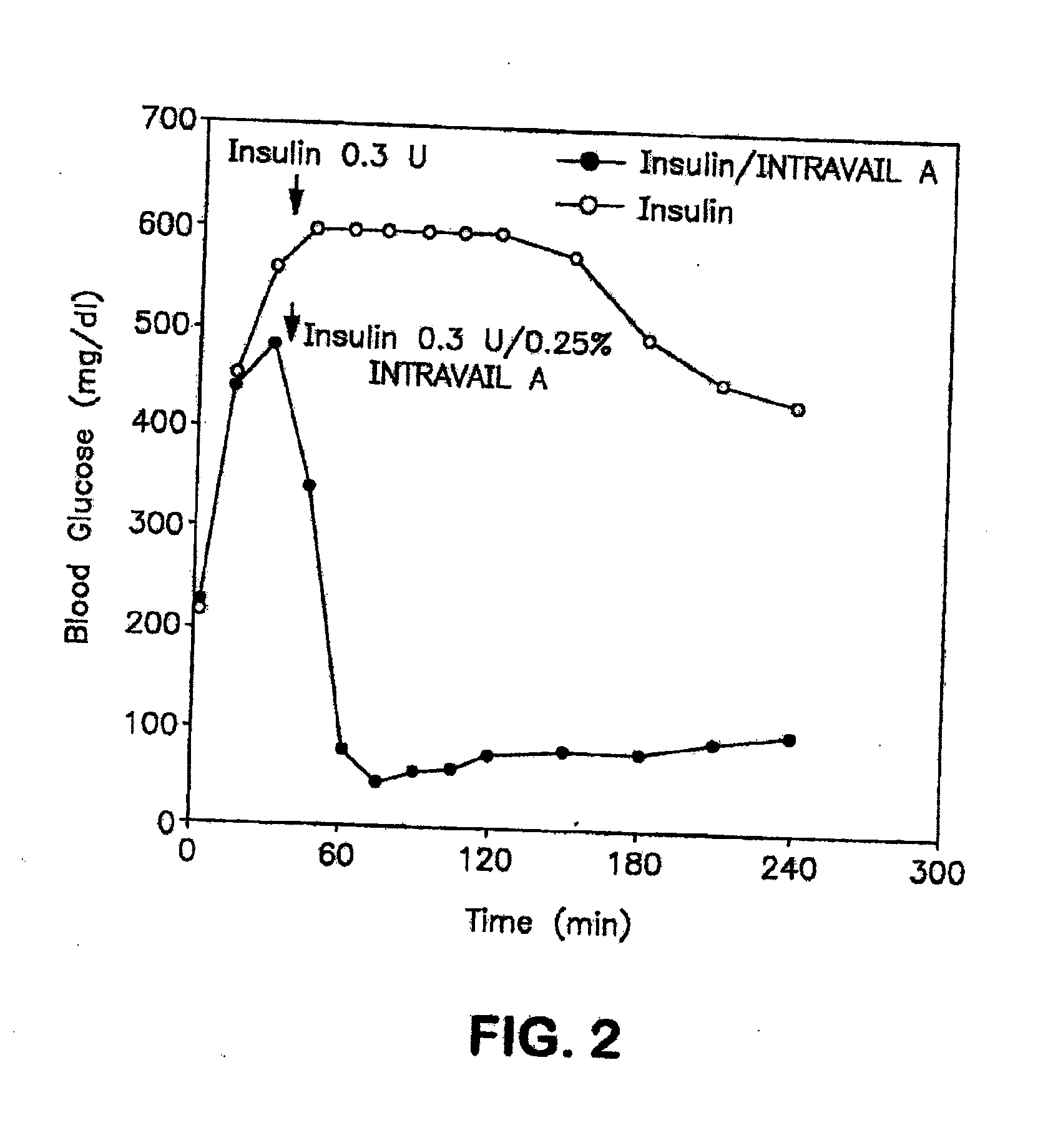
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms.
Owner:AEGIS THERAPEUTICS LLC
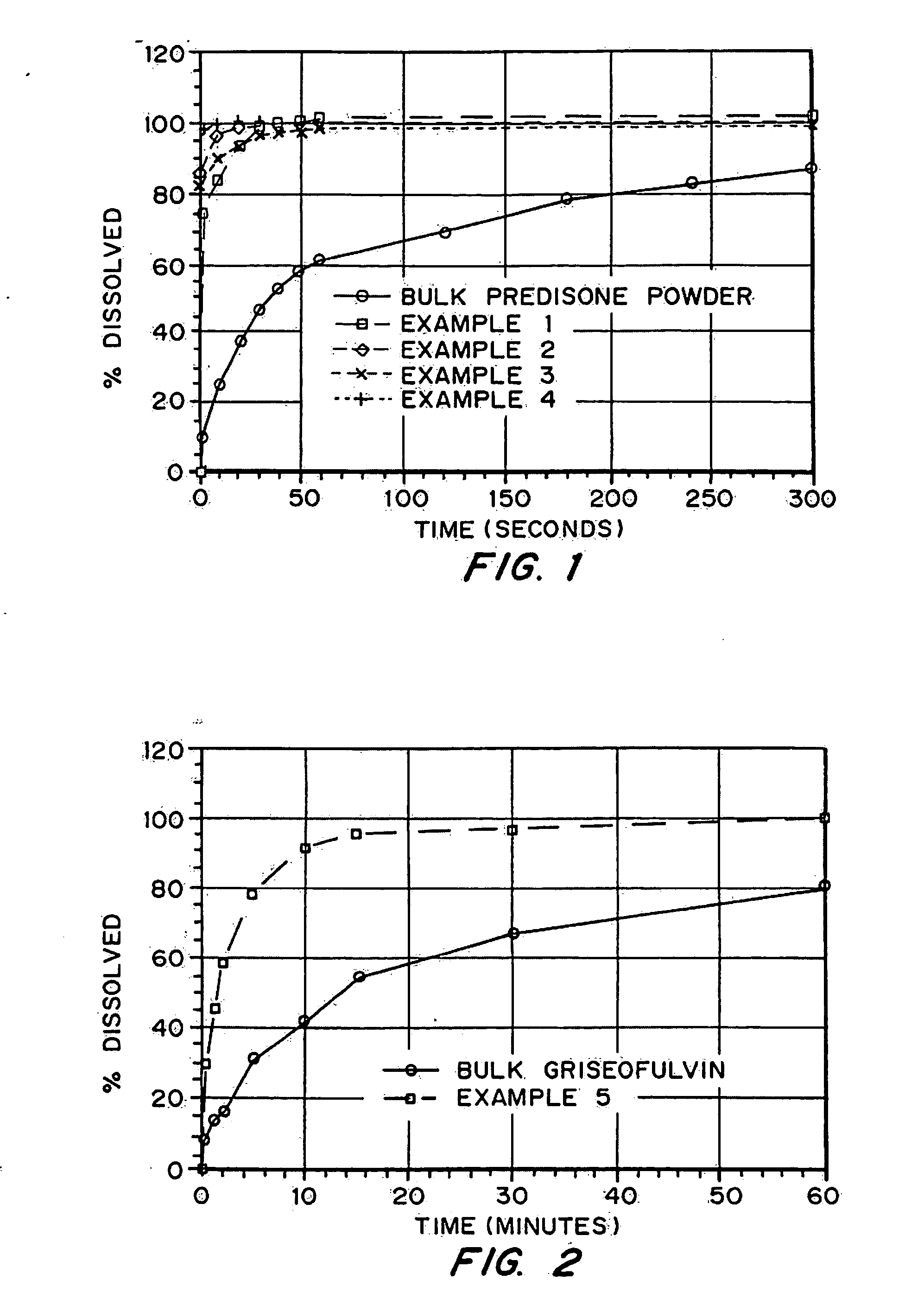
Solid pharmaceutical dispersions with enhanced bioavailability
InactiveUS8257741B2Improve solubilityEffective dispersionPowder deliveryBiocideAcetic acidHydroxypropylmethylcellulose acetate succinate
Spray dried solid dispersions comprising a sparingly soluble drug and hydroxypropylmethylcellulose acetate succinate (HPMCAS) provide increased aqueous solubility and / or biavailability in a use environment.
Owner:BEND RES
Protein-polymer-drug conjugates
ActiveUS20150104407A1Improve drug bioavailabilityImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsDrug conjugationPharmaceutical drug
A polymeric scaffold useful for conjugating with a protein based recognition-molecule (PBRM) to form a PBRM-polymer-drug conjugate is described herein. The scaffold includes one or more terminal maleimido groups. Also disclosed is a PBRM-polymer-drug conjugate prepared from the scaffold. Compositions comprising the conjugates, methods of their preparation, and methods of treating various disorders with the conjugates or their compositions are also described.
Owner:MERSANA THERAPEUTICS INC
Solid pharmaceutical dispersions with enhanced bioavailability
InactiveUS8263128B2Improve bioavailabilityPreventing and retarding ratePowder deliveryBiocideAcetic acidHydroxypropylmethylcellulose acetate succinate
Spray dried solid dispersions comprising a sparingly soluble drug and hydroxypropylmethylcellulose acetate succinate (HPMCAS) provide increased aqueous solubility and / or biavailability in a use environment.
Owner:BEND RES
Protein-Polymer-Drug Conjugates
ActiveUS20120321583A1High drug loadingImprove bindingPeptide/protein ingredientsAntibody ingredientsDrug conjugationPharmaceutical drug
A drug conjugate is provided herein. The conjugate comprises a protein based recognition-molecule (PBRM) and a polymeric carrier substituted with one or more -LD-D, the protein based recognition-molecule being connected to the polymeric carrier by LP. Each occurrence of D is independently a therapeutic agent having a molecular weight ≦5 kDa. LD and LP are linkers connecting the therapeutic agent and PBRM to the polymeric carrier respectively. Also disclosed are polymeric scaffolds useful for conjugating with a PBRM to form a polymer-drug-PBRM conjugate described herein, compositions comprising the conjugates, methods of their preparation, and methods of treating various disorders with the conjugates or their compositions.
Owner:MERSANA THERAPEUTICS INC
Compositions for drug administration
ActiveUS20100160378A1Improve absorption and bioavailabilityToxic effectsBiocideNervous disorderChain length5-HT receptor
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject, as well as compositions and methods for providing migraine pain relief. The compositions include at least one alkyl glycoside and at least one therapeutic agent, such as a 5-HT receptor agonist, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms.
