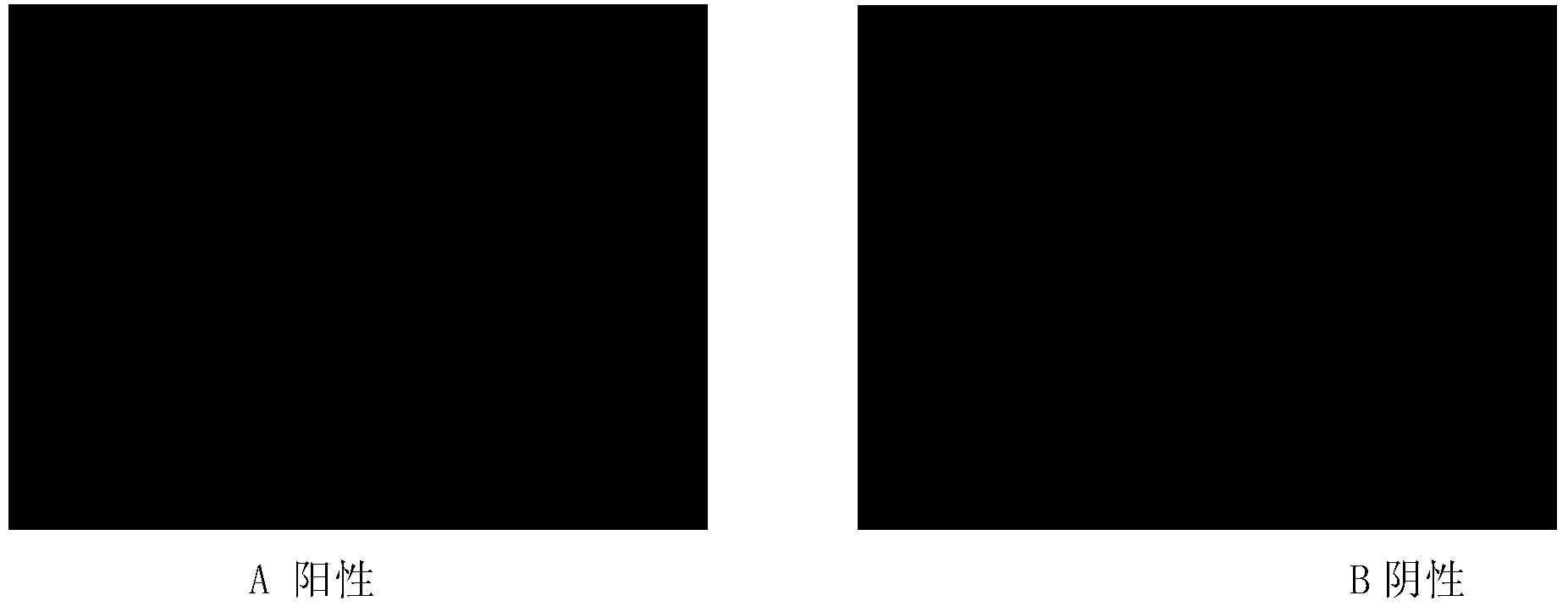Construction method and application of DNA (Deoxyribonucleic Acid) vaccine for avian leukosis virus subgroup J
A subgroup, microbial strain technology, applied in the field of veterinary nucleic acid vaccines, can solve the problems of low level and slow antibody production of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
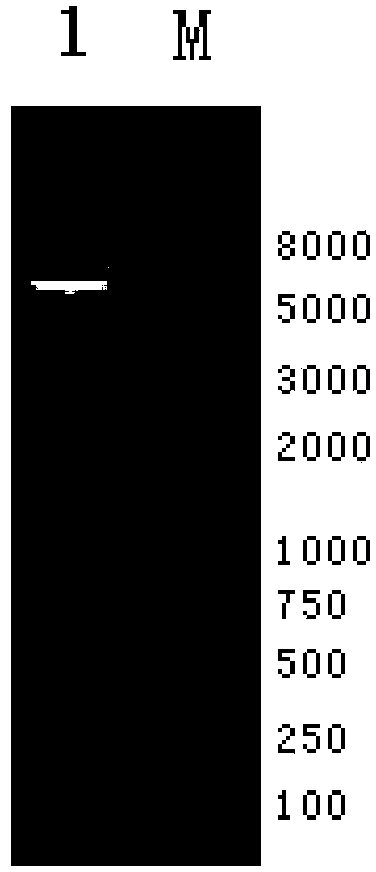
[0031] Example 1: Preparation of J subgroup avian leukemia DNA vaccine pcDNA3.1-Fc-env
[0032] (1) Cloning and identification of chicken IgG-Fc gene
[0033] According to the chicken IgG heavy chain sequence (X07174.1) published by NCBI, the primers were designed, the EcoR I restriction site and ATG start codon were added to the upstream primer, the terminator was removed from the downstream primer, and the chicken was obtained by RT-PCR method. IgG-Fc gene.
[0034] 1. Extraction of total RNA from chicken spleen
[0035] 1) Weigh an appropriate amount of spleen tissue into a 1.5ml centrifuge tube, add 500ul of Buffer LY and 10ul of β-mercaptoethanol, and shake vigorously on a shaker for 2 minutes. Then let stand for 2 minutes to sink the tissue fragments to the bottom of the tube.
