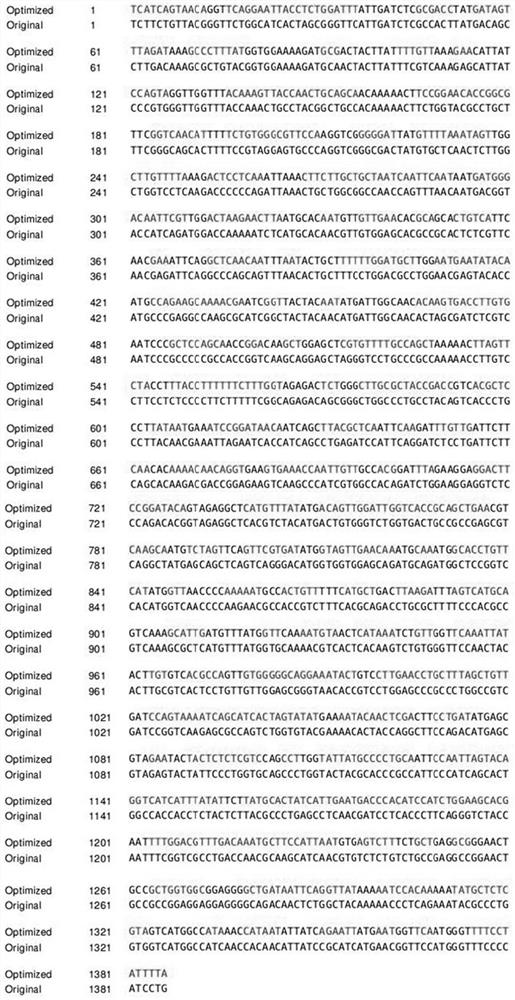
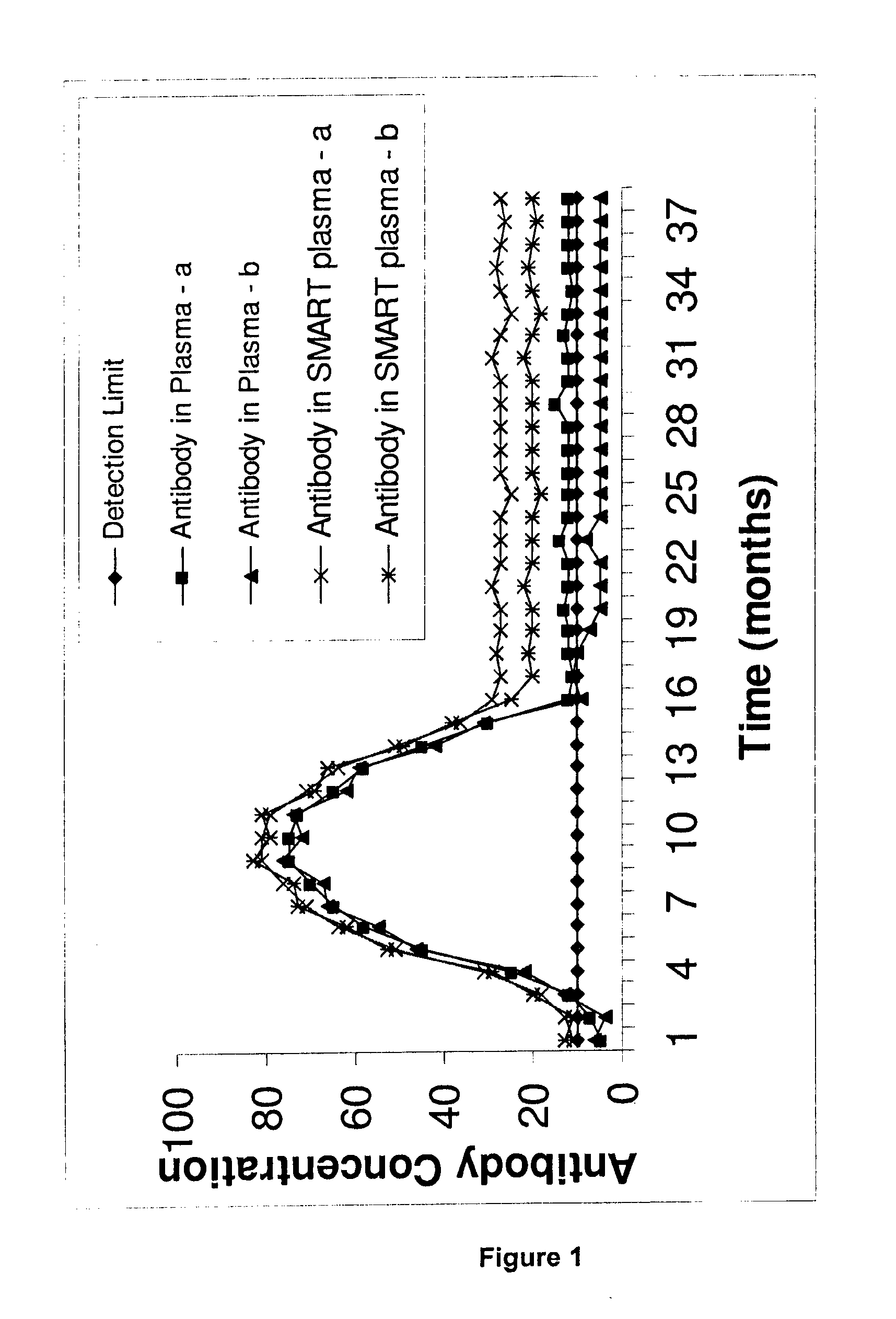
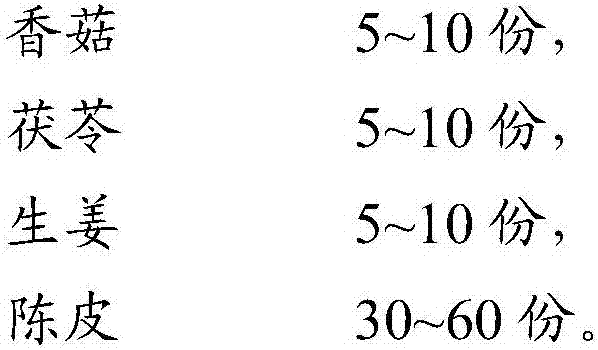Patents
Literature
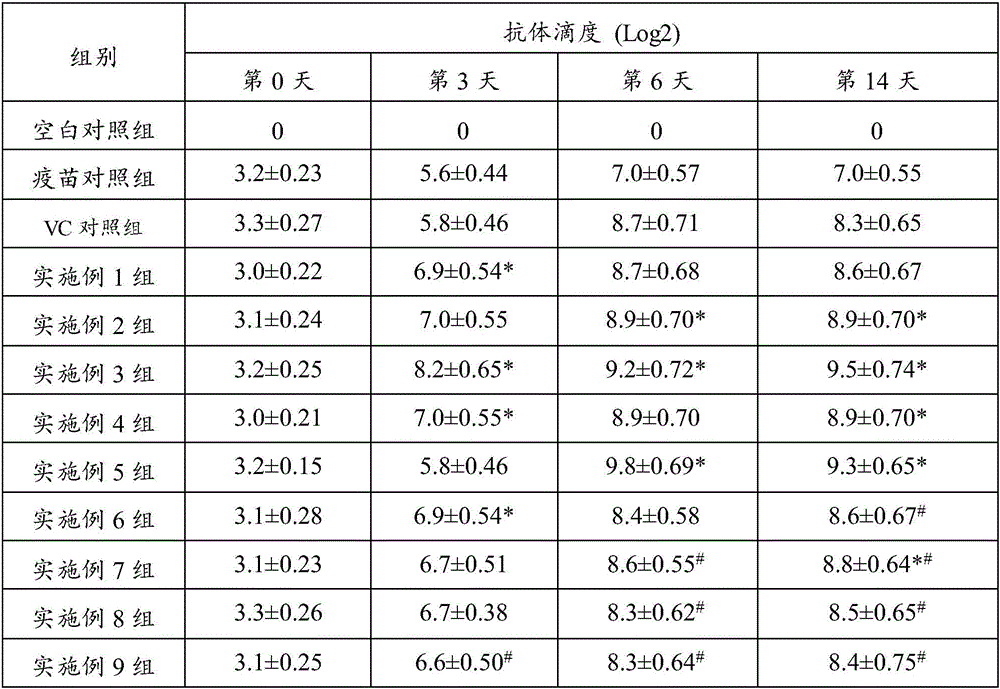
36 results about "Specific antibody level" patented technology
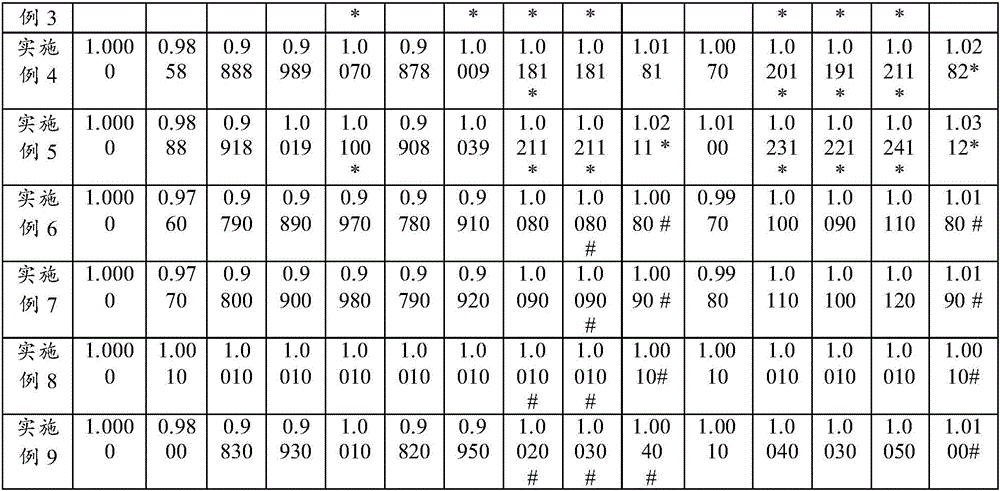
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
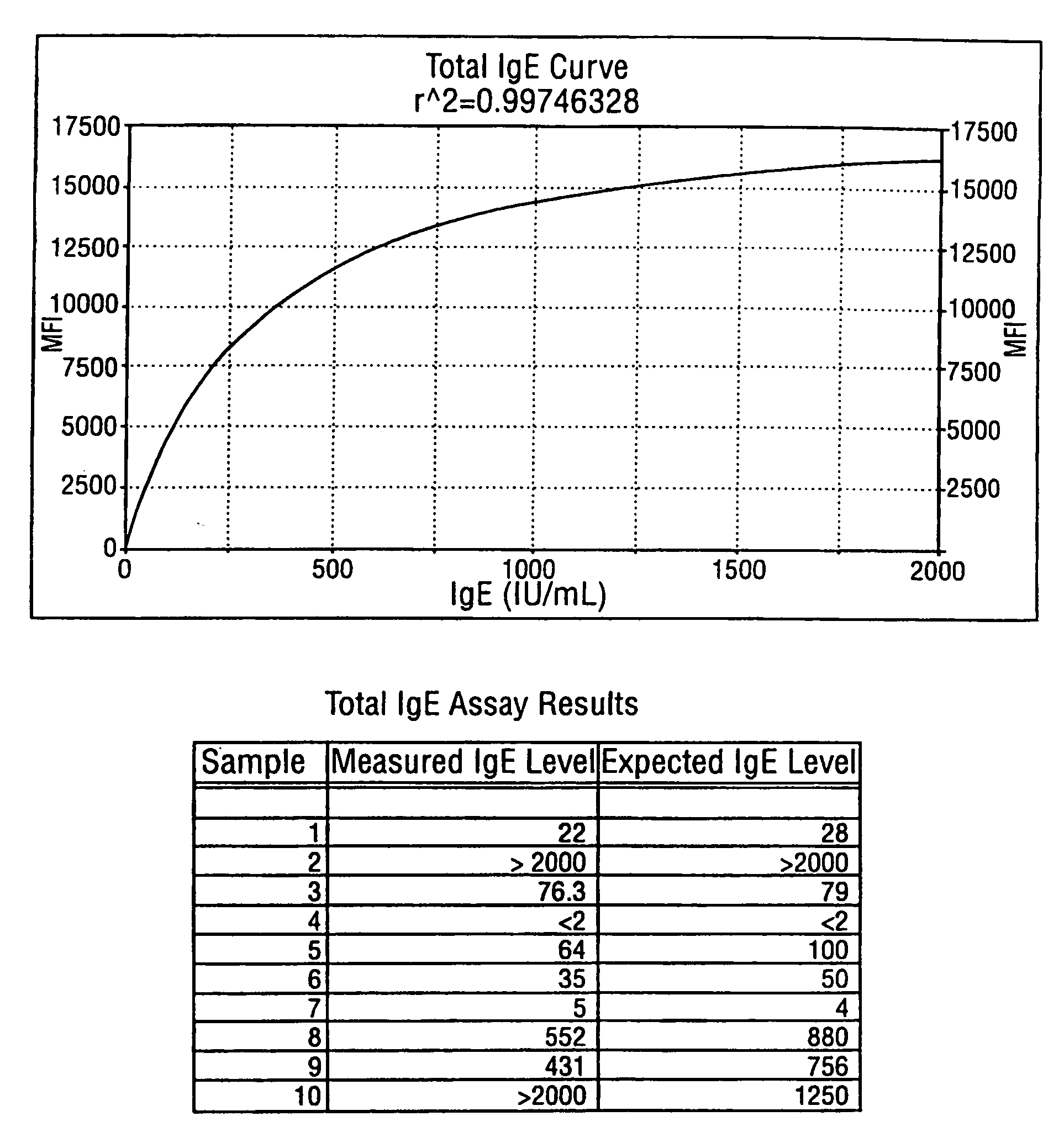
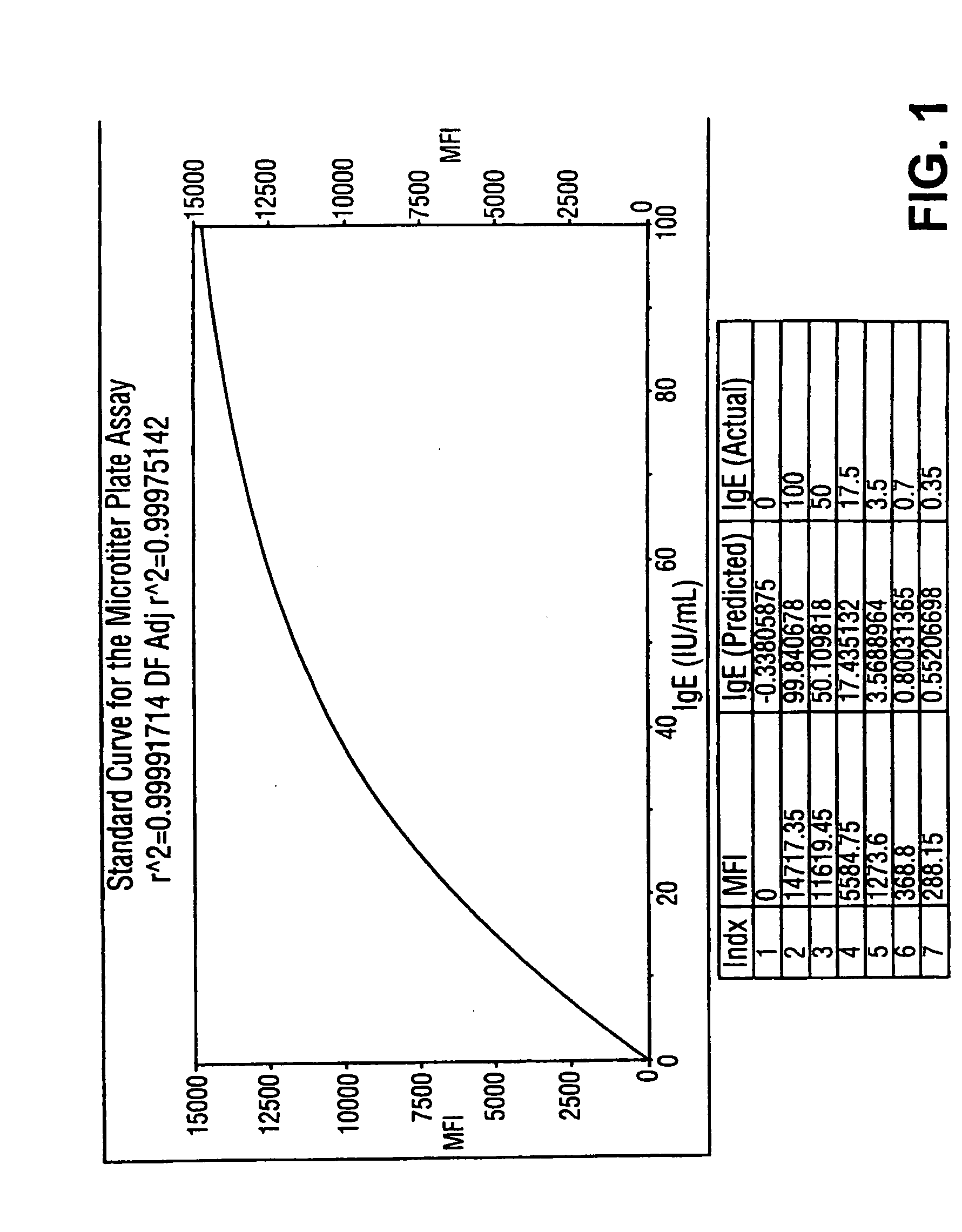
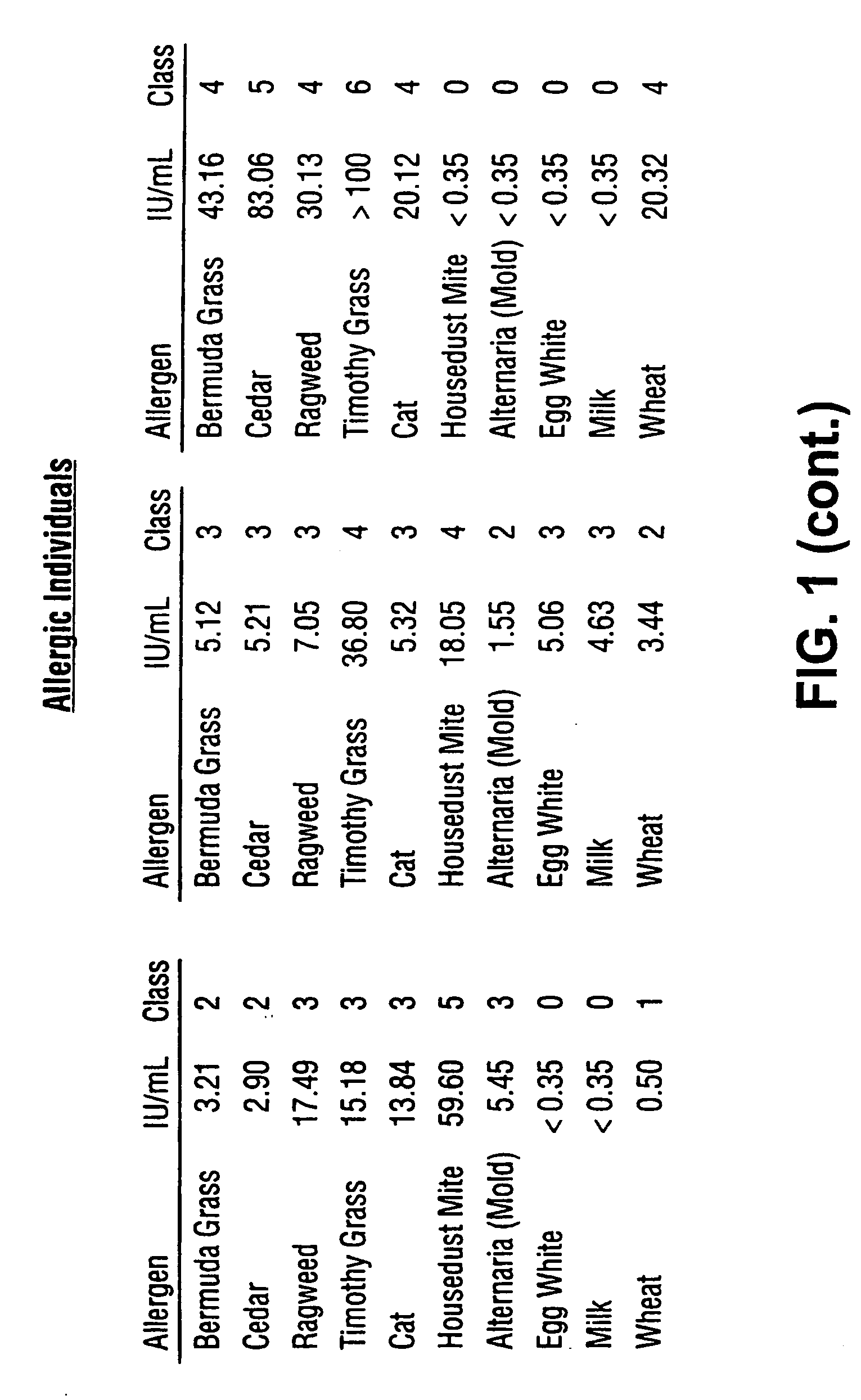
Homogeneous immunoassays for multiple allergens
InactiveUS7491553B2Chemiluminescene/bioluminescenceBiological testingQuantitative determinationMicroparticle
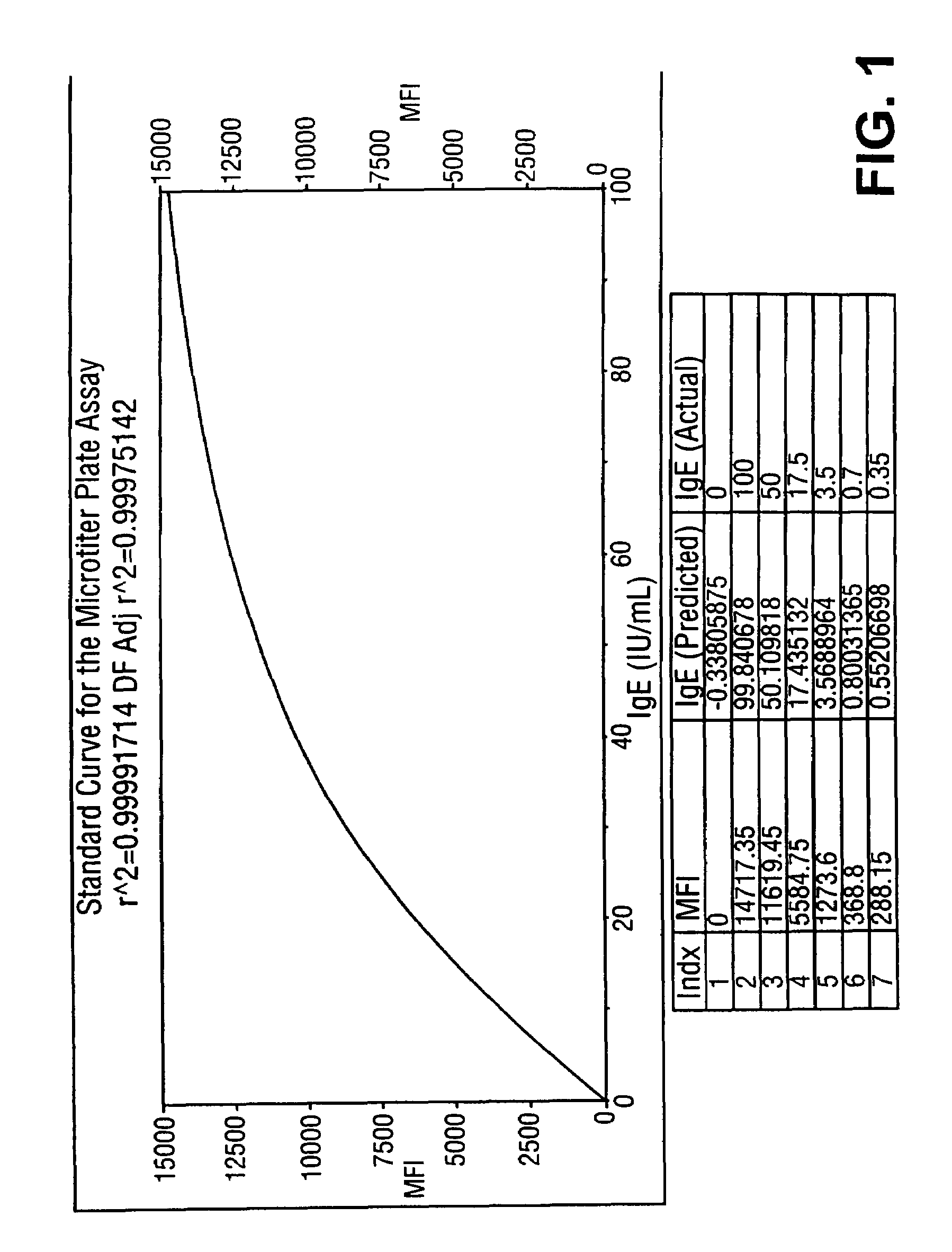
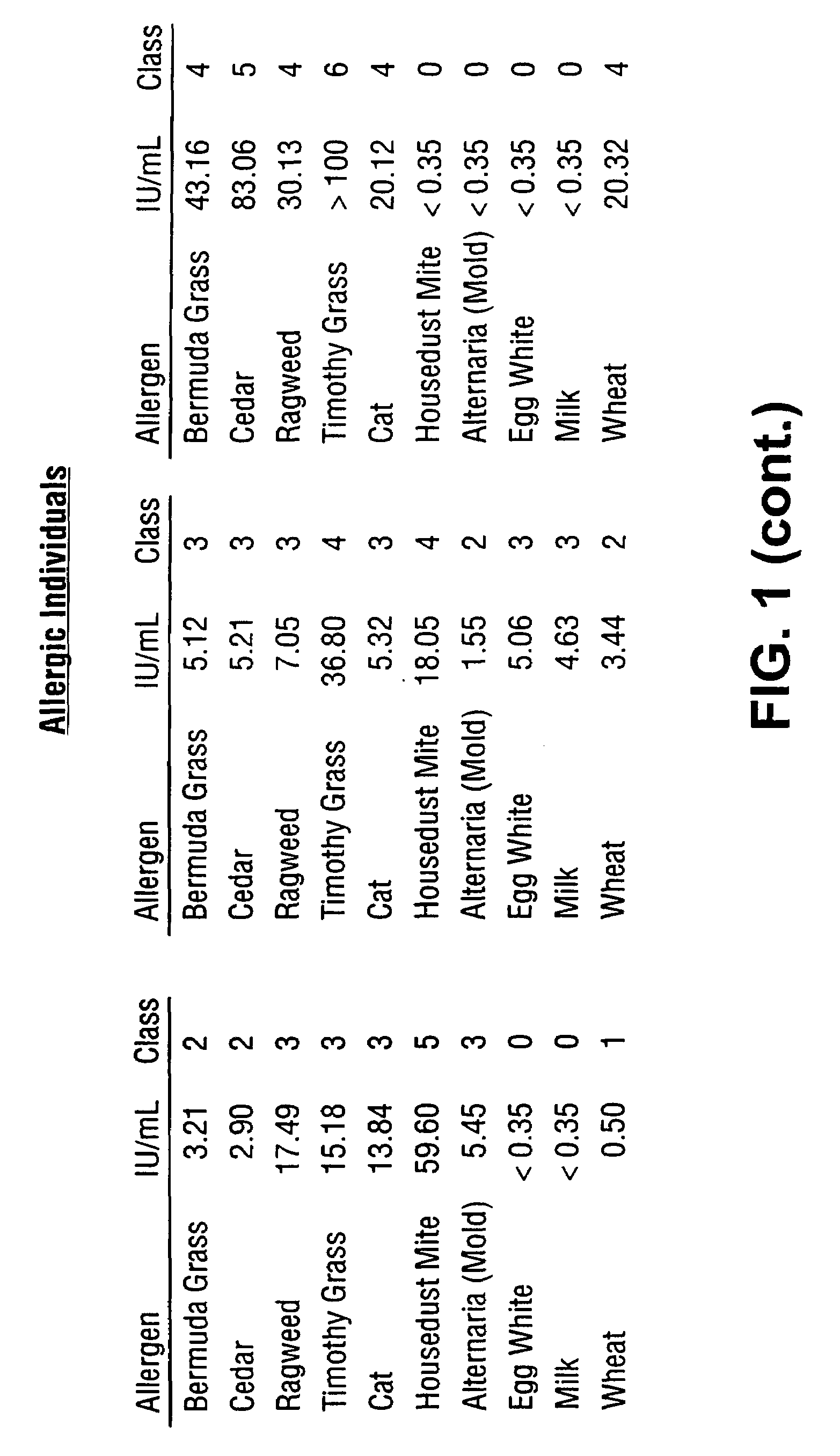
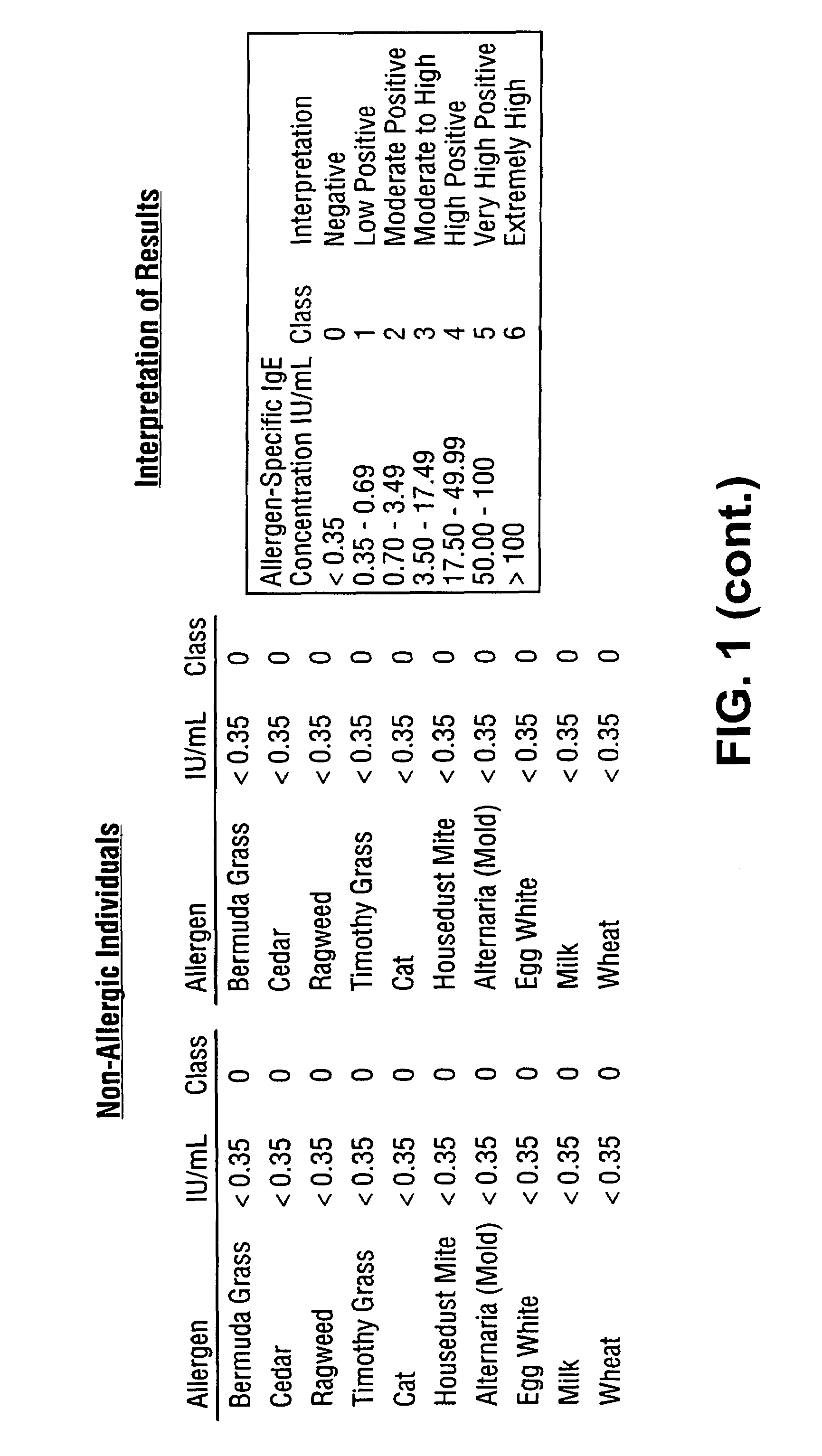
A homogeneous immunoassay method and system for quantitative determination of total immunoglobulin E and specific antibody levels to a plurality of allergens, in which a relatively small sampling of blood is required. The method utilizes relatively small microparticles in aqueous suspension. The immunoassay procedure is an immunometric sandwich procedure preferably utilizing biotin-streptavidin signal amplification techniques and R-phycoerytherin fluorescent labels.
Owner:IMMUNETECH
Subunit vaccine for bovine fusobacterium necrophorum and preparation method of subunit vaccine
ActiveCN110613842AImprove the level ofGood immune protectionAntibacterial agentsBacterial antigen ingredientsTGE VACCINEBiology
The invention relates to a subunit vaccine for bovine fusobacterium necrophorum. The subunit vaccine comprises three proteins: an outer membrane protein 43kDa OMP of bovine fusobacterium necrophorum,a leukotoxin truncated protein PL-4 of fusobacterium necrophorum and a hemolysin truncated protein H2 of fusobacterium necrophorum, wherein the dosage ratio of the three proteins is 1:1:1. The invention also relates to a preparation method of the subunit vaccine for bovine fusobacterium necrophorum. The subunit vaccine for