Owner:AEGIS THERAPEUTICS LLC
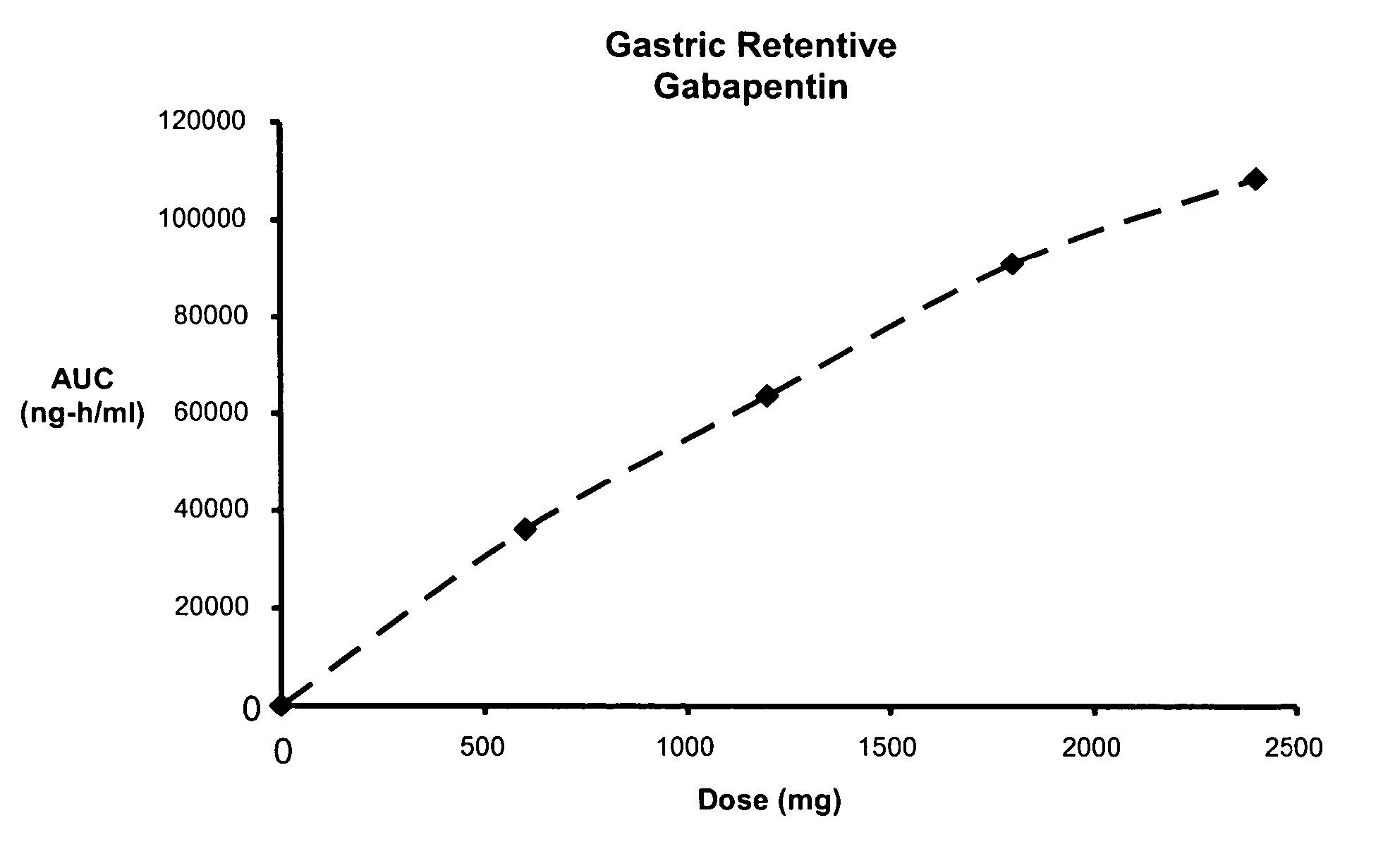
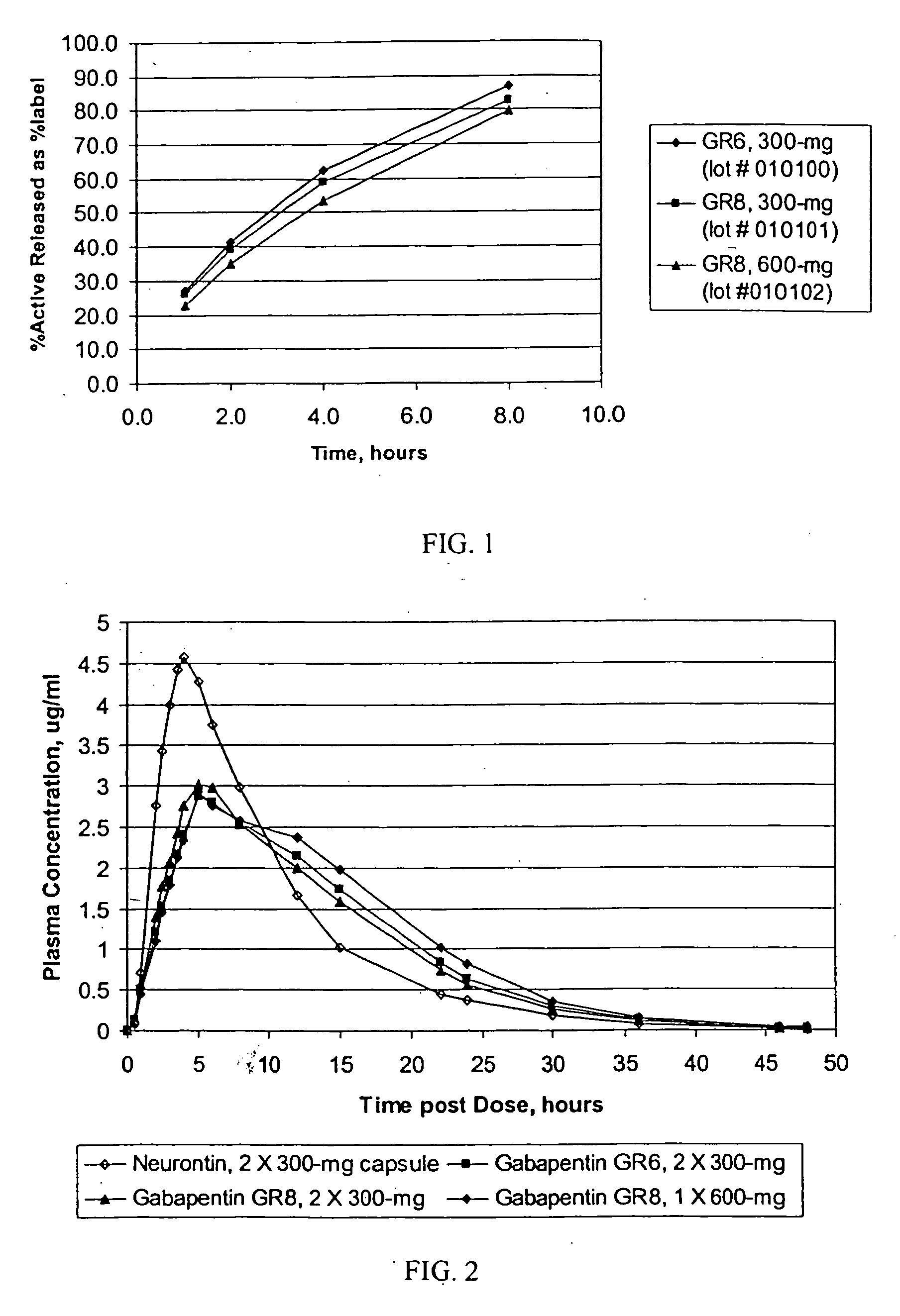
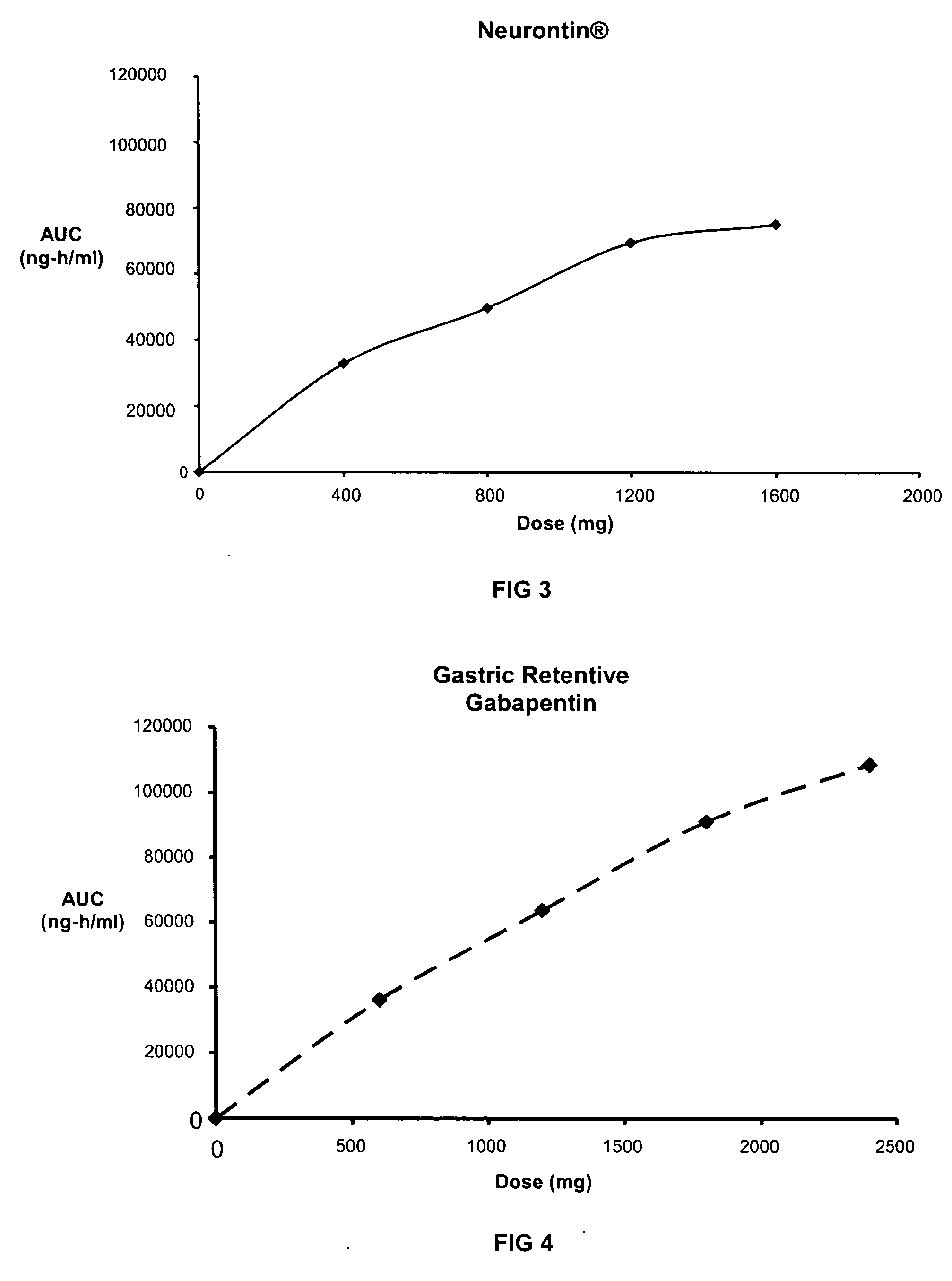
Methods of treating non-nociceptive pain states with gastric retentive gabapentin
InactiveUS20060159743A1Bioavailability (AUC)Improve complianceBiocideOrganic active ingredientsGabapentinDosing regimen
Provided is a method of treating a patient suffering from a pain state by administering to the patient a gastric retentive dosage form of gabapentin that is capable of administration in once-daily or twice daily dosing regimens. By reducing the need to administer gabapentin from the thrice-daily administrations characteristic of immediate release gabapentin, the gastric retentive gabapentin dosage forms provided herein have the advantages of improving patient compliance for gabapentin treatment. In addition to the foregoing, the gastric retentive gabapentin dosages forms also exhibit decreased blood plasma concentrations and increased bioavailability throughout the dosing regimen.