[0036] 2) Pour the supernatant into a DNA gap column, 13000rpm for two minutes, discard the DNA column (filter the DNA in the solution), and keep the liquid in the collection tube below.
[0...
Embodiment 2
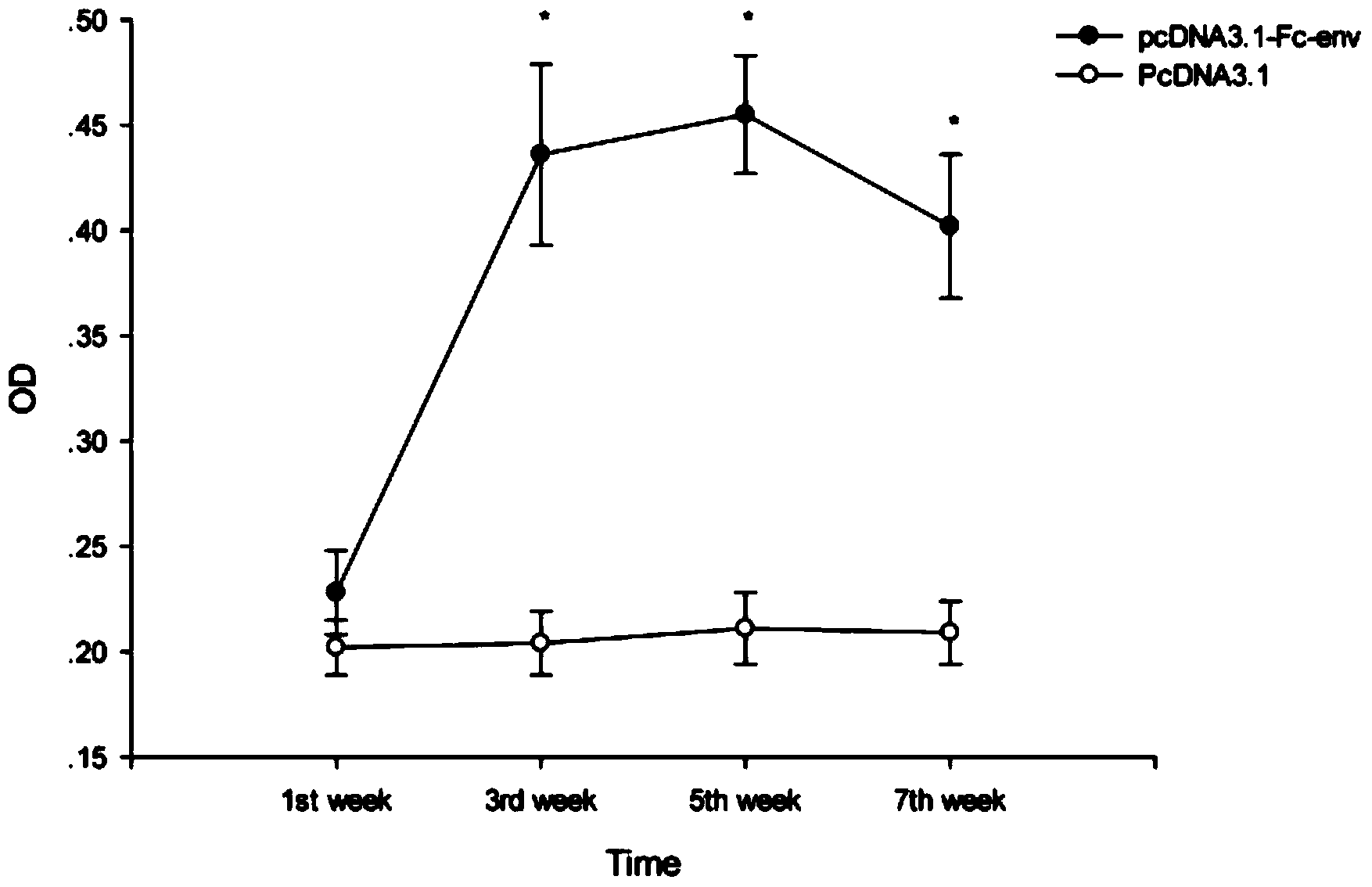
[0113] Example 2 Evaluation of the immune effect of DNA vaccine pcDNA3.1-Fc-env.
[0114] 1. Mass extraction of recombinant plasmids
[0115] (1) Take the recombinant ALVFc E. coli constructed in Example 1, inoculate 150 ml of LB medium, add the overnight cultured recombinant E. coli bacterial solution into a centrifuge tube, centrifuge at 10,000 rpm for 2 to 3 minutes to collect bacteria, and discard all the supernatant as much as possible.
[0116] (2) Add 12ml DNA purification buffer Buffer P1 (included in the commercial DNA purification kit) to the centrifuge tube with the bacterial cell pellets according to the instructions of the commercial DNA purification kit, and mix thoroughly with a pipette or a vortex shaker , suspended bacterial pellets.
[0117] (3) Add 12ml of Buffer P2 (included in a commercial DNA purification kit) to the centrifuge tube, gently invert and mix for 6-8 times to fully lyse the cells, and leave at room temperature for 3-5 minutes. At this point...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com