bovine fusobacterium necrophorum comprises the three proteins: the outer membrane protein 43kDa OMP of bovine fusobacterium necrophorum, the leukotoxin truncated protein PL-4 of fusobacterium necrophorum and the hemolysin truncated protein H2 of fusobacterium necrophorum that are used in combination to produce a high level of specific antibody after immunity, thereby provoking cellular immunity and humoral immunity well in mice and effectively protecting the mice from attack of the fusobacterium necrophorum. The subunit vaccine has a better immune protection effect comparable to that of a whole-bacteria inactivated vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Construction method and application of DNA (Deoxyribonucleic Acid) vaccine for avian leukosis virus subgroup J
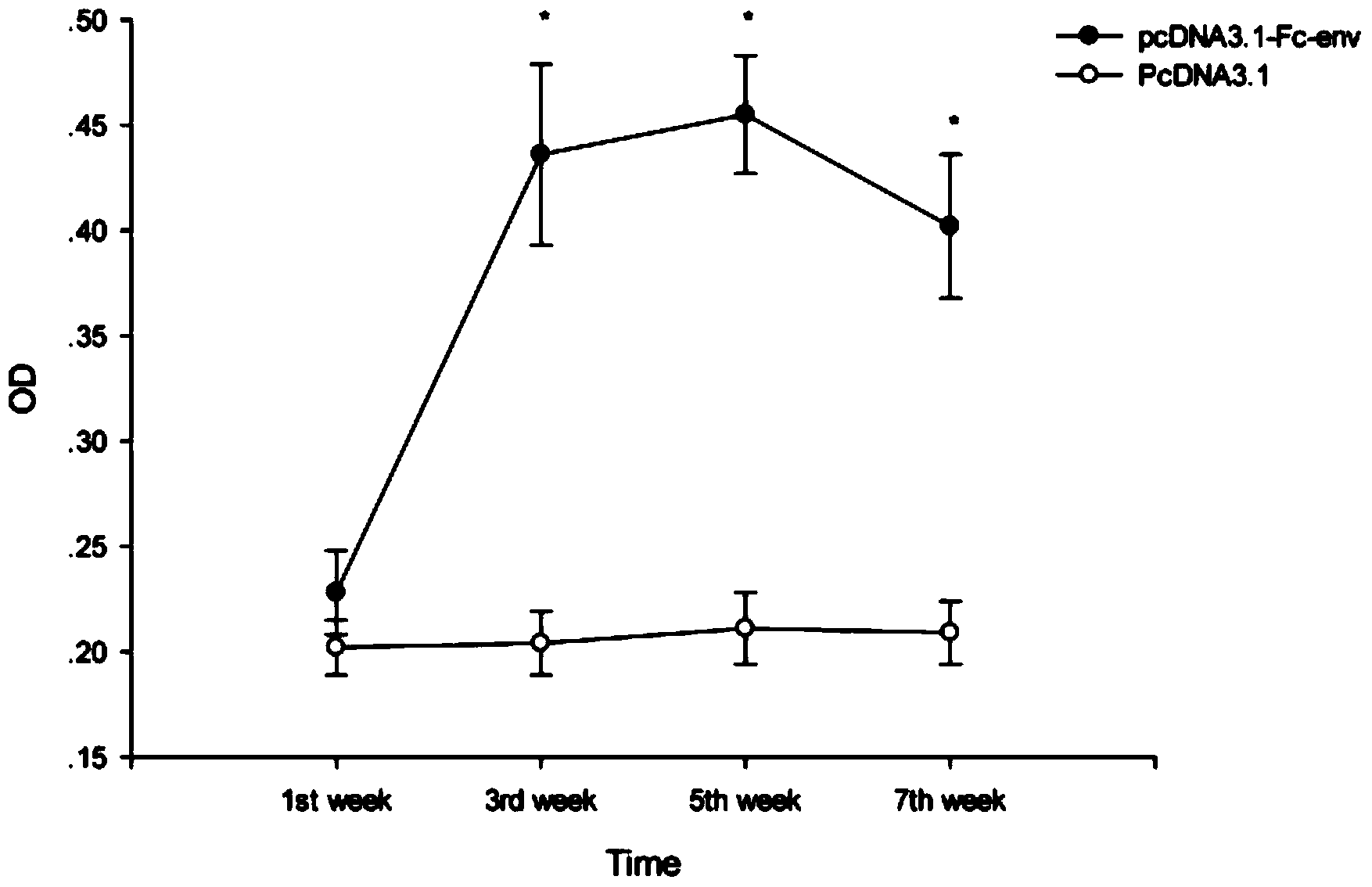
The invention relates to a construction method and application of a DNA (Deoxyribonucleic Acid) vaccine for avian leukosis virus subgroup J. A recombinant eukaryotic expression plasmid pcDNA3.1-Fc-env of an Fc fragment gene for expressing chicken immunoglobulin G and an envelope protein (env protein) gene of the avian leukosis virus subgroup J is constructed; transient transfection and indirect immunofluorescence assay prove that the pcDNA3.1-Fc-env can be accurately expressed in a 293T cell; a great number of plasmids are extracted, purified and quantified to 1mg / ml, then, the recombinant plasmids are used for immunizing mice, each mouse is immunized three times, 100mu g of recombinant plasmids are used in each immunization, and one immunization is carried out every two weeks; the level of an ALV-J env protein-specific antibody in serum is detected to show that the pcDNA3.1-Fc-env has the effect of preventing the avian leukosis virus subgroup J.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
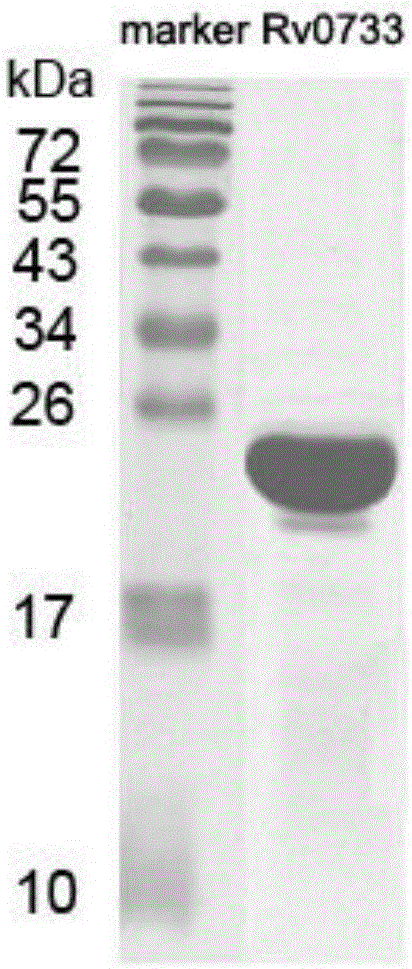
Reagent for detecting specific immune response of mycobacterium tuberculosis and use thereof