Owner:DEPOMED SYST INC
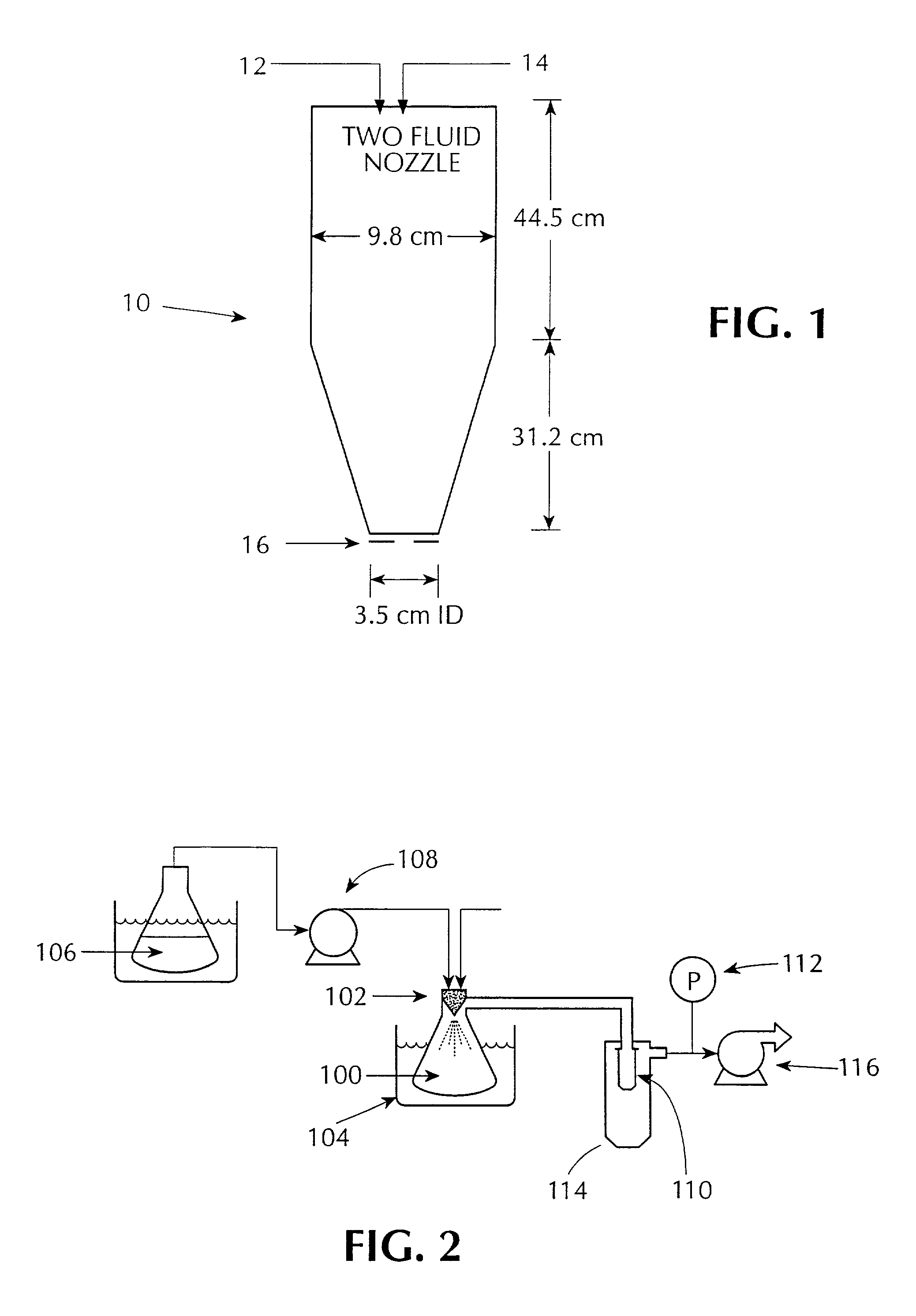
Process for producing nanoparticles by spray drying
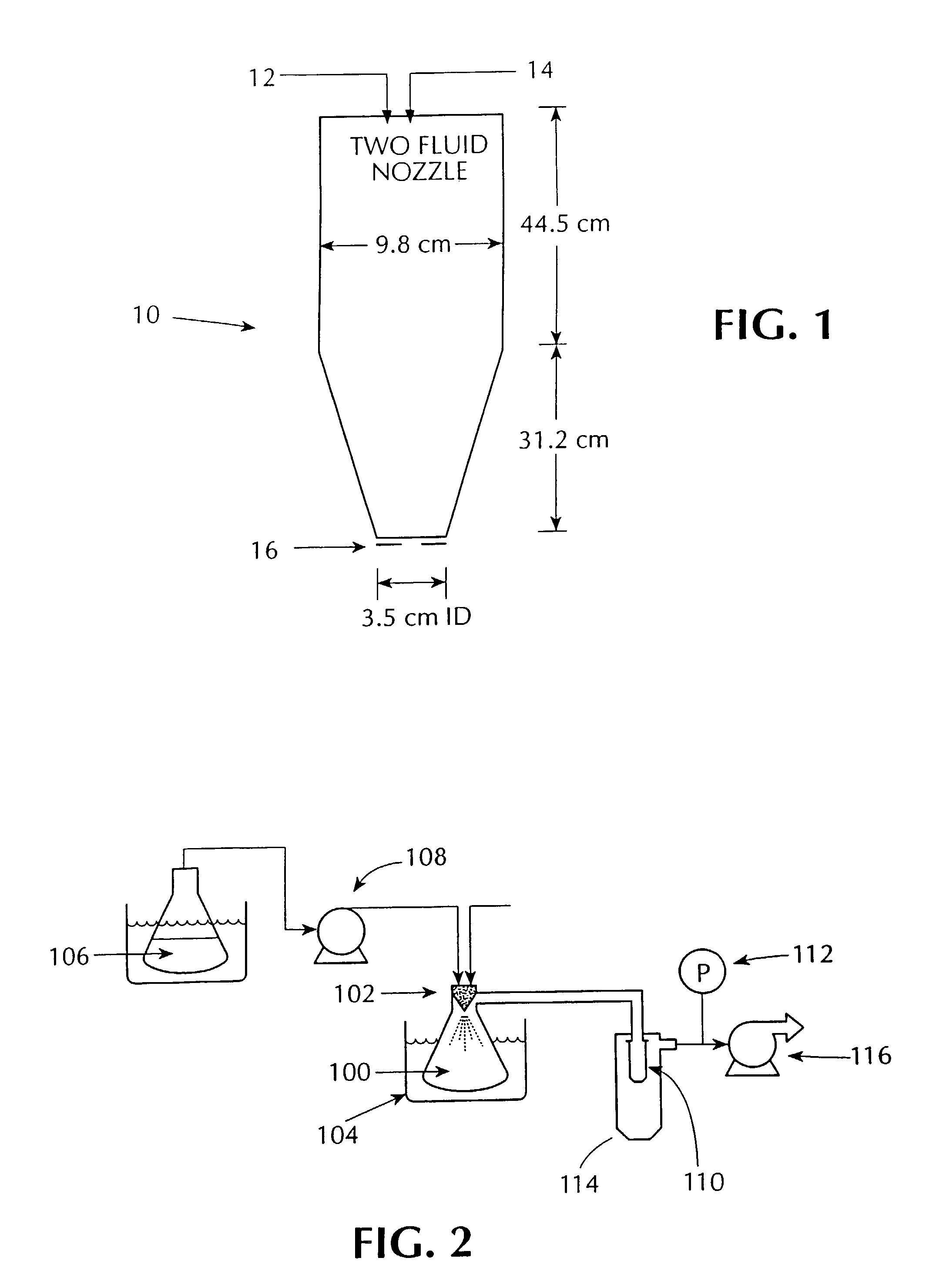
InactiveUS20060210640A1Improve drug bioavailabilityMass productionPowder deliveryCarbohydrate active ingredientsNanoparticleCompound (substance)
Owner:KERKHOF NICHOLAS J
Receptor mediated nanoscale copolymer assemblies for diagnostic imaging and therapeutic management of hyperlipidemia and infectious diseases
InactiveUS20050031544A1Effective dockingConducive to loadDispersion deliveryNanomedicineLipid formationActive agent
This invention relates to a method and system for improving diagnostic imaging and / or delivering therapeutically active agents for control of hyperlipidemia and infectious diseases (bacterial or viral), comprising nanoscale block copolymer assemblies carrying drug molecules in its core and receptor peptide in the corona surrounding the core, forming larger micelle or vesicle aggregates with target molecules such as LDL molecules and surface lipid of microorganisms.