ActiveCN103804499AEasy to operateHigh detection sensitivityHybrid peptidesMaterial analysisT cellInterferon alpha
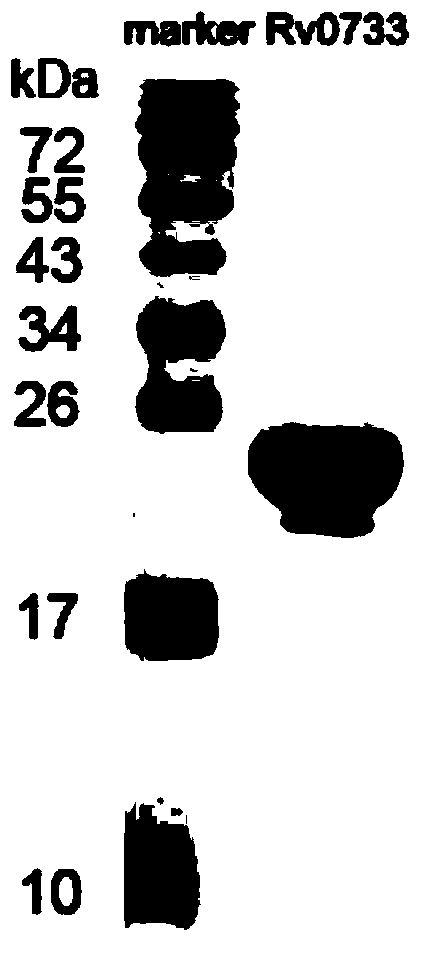
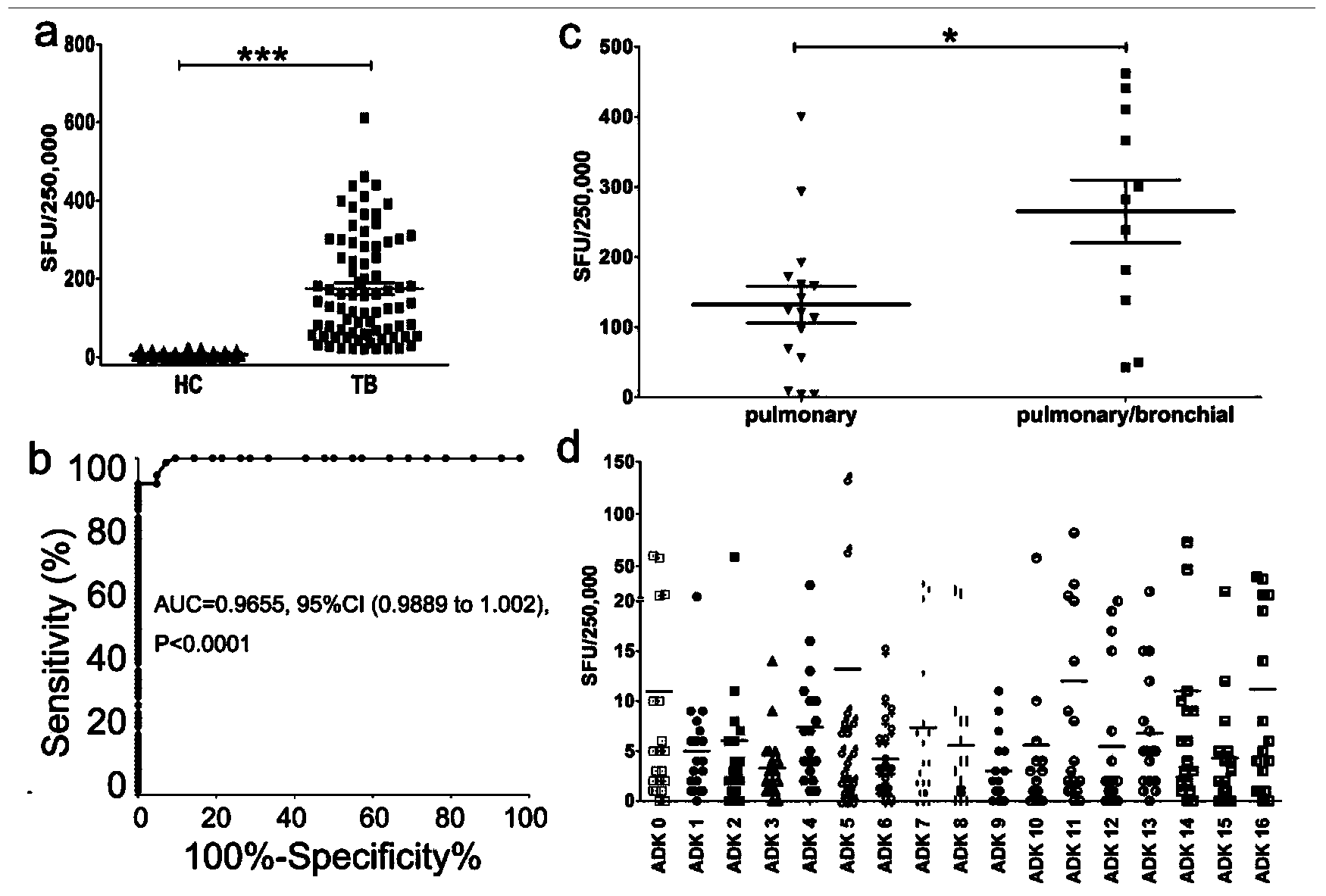
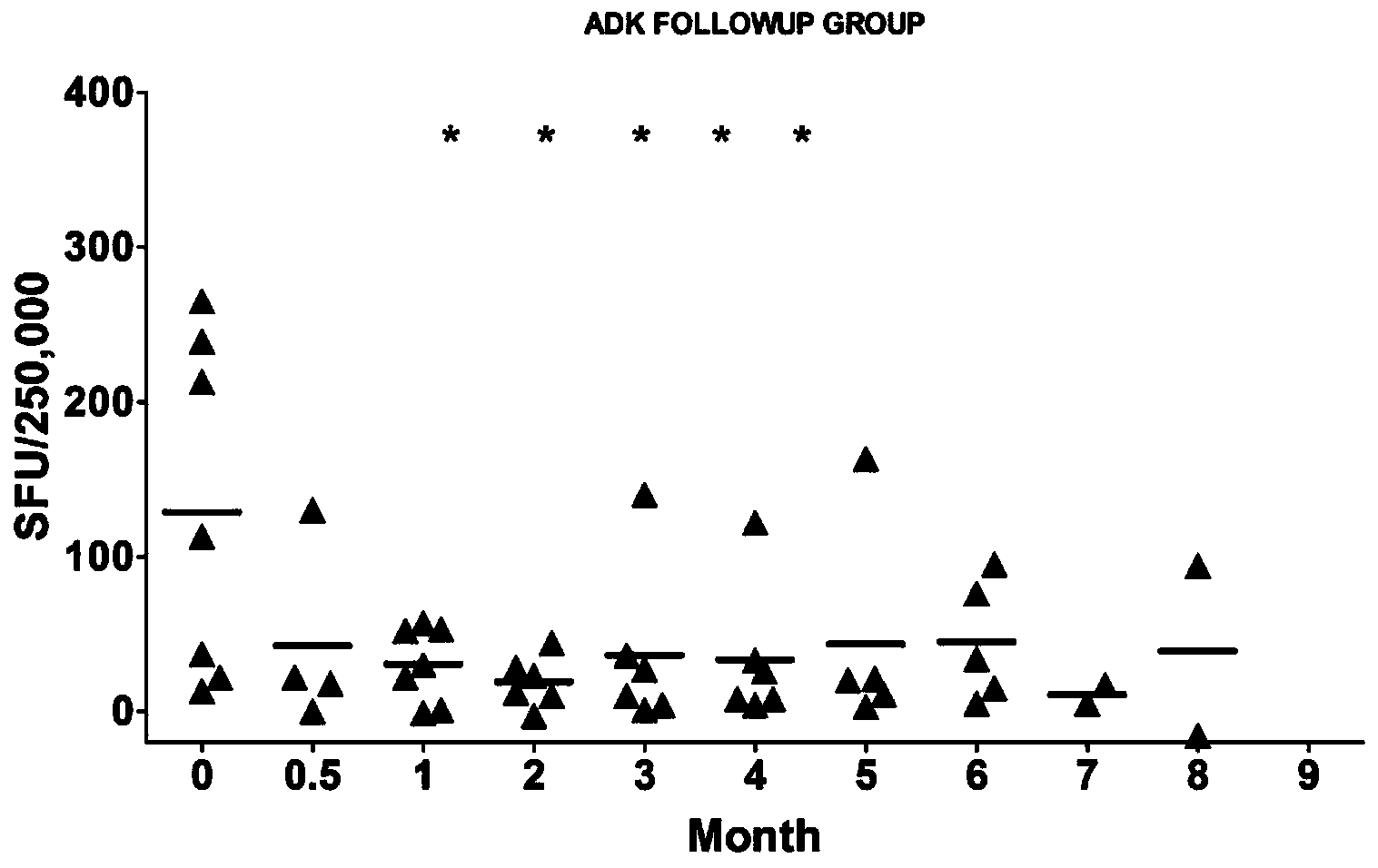
The invention discloses a reagent for detecting specific immune response of mycobacterium tuberculosis and use thereof. The invention discloses an immune positive reagent Rv0733 of mycobacterium tuberculosis by a gamma interferon release assay and a serum enzyme-linked immune method. The reagent comprises antigen or polypeptide and analogues thereof for detecting the specific cellular immunity of a tuberculosis sufferer and humoral immune response level. After the mice are immunized by using the reagent disclosed by the invention, the mice can generate specific cell factors such as gamma interferon and the like, and an antibody. T cells of tuberculosis patients or normal people are stimulated by using the reagent in vitro, the quantity of gamma interferon release cells is detected, or the content of gamma interferon in supernatant is cultivated, the tuberculosis patients can be distinguished from the normal people, the detection sensitivity and specificity of the tuberculosis sufferer can be improved by detecting the specific antibody level of resisting the reagent in the peripheral serum, and the clinical prognosis conditions of the tuberculosis patients can be disclosed by utilizing an Rv0733 specific immune response contrast experiment in the process of tracking treatment of the tuberculosis sufferer.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
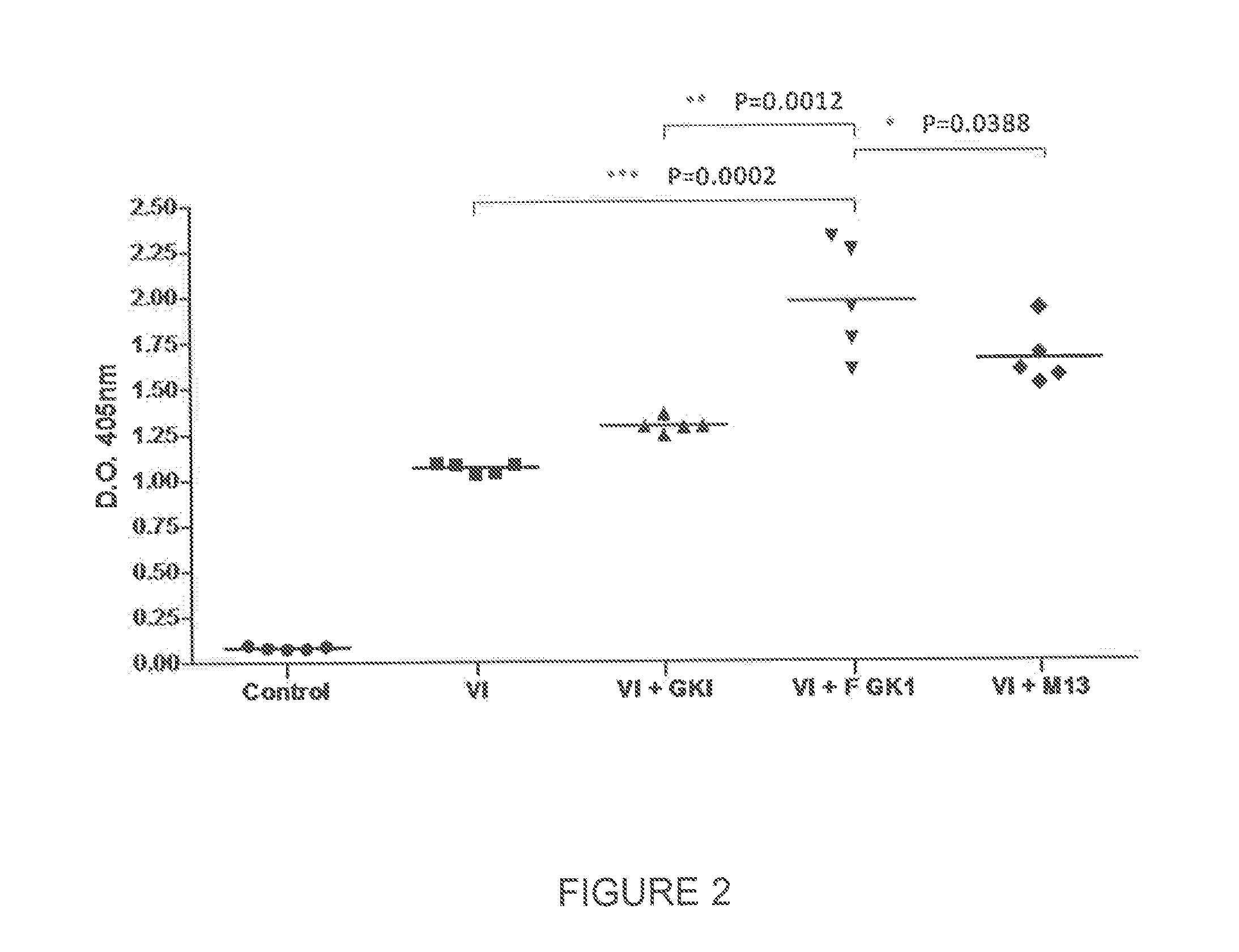
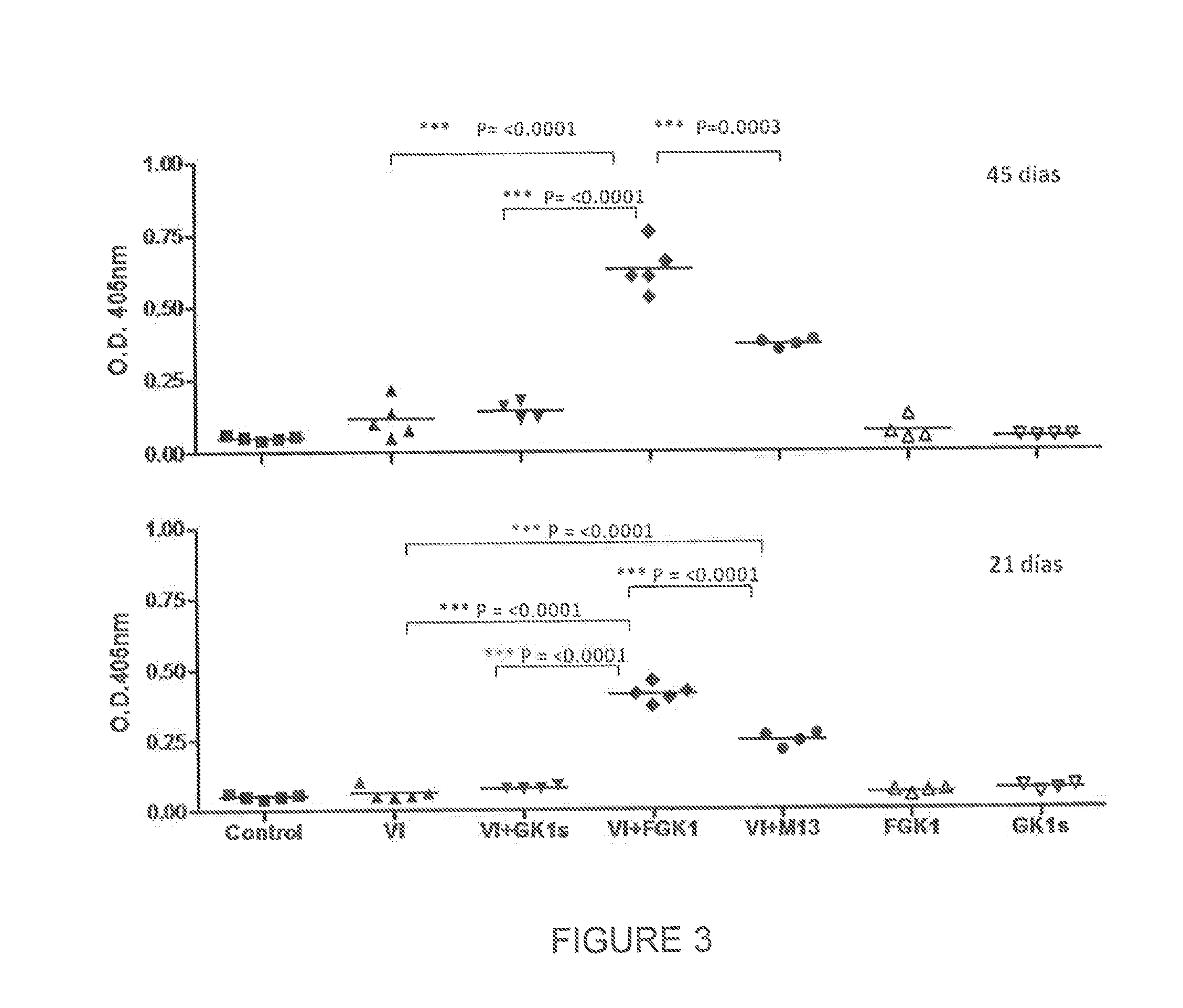
Use of gk-1 peptide expressed on m13 filamentous phage as pharmaceutical ingredient to enhance the efficiency of the immune response induced by vaccine or pathogen antigens
ActiveUS20140302085A1Enhance immune responsePotentiate antibody productionSsRNA viruses negative-sensePeptide/protein ingredientsAdditive ingredientVaccine antigen