Owner:NJEMANZE PHILIP CHIDI
Pharmaceutical compositions providing enhanced drug concentrations
InactiveUS8026286B2High dissolution rateImprove drug bioavailabilityAntibacterial agentsPowder deliverySolubilityPharmaceutical drug
A drug in a solubility-improved form is combined with a concentration-enhancing polymer in a sufficient amount so that the combination provides substantially enhanced drug concentration in a use environment relative to a control comprising the same amount of the same solubility-improved form of drug without the concentration-enhancing polymer.
Owner:BEND RES
Compositions for drug administration
InactiveUS20110257096A1Promote absorptionImprove bioavailabilityPeptide/protein ingredientsSomatostatinsChain lengthDrug administration
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms. In various aspects, the invention provides compositions and methods for oral delivery of peptides containing non-naturally occurring structures including D-amino acids and / or chain cyclization.
Owner:AEGIS THERAPEUTICS LLC
Alkylglycoside compositions for drug administration
ActiveUS8268791B2Improve absorption and bioavailabilityToxic effectsBiocideNervous disorderGlycosideDrug administration
Owner:AEGIS THERAPEUTICS LLC
Method of increasing drug oral bioavailability and compositions of less toxic orotate salts
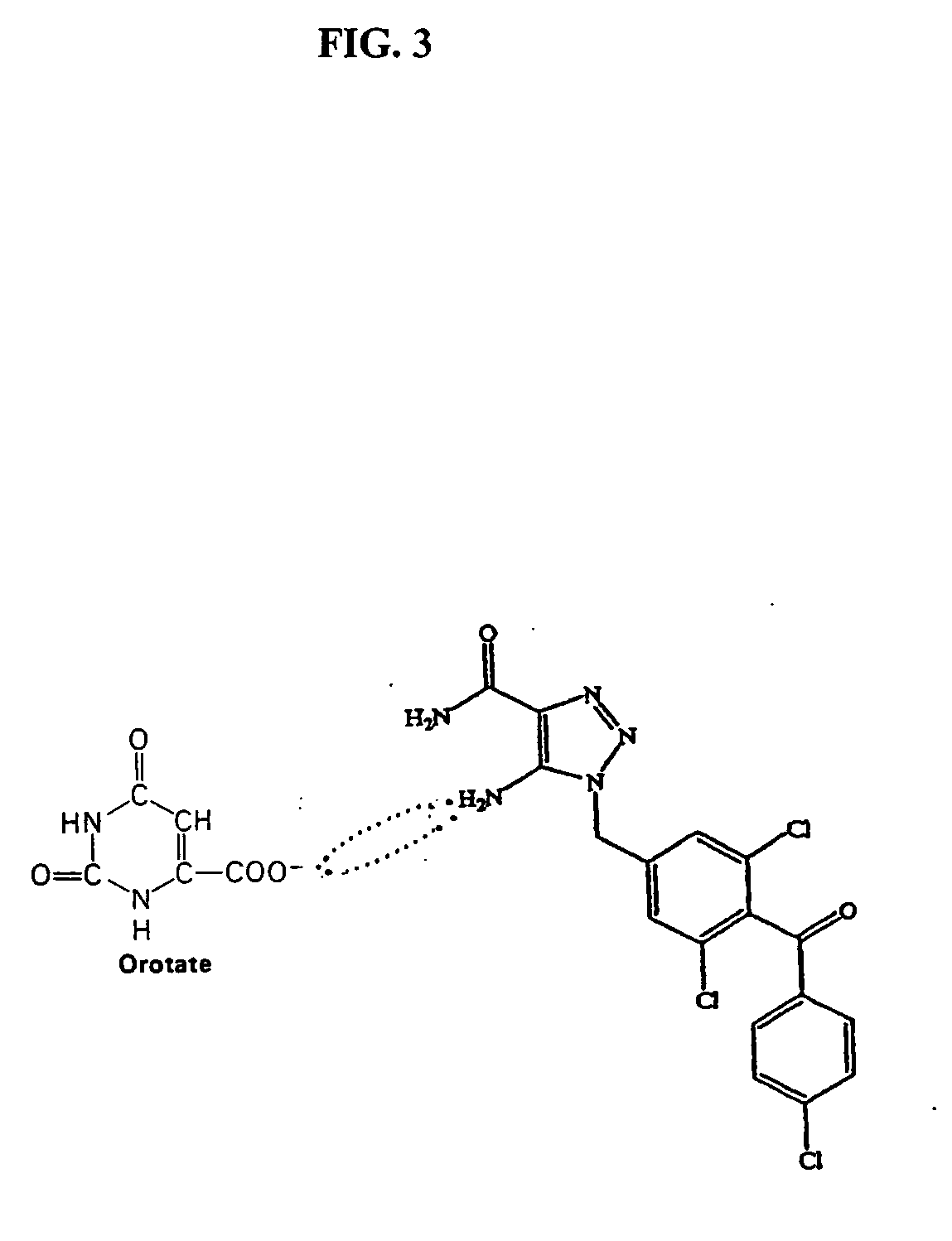
ActiveUS20060189640A1Improve bioavailabilityImprove drug bioavailabilitySalicyclic acid active ingredientsBiocideSide effectDrug interaction
The present invention relates generally to the method of increasing the oral bioavailability, reducing chemotherapy induced toxicity and side effects, and improving the effectiveness of pharmaceutical agents that are poorly absorbed from the gastrointestinal tract. Specifically, the invention relates to poorly absorbed pharmaceutical drugs and converting them to orotate salts. The orotate salts of the drugs can be dosed at lower doses to provide the efficacy benefits of a higher dose, while reducing the drugs' toxic effects at lower doses. Additionally, the orotate salts of pharmaceutical agents have better clearance and reduce the potential for drug-induced hepatic toxicity. Therefore, an especially useful formulation of the orotate salt of the pharmaceutical agent can provide rapid and consistent action using a lower dose while reducing drug interactions and side-effects.
Owner:SAVVIPHARM INC
Methods of treating drug-resistant cancers
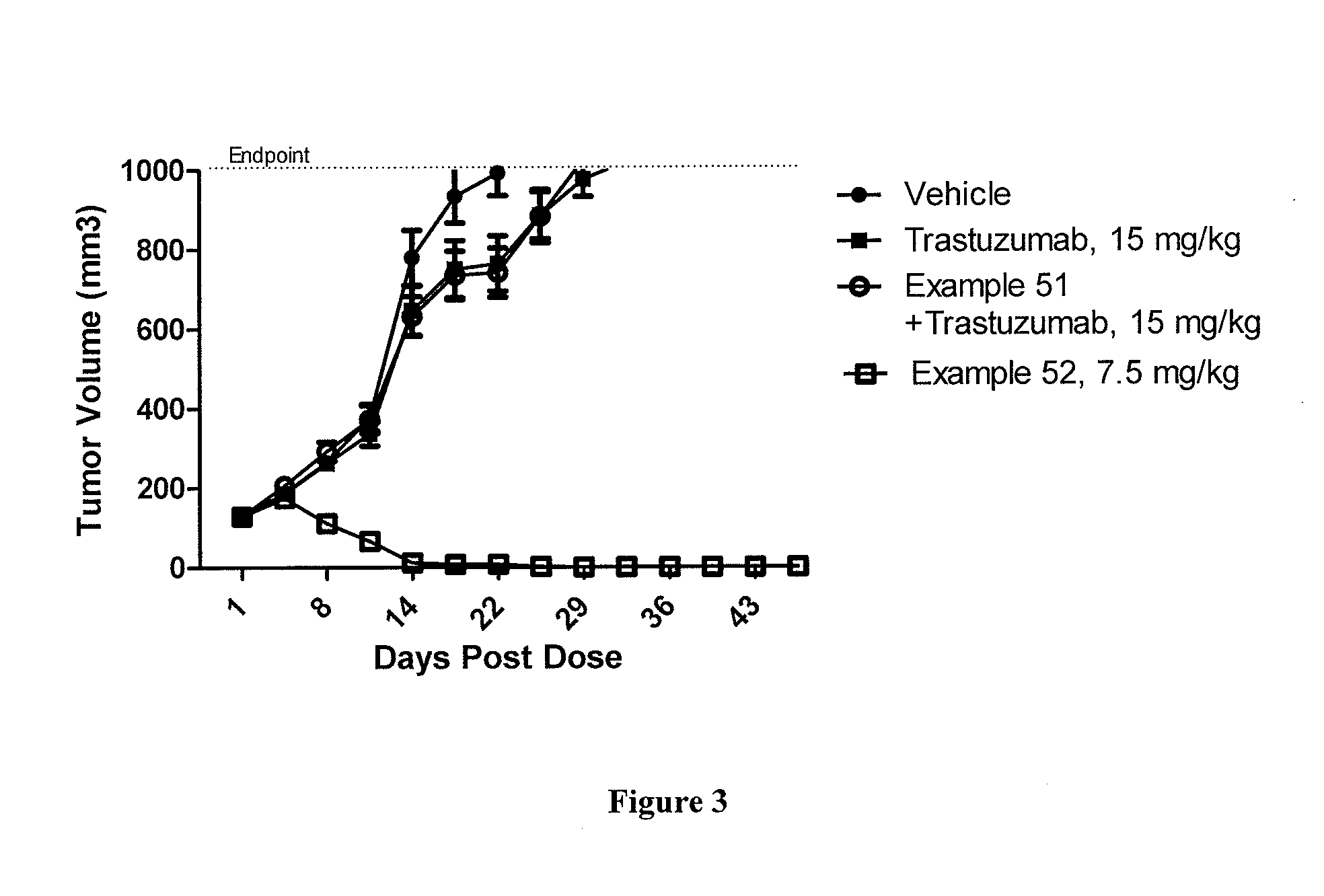
ActiveUS20100260786A1Improve drug bioavailabilityImprove bioavailabilityPeptidesAntibody ingredientsRefractoryOncology
Methods of treating a refractory or drug resistant cancer, cell proliferative disorder and tumor cells are provided.