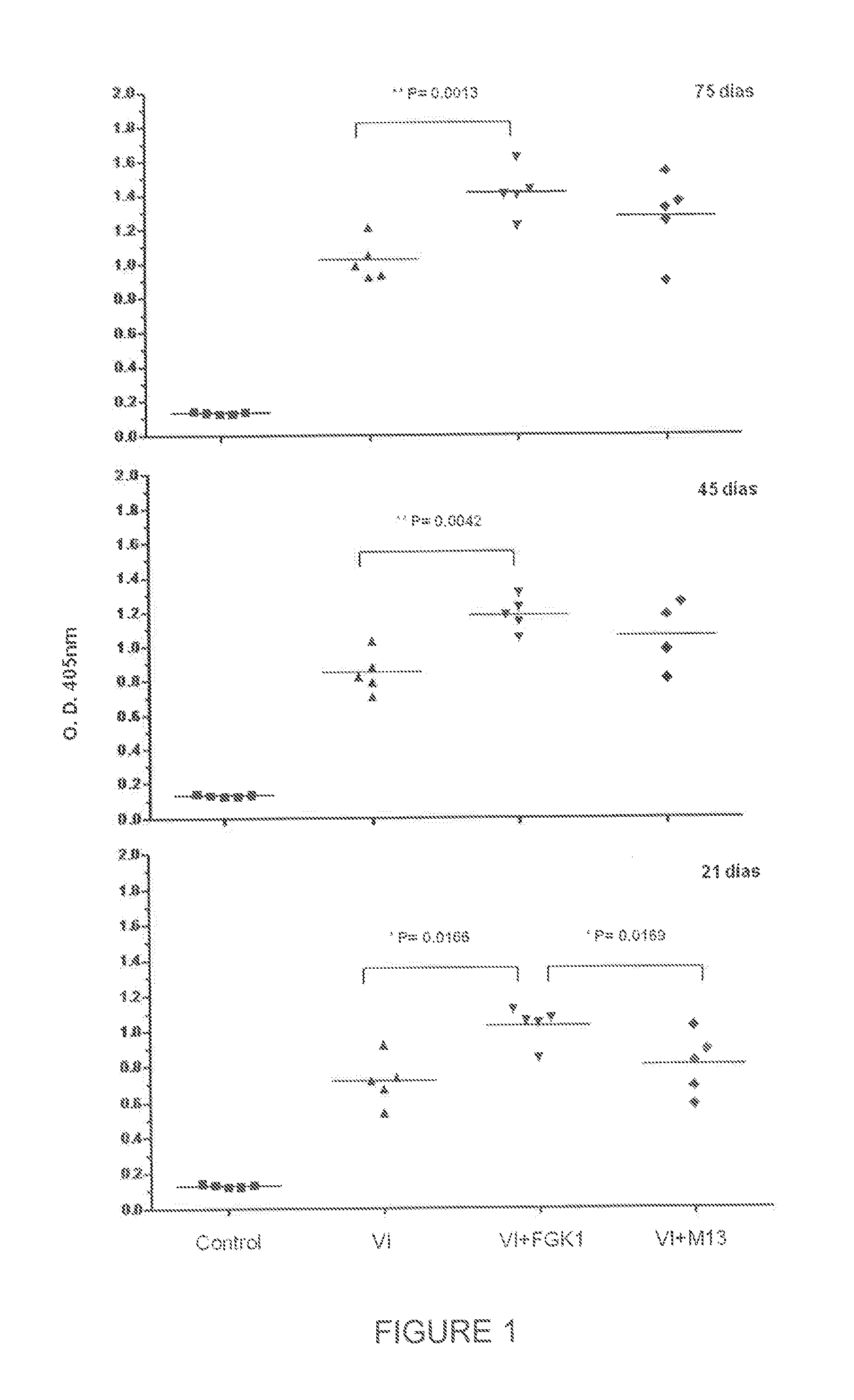
The present invention is directed to the use of FGK-1 immunopotentiator, composed by the peptide named GK-1, characterized by the sequence G-Y-Y-Y-P-S-D-P-N-T-F-Y-A-P-P-Y-S-A and linked to the pVIII surface protein of M13 filamentous phage, to prepare pharmaceutical products potentiating the protective immune response of vaccine antigens when used by itself or conjointly with these antigens administered either intranasally, subcutaneously, or intramuscularly, yielding an increase in the level of specific antibodies against vaccine antigens in serum and in bronchoalveolar lavages.
Owner:UNIV NAT AUTONOMA DE MEXICO
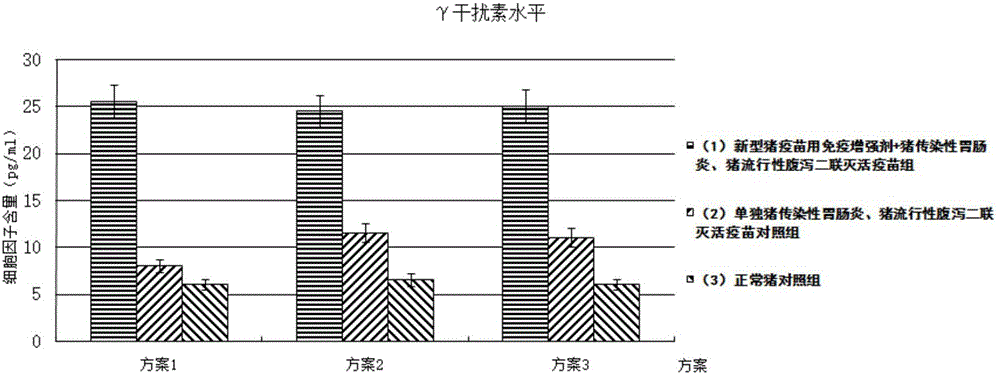
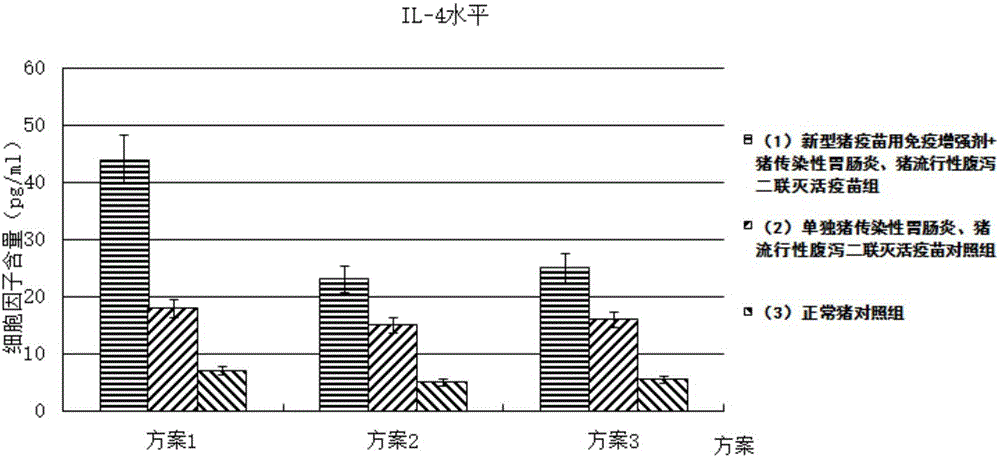
Immune enhancer for pig vaccine, and preparation method and application thereof
InactiveCN106177945AIncrease the level of humoral immune responseImprove the level ofImmunological disordersAntibody medical ingredientsFreeze-dryingWindow period
The invention provides an immune enhancer for pig vaccine, and a preparation method and application thereof. The immune enhancer for pig vaccine comprises a pig gene engineering cell factor compound and a freeze-drying protective additive, wherein the pig gene engineering cell factor compound comprises the following ingredients in parts by weight: 50 to 70 parts of recombinant porcine IFN-Alpha, 15 to 25 parts of recombinant porcine IFN-Gamma and 15 to 25 parts of recombinant porcine IL-2. The immune enhancer has the following effects that after the combined use of the immune enhancer and the vaccine, the vaccine protective period specificity antibody level is improved; the antibody generating window period is shortened by 30 to 50 percent; the secretory volume of specific humoral immunity response markers 1L-4 can be improved by 1 to 5 times through being compared with that during the single use of the vaccine; the cellullar immunologic response level of the pig vaccine can be improved; the secretory volume of the cellullar immunologic response marker interferon Gamma is improved by 1 to 5 times through being compared with that during the single use of the vaccine; the safety is high; the immune enhancer can be prepared into a water soluble state; the problems that on the existing market, the vaccine immunologic adjuvant has the injection abscess, nodule and ulceration, so that the acute or chronic inflammation reaction and the like can be caused are solved.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
IgY From Norovirus P Particles And Their Derivatives
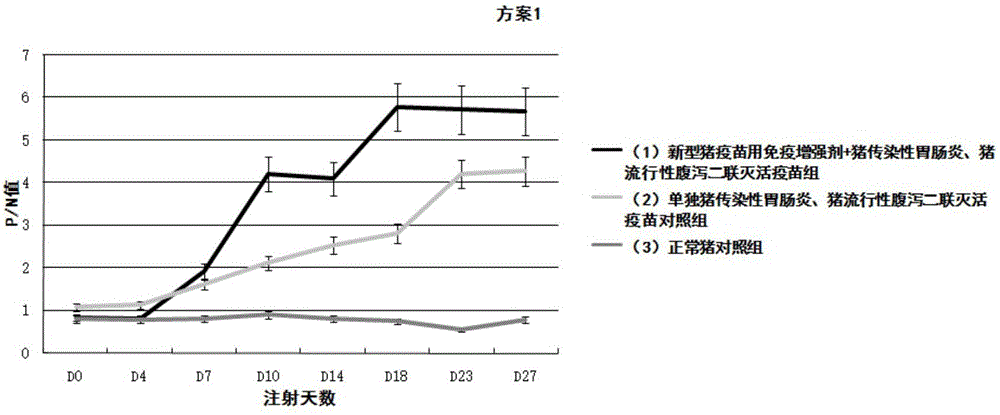
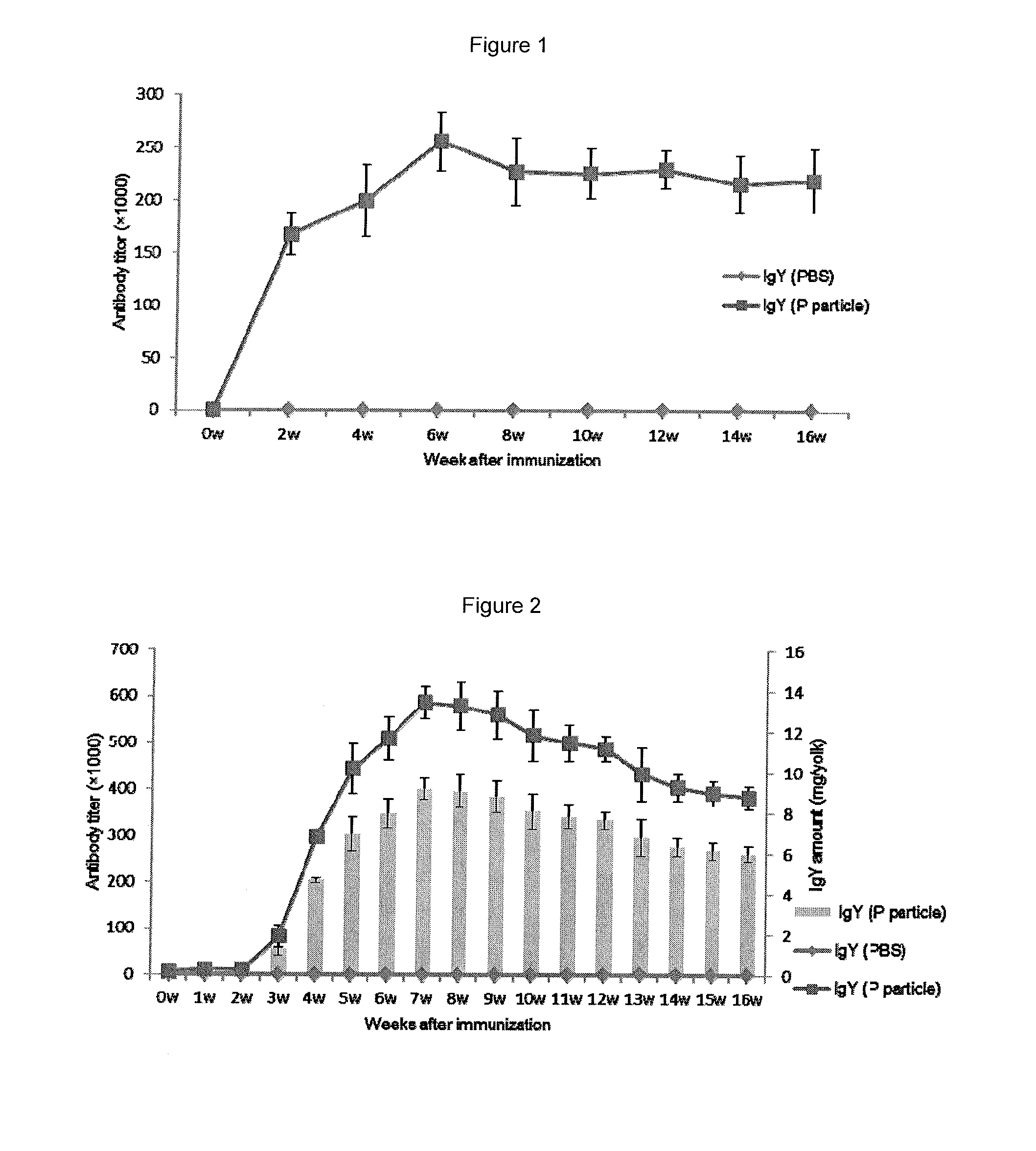
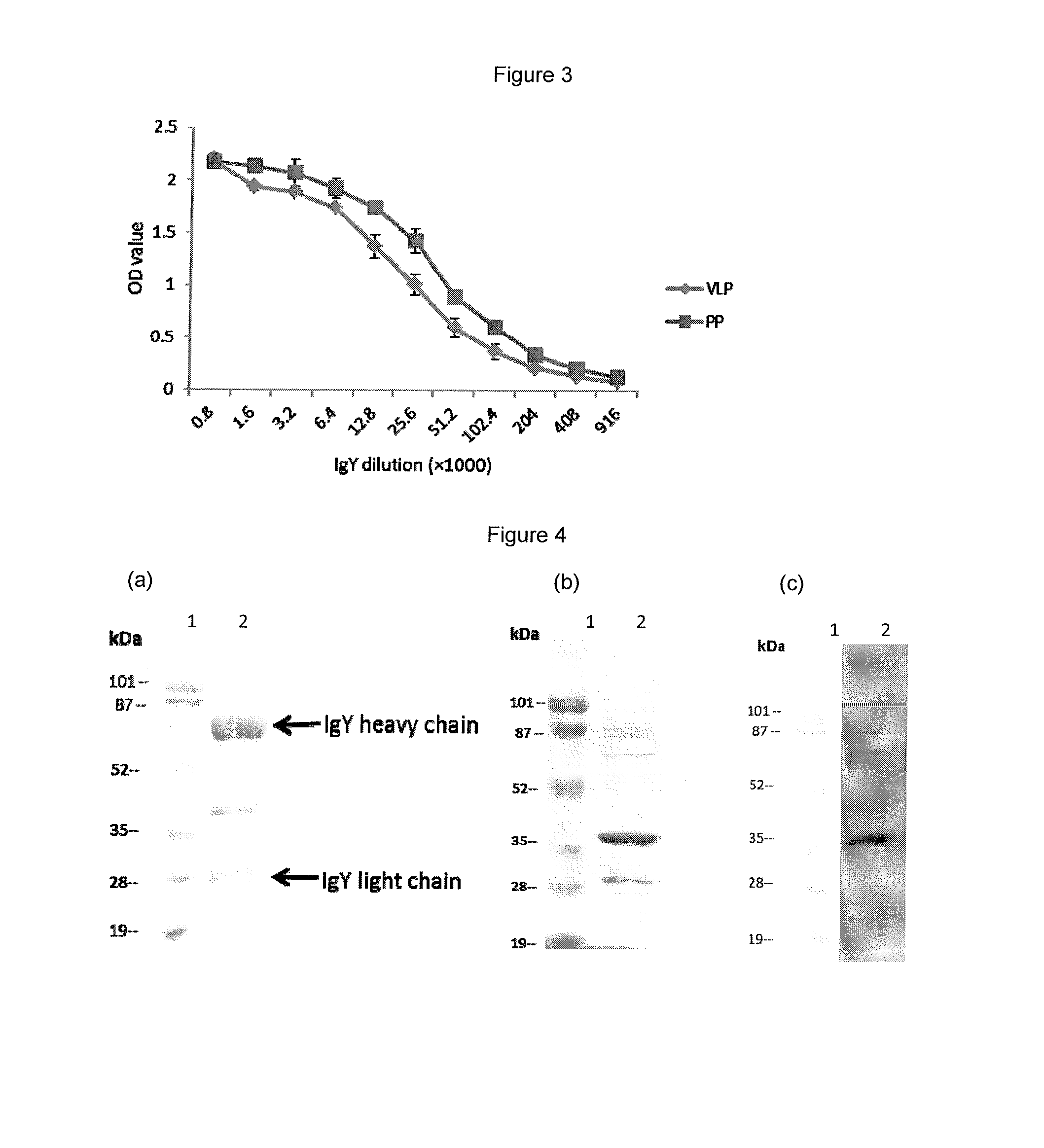
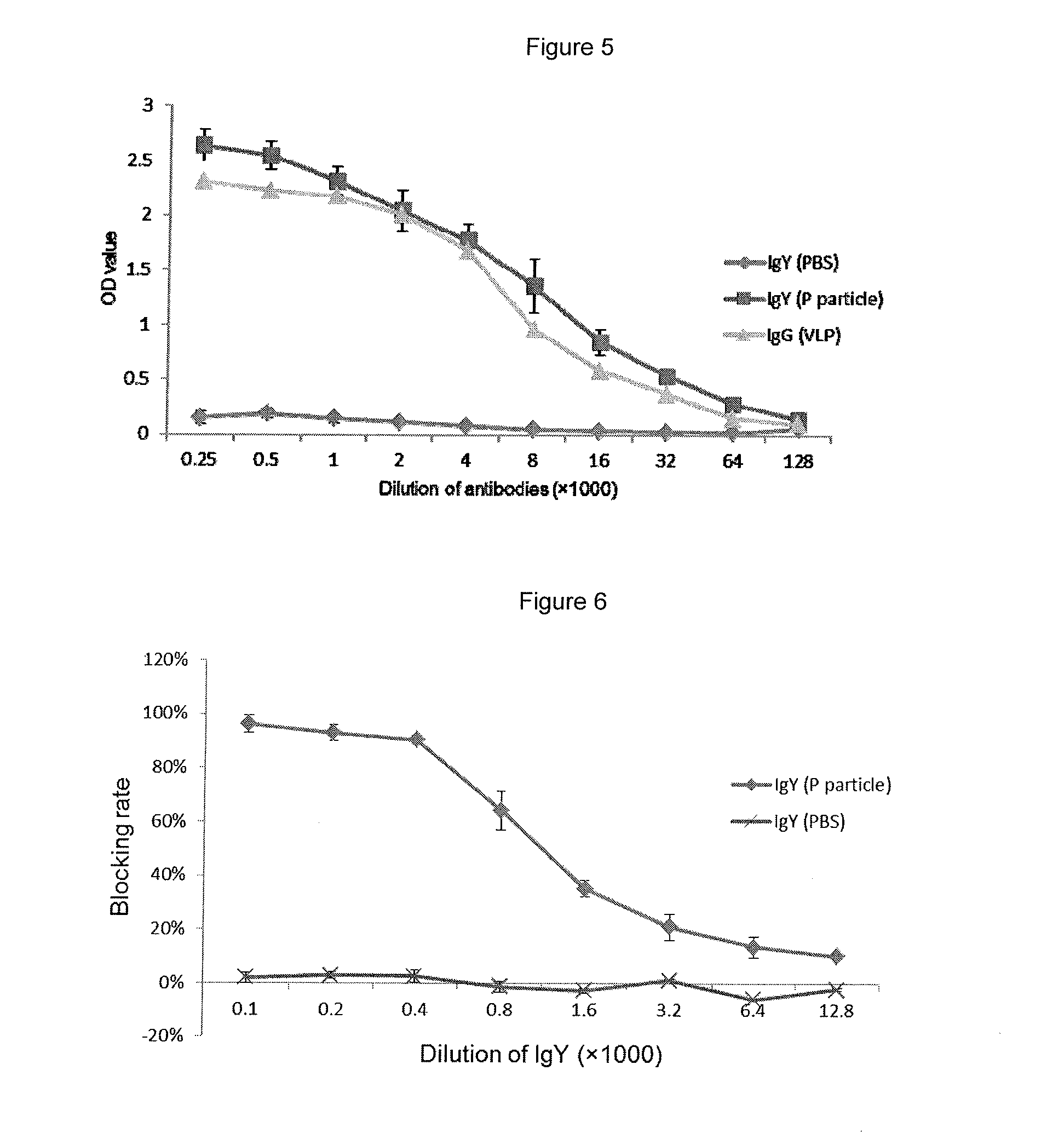
InactiveUS20140017257A1Easy to produceSafe and effectiveEgg immunoglobulinsImmunoglobulins against virusesPassive ImmunizationsDisease
A method for large-scale production of anti-NoV antibodies for use as a potential treatment for NoV disease using passive immunization. NoV-specific immunoglobulins (IgY) can be produced by immunizing chickens with NoV P particles. The birds continuously produced high titers of antibodies in their eggs for at least 3 months, in which NoV-specific antibody levels reached 4.7-9.2 mg / egg yolk. The egg yolk antibodies strongly reacted with NoV P particles by both ELISA and Western blot and blocked NoV virus-like particle (VLP) and P particle binding to the histo-blood group antigen (HBGA) receptors with a BT50 of about 1:800. The chicken IgY remain stable at 70° C. for 30 min or treatment at pH 4 to 9 for 3 hr, demonstrating that chicken IgY provides large-scale production of anti-NoV antibodies for use in passive immunization against NoV infection.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Vaccine for preventing porcine epizootic diarrhea disease