Owner:SEAGEN INC
Pharmaceutical compositions comprising drug and concentration-enhancing polymers
InactiveUS7887840B2High dissolution rateImprove solubilityPowder deliveryOrganic active ingredientsSolubilityPolymer
A solubility-improved drug form is combined with a concentration-enhancing polymer in a sufficient amount so that the combination provides substantially enhanced drug concentration in a use environment relative to a control comprising the same amount of the same drug form without the concentration-enhancing polymer.
Owner:BEND RES
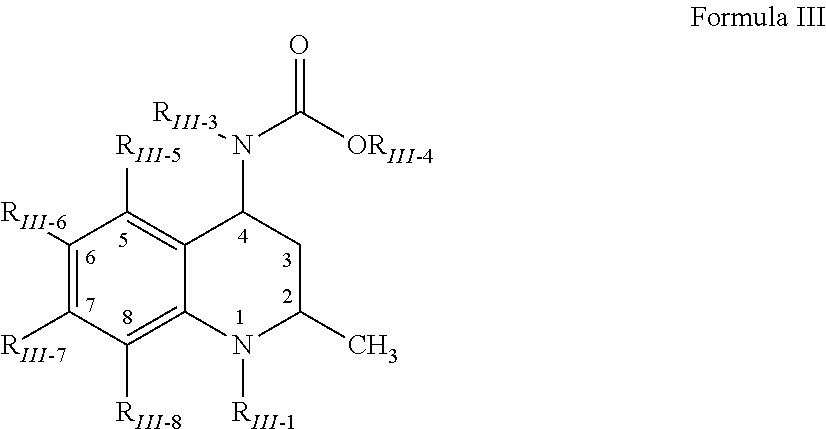
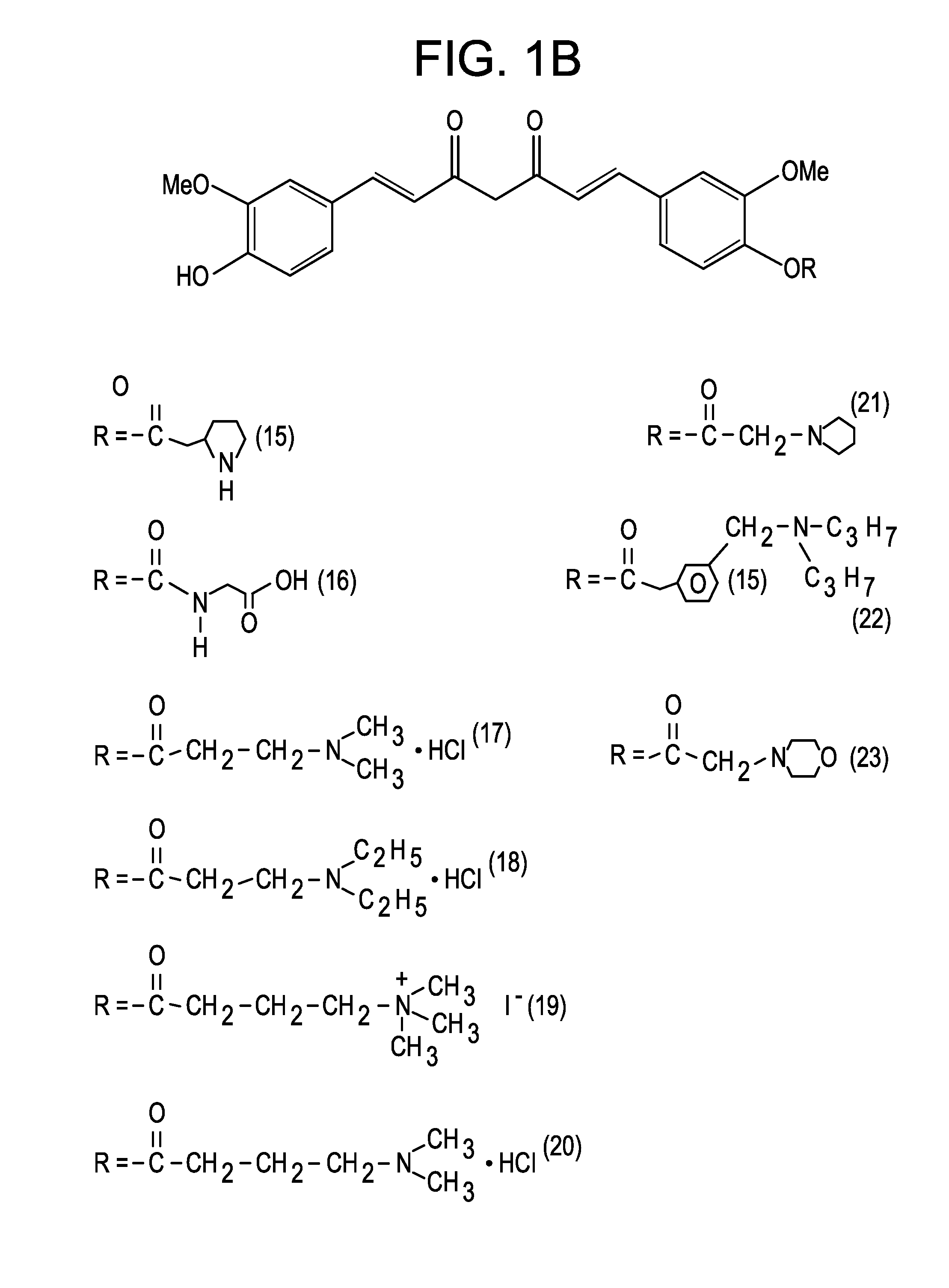
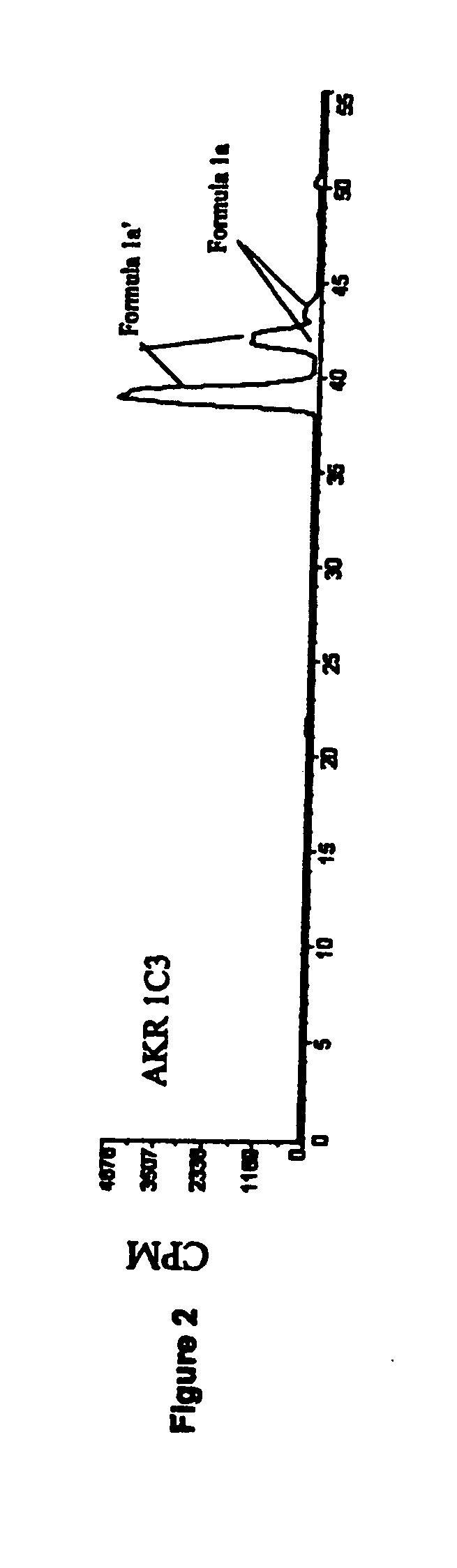
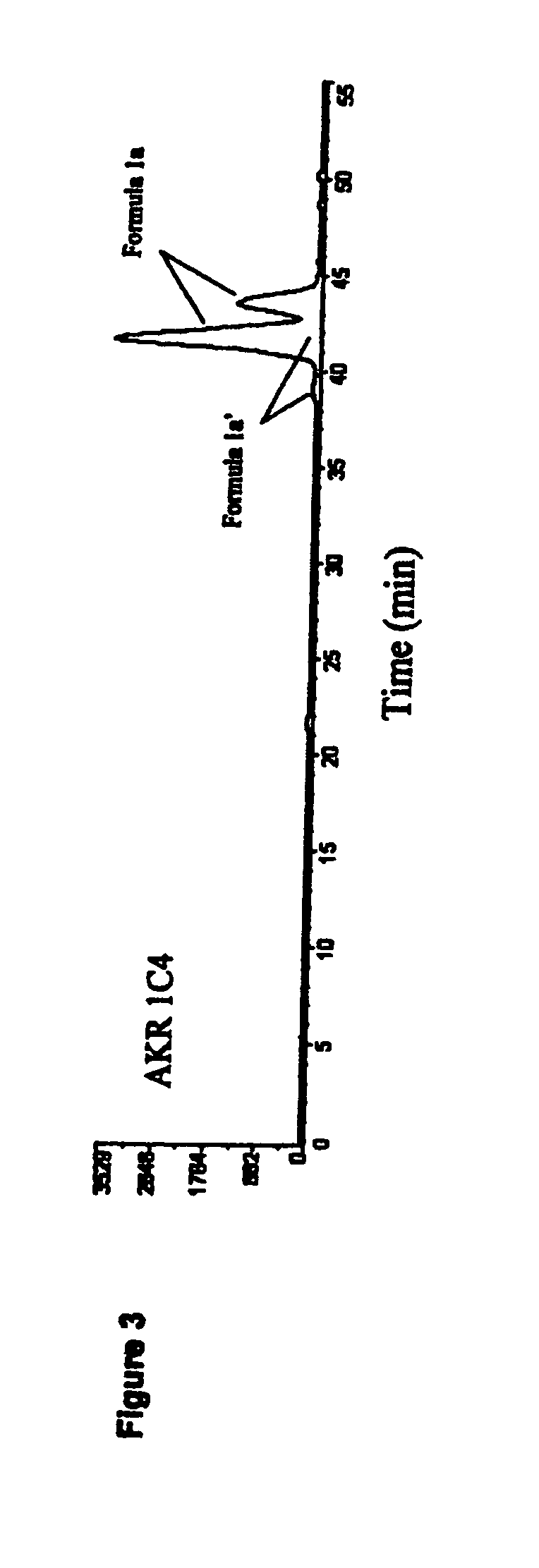
Medicaments and methods combining a HCV protease inhibitor and an AKR competitor
InactiveUS20070207949A1Improve bioavailabilityImprove drug bioavailabilityOrganic active ingredientsBiocideDiseaseProteinase activity
Disclosed are medicaments, pharmaceutical compositions, pharmaceutical kits, and methods based on combinations of a hepatitis C virus (HCV) protease inhibitor and an aldo-keto reductase (AKR) competitor, for concurrent or consecutive administration in treating, preventing, or ameliorating one or more symptoms of HCV, treating disorders associated with HCV, or inhibiting cathepsin activity in a subject.
Owner:SCHERING CORP
Intranasally administering curcumin prodrugs to the brain to treat alzheimer's disease
InactiveUS20080076821A1Reduce dose requireHighly lipophilicBiocidePharmaceutical delivery mechanismDiseaseCurcumin
Owner:DEPUY SYNTHES PROD INC
Medicaments and methods combining a HCV protease inhibitor and an AKR competitor
InactiveUS20070232527A1Improve efficacyExtended durationBiocideDipeptide ingredientsDiseaseCathepsin
Disclosed are medicaments, pharmaceutical compositions, pharmaceutical kits, and methods based on combinations of a hepatitis C virus (HCV) protease inhibitor and an aldo-keto reductase (AKR) competitor, for concurrent or consecutive administration in treating, preventing, or ameliorating one or more symptoms of HCV, treating disorders associated with HCV, or inhibiting cathepsin activity in a subject.
Owner:SCHERING CORP
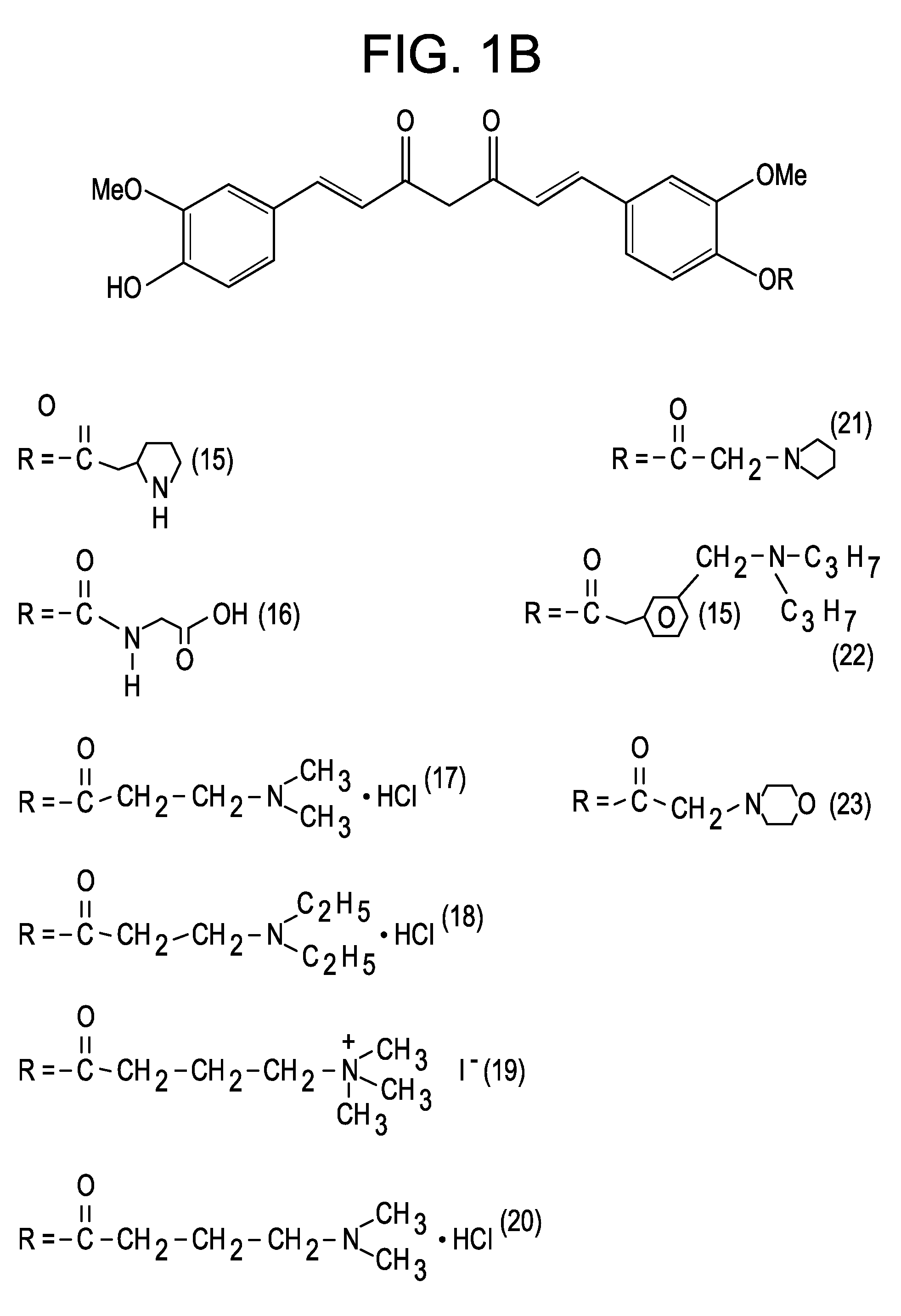
Curcumin-Resveratrol hybrid molecule
InactiveUS7745670B2Reduced plasma concentrationAvoiding extensive hepatic first-pass metabolismNervous disorderOrganic chemistryDiseaseBiology
Novel molecules based upon hybridization of curcumin and hydroxystilbenes, such as resveratrol. It is believed that these novel molecules will have special application in treating Alzheimer's Disease.
Owner:DEPUY SYNTHES PROD INC
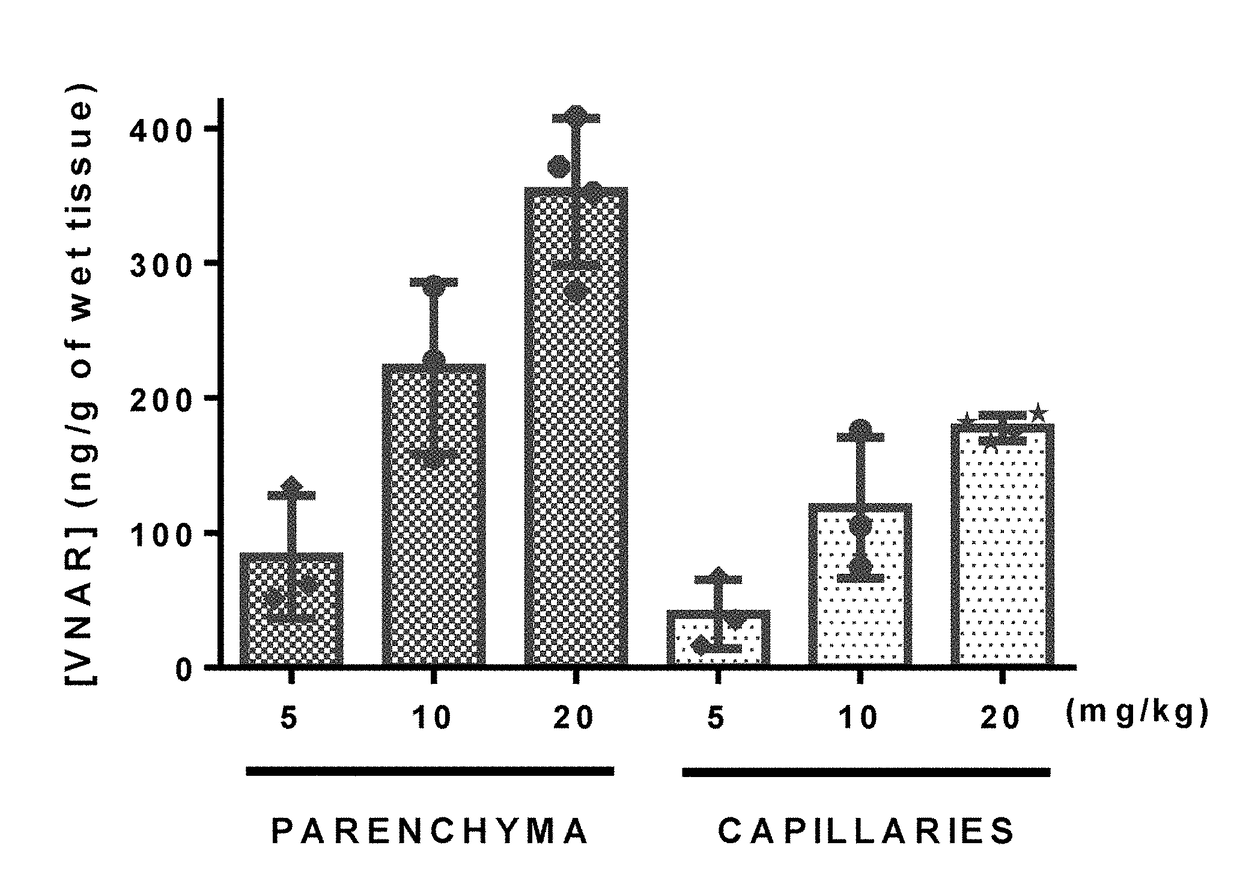
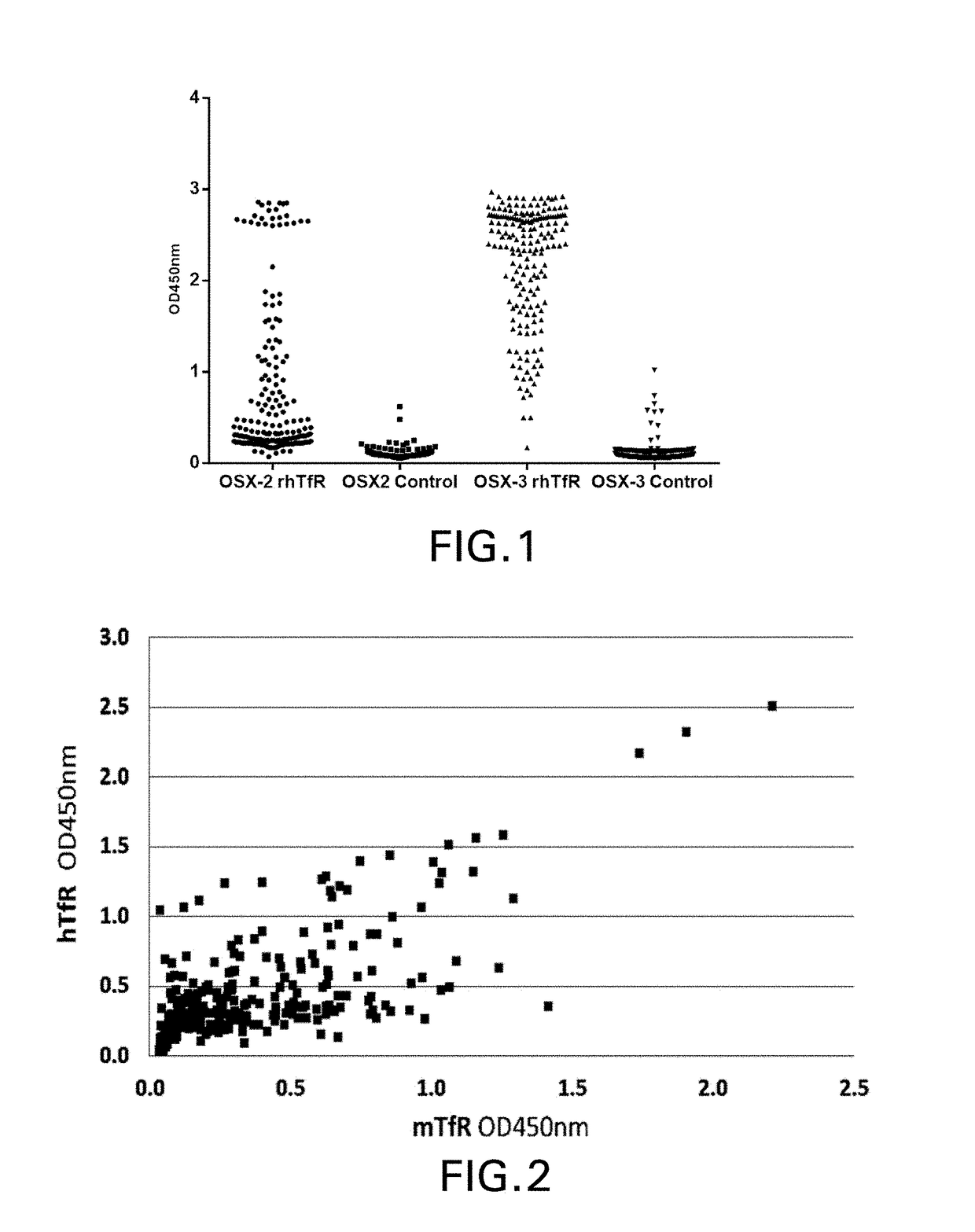
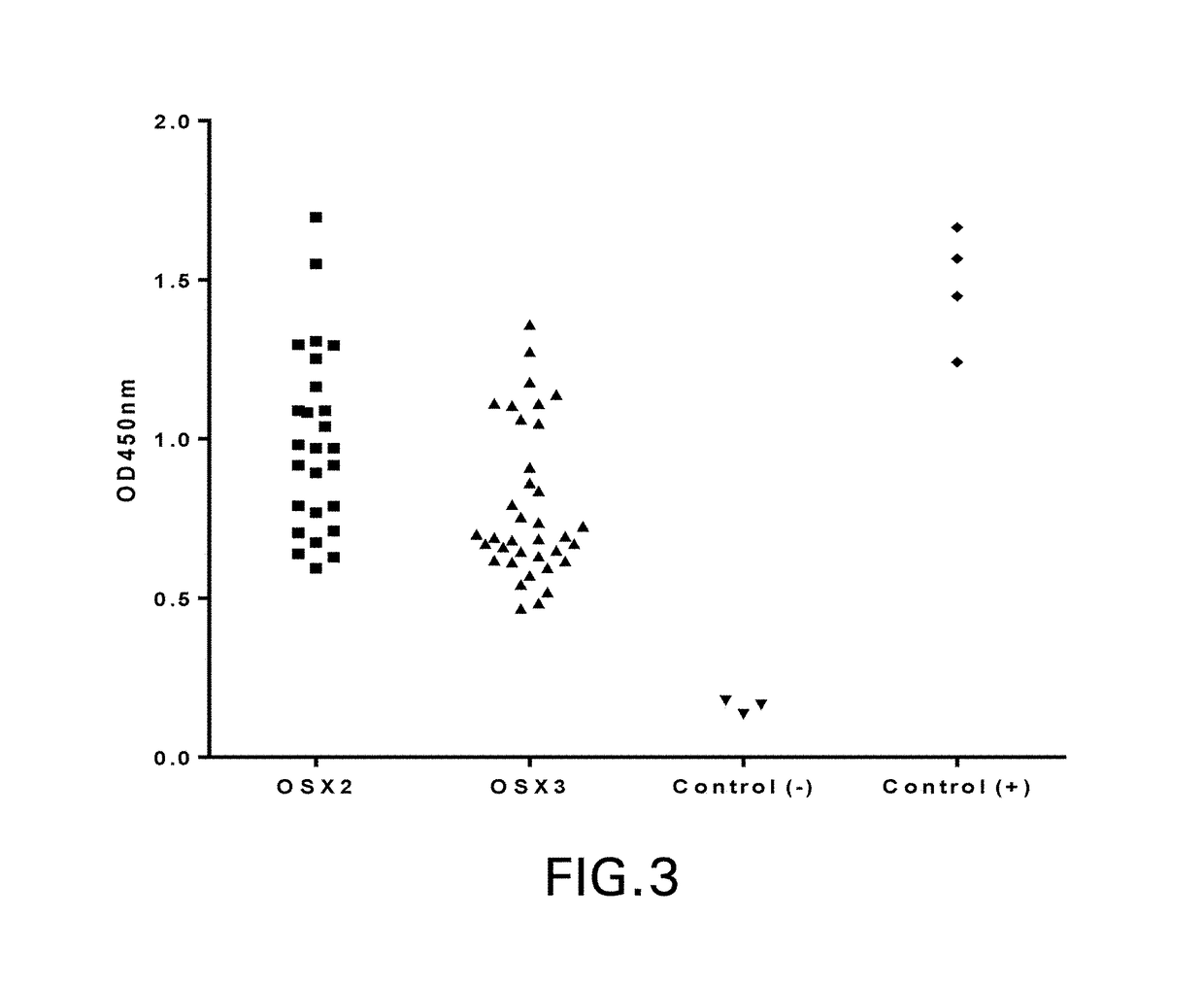
TfR SELECTIVE BINDING COMPOUNDS AND RELATED METHODS
ActiveUS20170348416A1Improve drug bioavailabilityNervous disorderHybrid immunoglobulinsChemistryAntibody
The present invention relates to peptides that bind with high specificity and which functionally interact with the transferrin receptor (“TfR”) and which may be used in making molecular vehicles that carry biomolecules across membranes, including, e.g., across the blood brain barrier or the gastrointestinal tract. TfR specific binding moieties may also be used alone or as components in specific molecules that target the transferrin / transferrin receptor transport system. The invention relates more specifically to VNAR single chain antibodies derived from nurse shark that bind to TfR, compounds and compositions comprising a TfR specific VNAR binding moiety, methods for preparing them, diagnostic and therapeutic methods of use in vitro or in vivo, e.g., to diagnose, treat and / or prevent a pathological condition, disorder or disease in which it is beneficial to deliver a heterologous biomolecule across the blood brain barrier by association with a TfR specific VNAR binding moiety. Other uses for TfR specific VNAR binding moieties of the invention include, e.g., regulating the interaction of iron-charged transferrin with TfR (receptor cycling or cell surface presentation), such as may be therapeutic in treatment of certain cancer cells and tumors of various tissue types.