InactiveCN104645326AImprove protectionImprove stabilityBacteriaGenetic material ingredientsNucleotide sequencingDiarrhea
The invention discloses an application of a recombinant salmonella containing a nucleotide sequence shown in SEQ ID NO:1 in preparation of a medicine for preventing a porcine epizootic diarrhea disease and also discloses a vaccine which is used for preventing the porcine epizootic diarrhea disease and is prepared from the recombinant salmonella containing the nucleotide sequence shown in SEQ ID NO:1 and pharmaceutically acceptable auxiliary materials or a carrier. The vaccine for preventing the porcine epizootic diarrhea disease can produce a high-level antibody specific to porcine epizootic diarrhea viruses, has a good protective effect on the porcine epizootic diarrhea disease and has a good clinical application prospect.
Owner:SICHUAN AGRI UNIV
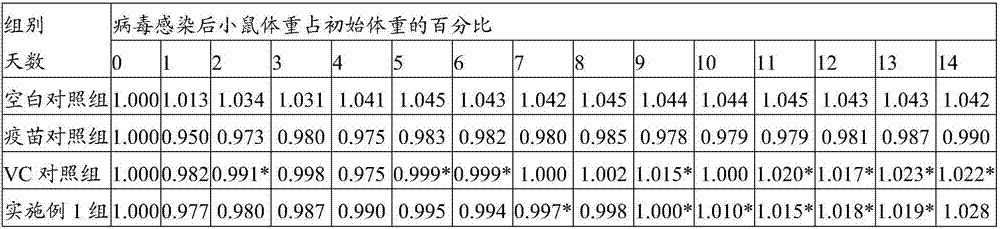
Traditional Chinese medicine composition and application thereof
InactiveCN106421722AHigh activityImprove the quality of lifeMetabolism disorderAntiviralsH1n1 virusTangerine Peel
The invention discloses a prescription, which contains aqueous extracts of shiitake mushrooms, poria cocos, zingiber officinale and dried tangerine peels and plays a role in resisting H1N1 viruses, and application thereof. The prescription is mainly made up of the shiitake mushrooms, the poria cocos, the zingiber officinale and the dried tangerine peels. Proven by antiviral tests, the prescription disclosed by the invention can be used for remarkably improving the immunologic function of H1N1 virus infected mice and improving the pneumonia pathological change degree of the H1N1 virus infected mice and can be used for remarkably improving the specific antibody level in blood serum of the H1N1 virus infected mice, remarkably lowering the content of viruses in lungs of the H1N1 virus infected mice and improving the level of lung cell factors IL-2, so that the prescription has a good H1N1 influenza virus resisting action.
Owner:INFINITUS (CHINA) CO LTD
Immunoprotection combined protein and immune vaccine thereof
ActiveCN108727505AGood immune protectionHave synergistic effectAntibody mimetics/scaffoldsAntiparasitic agentsDiseaseAntibody
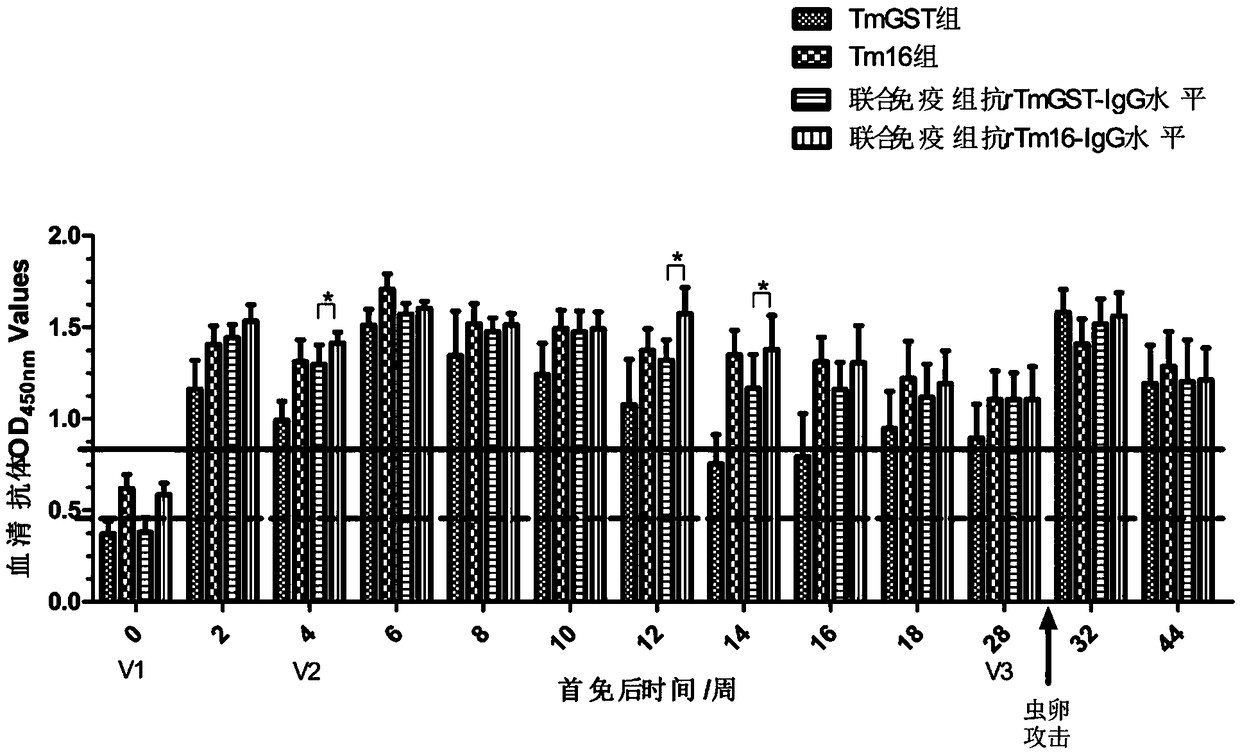
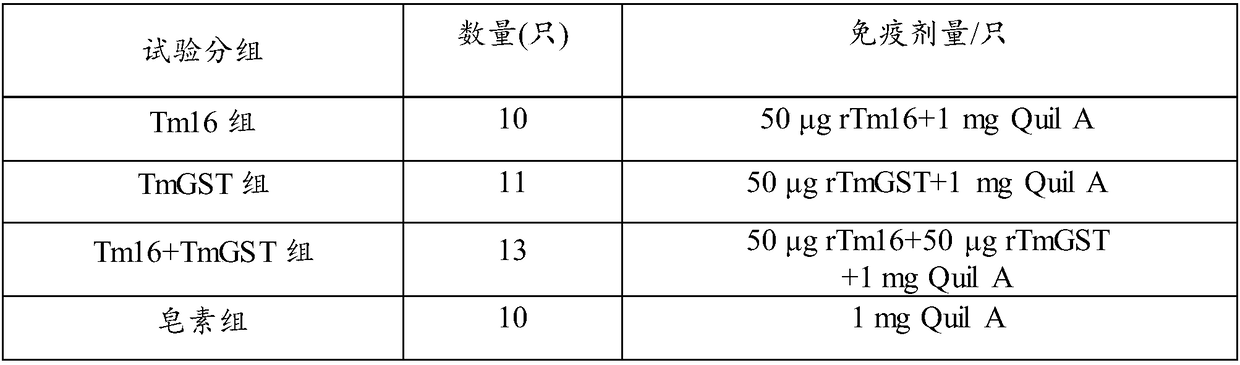
The invention relates to the technical field of biology, and discloses immunoprotection combined protein and an immune vaccine thereof. The immunoprotection combined protein includes Tm16 protein andTm-GST protein, or is Tm16 and Tm-GST fusion protein. The Tm16 protein and the Tm-GST protein are combined to be used as an immunogen immune animal; the immunoprotection effect by aiming at optimum cysticercus cerebralis is generated on animals; the synergistic effect is achieved; higher specific antibody level can be induced; a novel measure for preventing and controlling cysticercus cerebralis diseases is formed.
Owner:SICHUAN AGRI UNIV
Cell adaptation strain MJ of QX type IBV (Infectious Bronchitis Virus) and application of cell adaptation strain MJ
InactiveCN109504653AHigh biosecurityProtectSsRNA viruses positive-senseViral antigen ingredientsToxicantOrganism
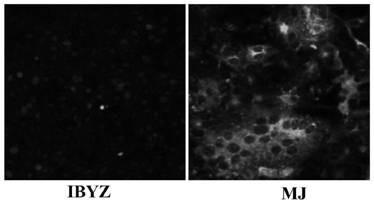
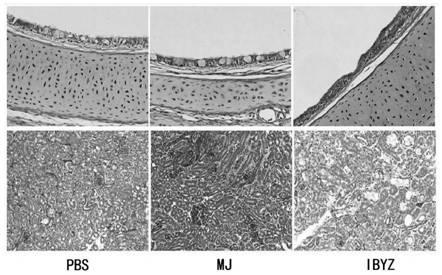
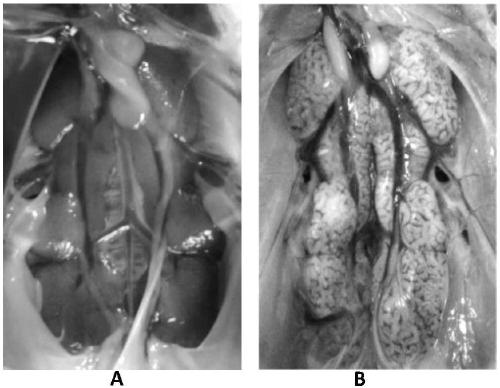
The invention provides a cell adaptation strain MJ of QX type IBV (Infectious Bronchitis Virus) and application of the cell adaptation strain MJ and belongs to the technical field of avian IBV. The cell adaptation strain MJ has the collection number of CGMCC No. 14681. Proven by tests, TCID50 of cell toxicant fluid, gathered after a Vero cell adaptation strain MJ is inoculated with single-layer Vero cells, reaches 106.5 / mL or more; the strain MJ does not have pathogenicity to SPF (specific pathogen free) chicks, and the toxicity of a virus does not become high after the virus is subjected to continuous passage for 5 generations or more in chick bodies; after the SPF chicks are immunized by adopting cell toxicants gathered after the strain MJ is inoculated with Vero cells, the specific antibody level of organisms rises, and good protection is provided for attack of homotype powerful toxicants. The QX type avian IBV can be extensively prepared by using the Vero cell adaptation strain MJprovided by the invention, and the cell adaptation strain MJ is applicable to large-scale industrial production; the cell adaptation strain MJ can also be applied to preparation of QX type avian infectious bronchitis vaccines.
Owner:JIANGSU INST OF POULTRY SCI
Vaccine for preventing porcine epizootic diarrhea
InactiveCN104667296AImprove protectionImprove stabilityGenetic material ingredientsDigestive systemDiseaseNucleotide
The invention discloses an application of a recombinant salmonella having a nucleotide sequence as shown in SEQ ID NO: 1 in preparation of a medicine for preventing porcine epizootic diarrhea, and also discloses a vaccine which is prepared from the recombinant salmonella having the nucleotide sequence as shown in SEQ ID NO: 1 and pharmaceutically acceptable auxiliary materials or carriers and is used for preventing the porcine epizootic diarrhea. The specific antibody level of the vaccine disclosed by the invention for porcine epidemic diarrhea virus is high, so that the vaccine has a good protection effect on the porcine epizootic diarrhea disease; and the vaccine is good in clinical application prospect.
Owner:SICHUAN AGRI UNIV
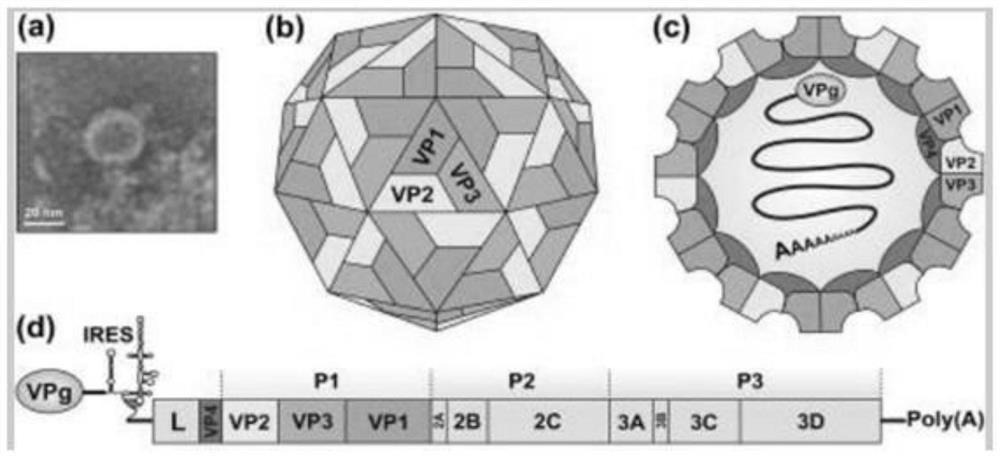
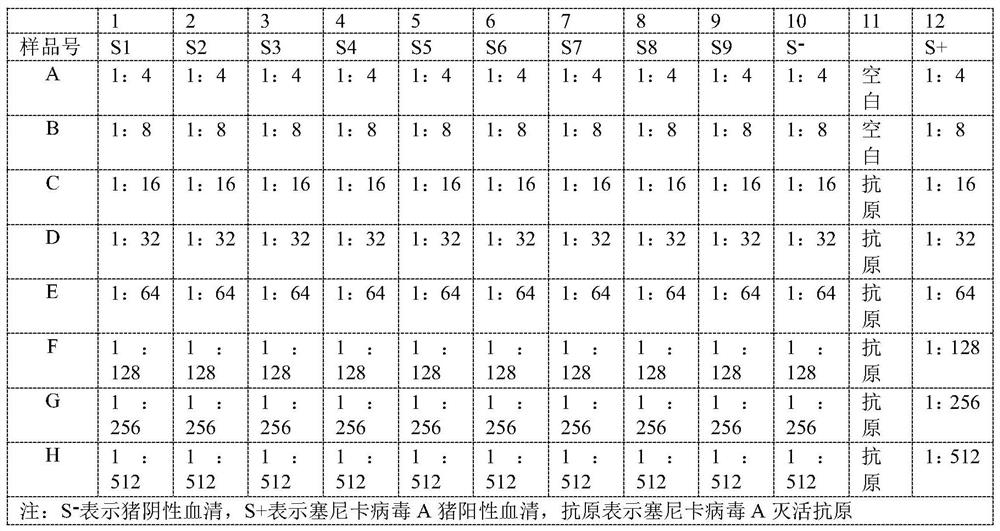
Kit for detecting Seneca virus A antibody
The invention discloses a kit for detecting a porcine Seneca virus A antibody. The kit for detecting the Seneca virus A antibody at least comprises an elisa plate coated with Seneca virus A rabbit antiserum and Seneca virus A guinea pig antiserum. The kit has the advantages of high sensitivity, good specificity, simple operation and stable result, is suitable for batch detection, and can evaluate the infection condition of Seneca virus A in unimmunized swinery and the specific antibody level of swinery after vaccine immunization.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Traditional Chinese medicine composition and application thereof
The invention discloses a formula which is prepared from radix ginseng, bighead atractylodes rhizome, poria cocos and pericarpium citri reticulatae and has the function of resisting H1N1 viruses and application of the formula. The formula is mainly prepared from the radix ginseng, the poria cocos, the bighead atractylodes rhizome and the pericarpium citri reticulatae. According to the formula disclosed by the invention, an anti-virus test verifies that the survival rate and the life quality of H1N1 virus-infected mice can be remarkably increased, the pneumonic lesion degree of the H1N1 virus-infected mice is improved, the level of a specific antibody in serum of the H1N1 virus-infected mice can be remarkably improved, the content of viruses in the lung of the H1N1 virus-infected mice is remarkably reduced, and the level of cytokines IL-2 of the lung can be remarkably improved, so that the anti-virus test shows that the formula has a good H1N1 influenza virus resisting function.
Owner:INFINITUS (CHINA) CO LTD
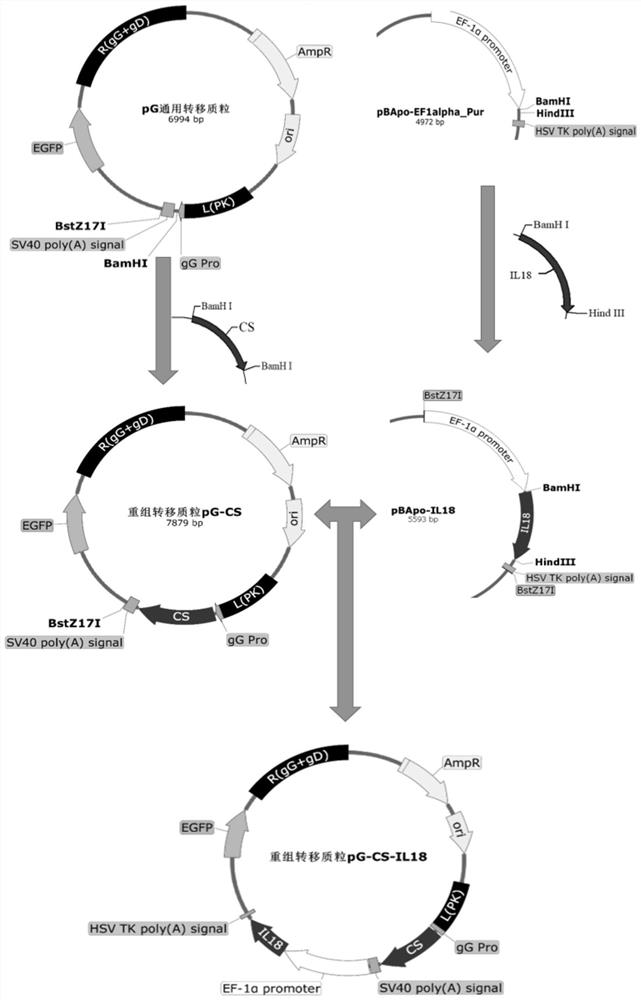
Recombinant porcine pseudorabies virus strain capable of simultaneously expressing PEDV variant S1 gene CS region and porcine IL-18 and application of recombinant porcine pseudorabies virus strain
PendingCN113637648AGood genetic stabilityFree from virulent lethal attacksSsRNA viruses positive-sensePeptide/protein ingredientsEpidemic diarrheaRabies
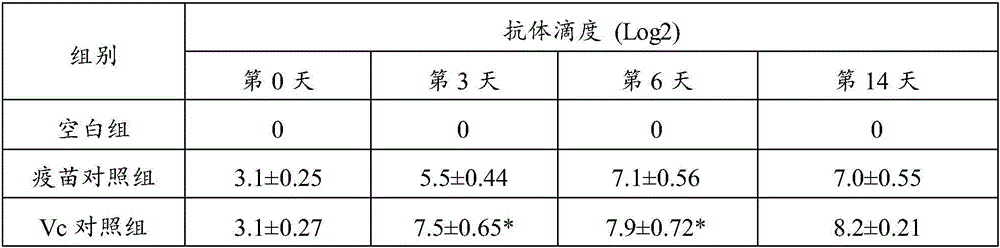
The invention belongs to the field of molecular biology, and particularly relates to a recombinant porcine pseudorabies virus strain capable of simultaneously expressing a PEDV variant S1 gene CS region and a porcine IL-18 and an application of the recombinant porcine pseudorabies virus strain. The classification name of the strain is rPRV-PEDV S1-IL18, the preservation number is CCTCC NO: V201740, the preservation date is October 31, 2017, and the strain is preserved in the China Center for Type Culture Collection, Wuhan University, Wuhan, China. The recombinant porcine pseudorabies virus strain is successfully obtained, can induce piglets to generate specific antibodies aiming at the PEDV and the PRV, and can protect the piglets from virulent lethal attacks of the PEDV and the PRV. The level of a PRV specific antibody generated by a recombinant strain group is higher than that of a recombinant virus rPRV-PEDV S1 group without porcine IL-18, and the recombinant strain group is expected to become a candidate strain of a novel porcine epidemic diarrhea and porcine pseudorabies bigeminy attenuated vaccine.