Owner:OSSIANIX
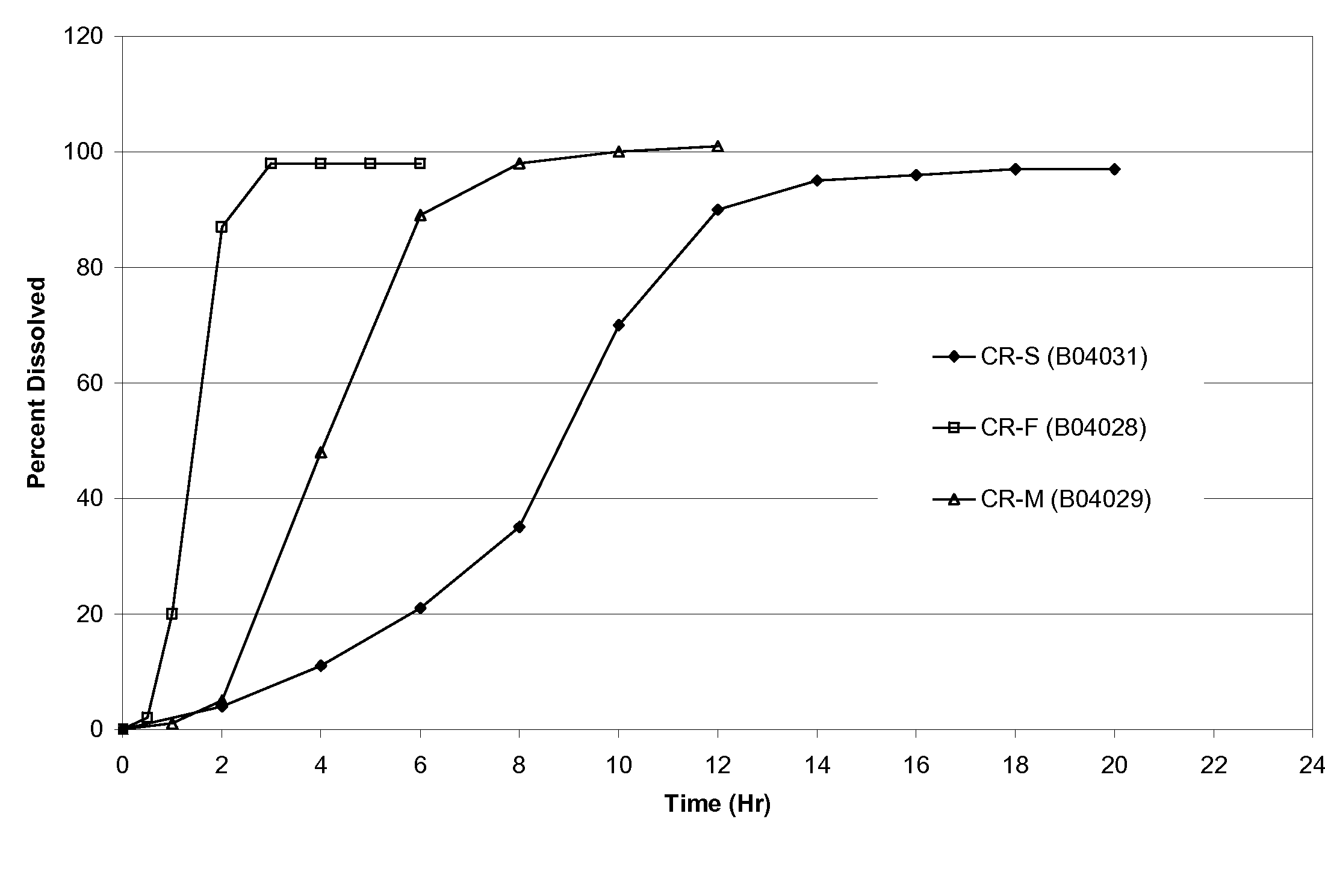
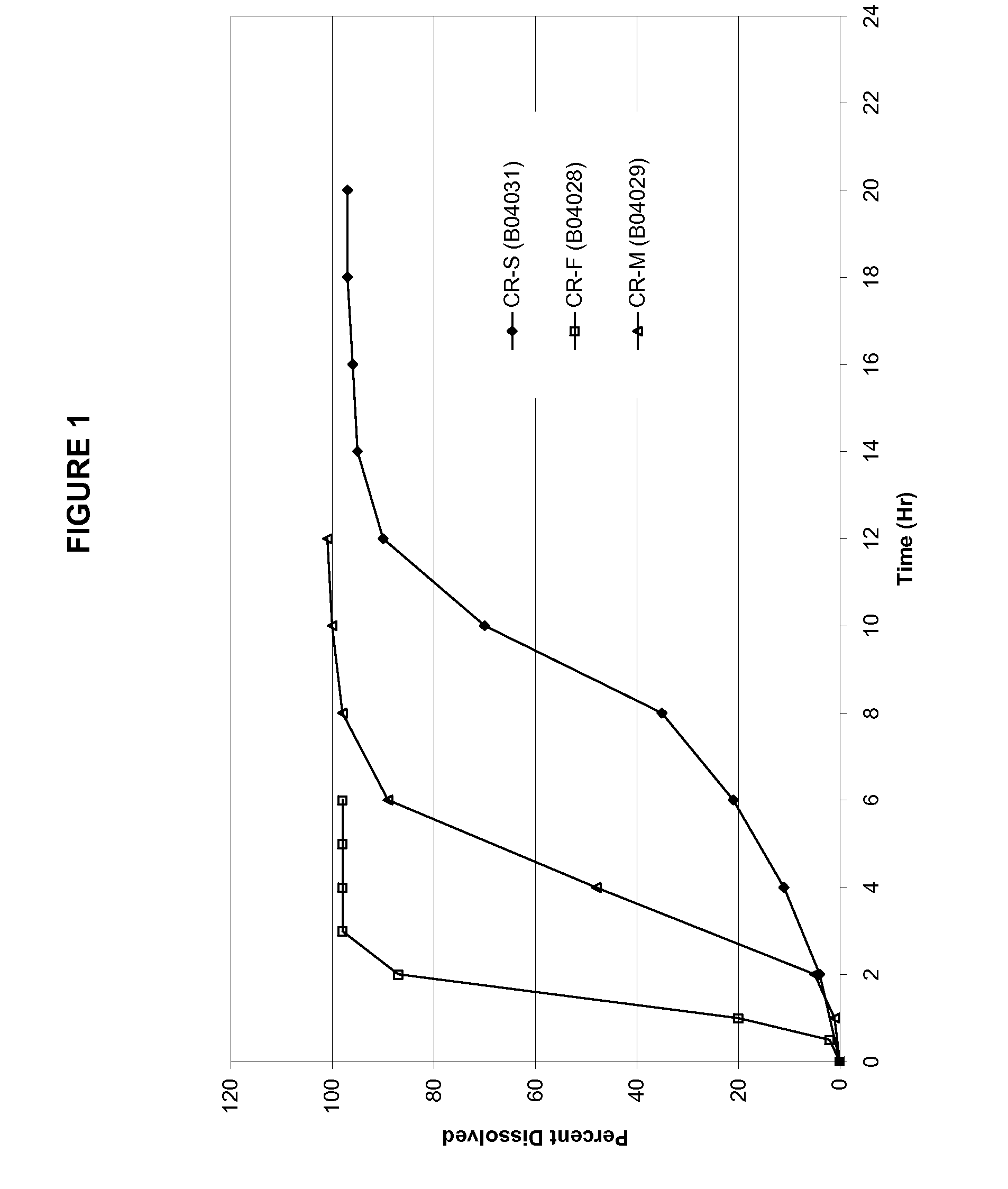
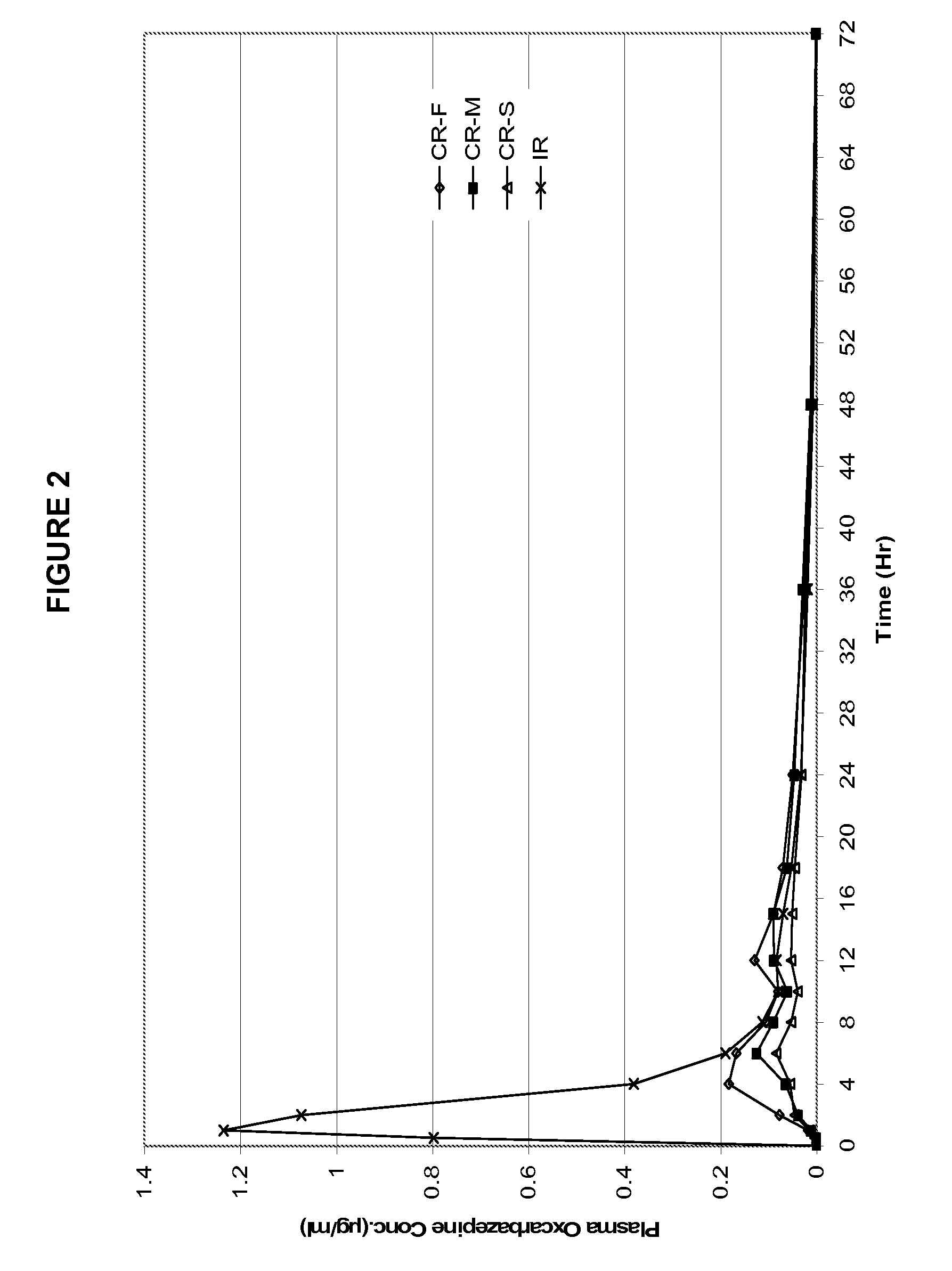
Modified-release preparations containing oxcarbazepine and derivatives thereof
ActiveUS20070254033A1Reduce volatilityBetter therapeutic profilePowder deliveryNervous disorderSolubilityOxcarbazepine
Controlled-release preparations of oxcarbazepine and derivatives thereof for once-a-day administration are disclosed. The inventive compositions comprise solubility-and / or release enhancing agents to provide tailored drug release profiles, preferably sigmoidal release profiles. Methods of treatment comprising the inventive compositions are also disclosed.
Owner:SUPERNUS PHARM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com