Owner:HENAN AGRICULTURAL UNIVERSITY
Methods and kits for the detection of an infection in subjects with low specific antibody levels
This invention relates to methods that enable the detection of antibodies against a latent infection, a chronic infection, a re-infection, and / or a breakthrough infection; enable the diagnosis of a latent infection, a chronic infection, a re-infection, and / or a breakthrough infection; and increase low anti-viral antibody levels, and a kit for the detection of virus-specific antibodies expressed at low levels.
Owner:智能生物科技有限公司
Fusion gene, protein coded by fusion gene and application of fusion gene in fish iridovirus oral vaccine
ActiveCN114480438AHigh expressionVerify validityBacteriaAntibody mimetics/scaffoldsStaphylococcus lactisTGE VACCINE
The invention discloses a fusion gene, a protein coded by the fusion gene and application of the fusion gene in fish iridovirus oral vaccines. The fusion gene contains a micropterus salmoides iridovirus capsid protein MCP antigen optimization gene, a connection sequence and a tag sequence. The recombinant lactococcus lactis NZ9000 pNZ8148-MCP prepared on the basis of the recombinant lactococcus lactis NZ9000 pNZ8148-MCP is preserved in the China Center for Type Culture Collection (CCTCC), and the preservation number is CCTCC NO: M20211160. After the oral vaccine prepared from the recombinant lactococcus lactis is used for immunization, the serum specific antibody level of largemouth bass can be remarkably improved, the IgM content in the kidney can also be remarkably improved after the oral vaccine is used for immunization, the relative immune protection rate can reach 60.47%, injection is not needed, oral administration can be achieved, operation is easy, and universality and applicability are high.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Methods and kits for the detection of an infection in subjects with low specific antibody levels
InactiveUS20130216999A1Lower antibody levelsMicrobiological testing/measurementImmunoassaysBreakthrough infectionRe infection
This invention relates to methods that enable the detection of antibodies against a latent infection, a chronic infection, a re-infection, and / or a breakthrough infection; enable the diagnosis of a latent infection, a chronic infection, a re-infection, and / or a breakthrough infection; and increase low anti-viral antibody levels, and a kit for the detection of virus-specific antibodies expressed at low levels.
Owner:SMART BIOTECH
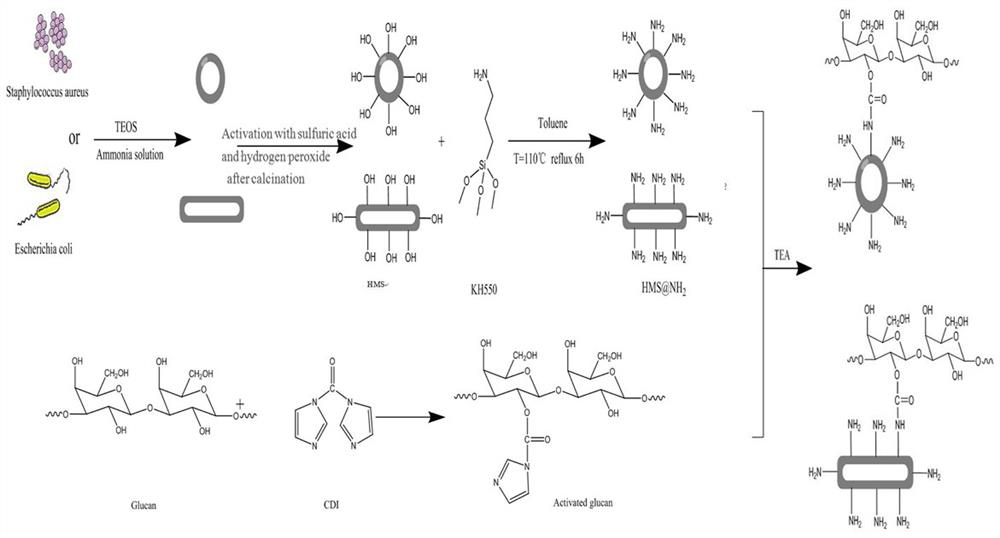
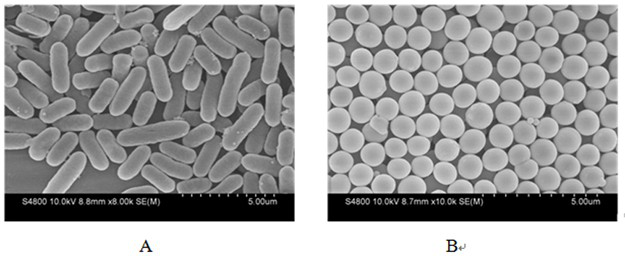
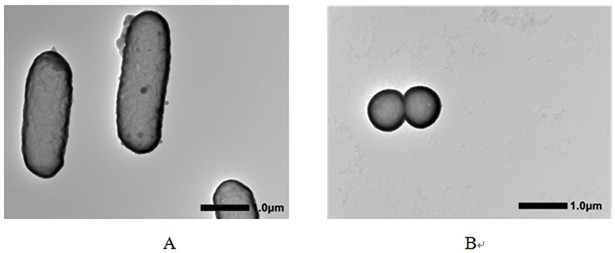
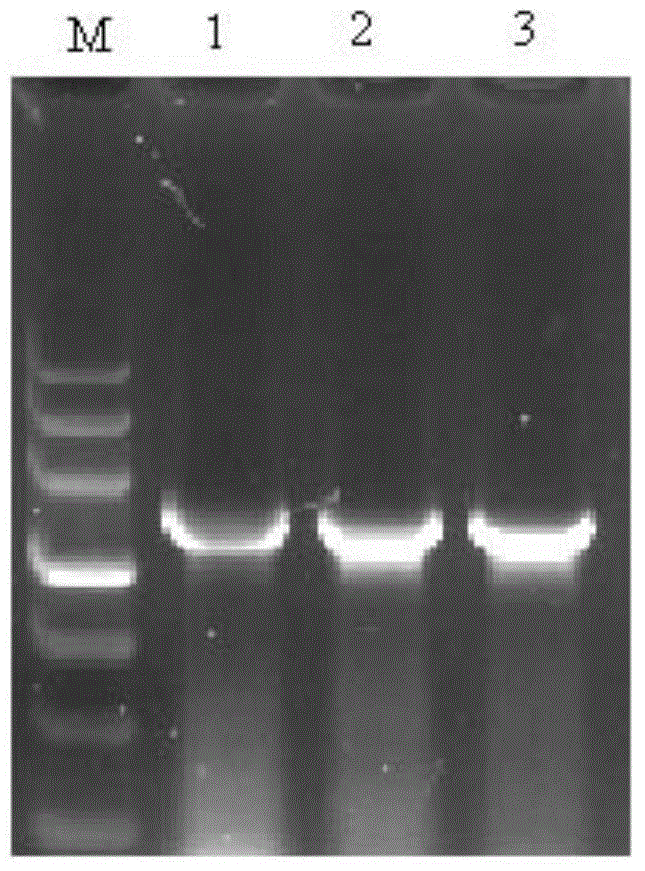
A biomimetic hollow silica composite particle modified by β-1,3-d-glucan and its application
ActiveCN108653727BEasy to passSustained releaseInorganic non-active ingredientsAntibody medical ingredientsFreund's adjuvantPharmaceutical drug
Owner:SUN YAT SEN UNIV
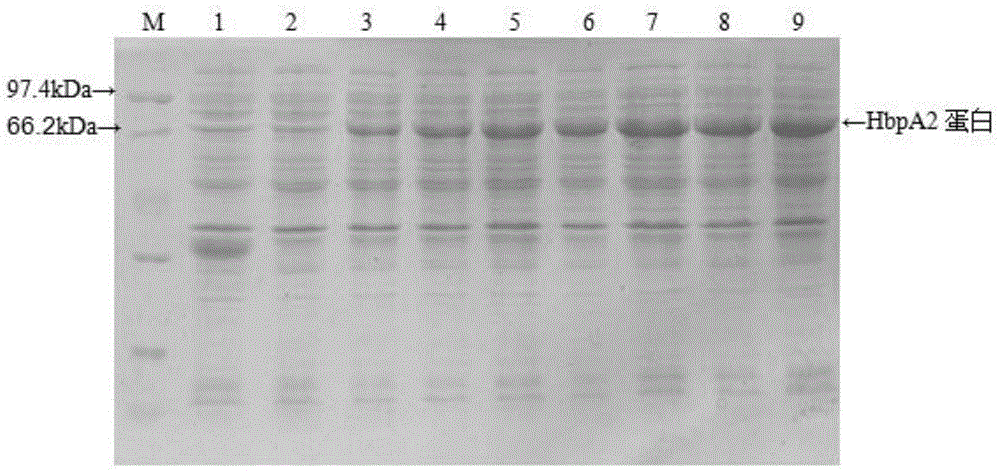
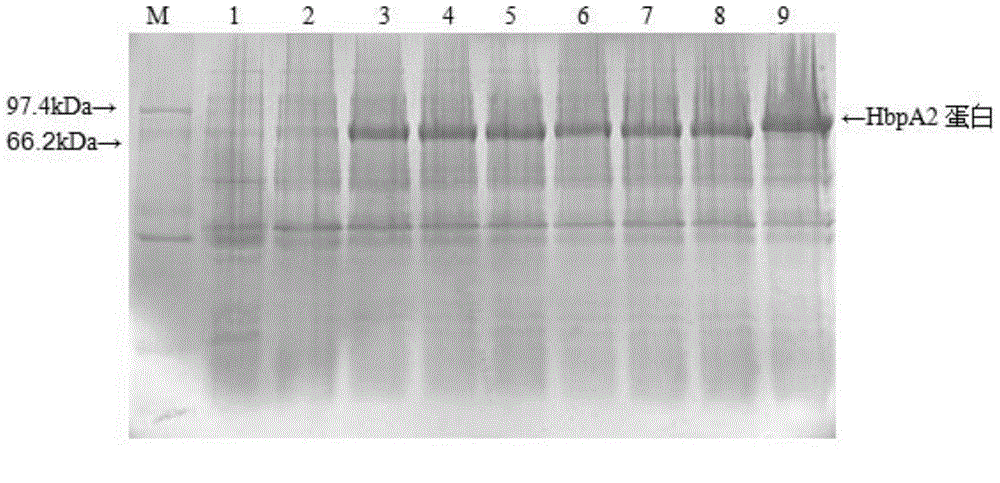
Recombinant haemophilus parasuis immunoprotecive antigen HbpA2 and preparation method thereof
ActiveCN104404056AImproving immunogenicityImprove protectionBacteriaMicroorganism based processesProtective antigenSerum ige
The invention discloses a nucleotide sequence as shown in SEQ ID NO:1 and discloses a recombinant vector and recombinant bacteria containing the recombinant bacteria, a recombinant HbpA2 protein coded by the nucleotide sequence as shown in the SEQ ID NO:1 and a preparation method thereof. The recombinant HbpA2 protein has good immunogenicity and protection genicity. The HbpA2 generated after immunization has high specific antibody level and can remarkably protect attacks of mouse anti-haemophilus parasuis serum type-5 strain with strong virulence. It shows that the recombinant protein HbpA2 is a protective antigen for haemophilus parasuis and can be made into a vaccine.
Owner:SICHUAN AGRI UNIV
A kind of reagent for detecting specific immune response of Mycobacterium tuberculosis and use thereof
ActiveCN103804499BEasy to distinguishHuge development potentialHybrid peptidesMaterial analysisT cellInterferon alpha
The invention discloses a reagent for detecting specific immune response of mycobacterium tuberculosis and use thereof. The invention discloses an immune positive reagent Rv0733 of mycobacterium tuberculosis by a gamma interferon release assay and a serum enzyme-linked immune method. The reagent comprises antigen or polypeptide and analogues thereof for detecting the specific cellular immunity of a tuberculosis sufferer and humoral immune response level. After the mice are immunized by using the reagent disclosed by the invention, the mice can generate specific cell factors such as gamma interferon and the like, and an antibody. T cells of tuberculosis patients or normal people are stimulated by using the reagent in vitro, the quantity of gamma interferon release cells is detected, or the content of gamma interferon in supernatant is cultivated, the tuberculosis patients can be distinguished from the normal people, the detection sensitivity and specificity of the tuberculosis sufferer can be improved by detecting the specific antibody level of resisting the reagent in the peripheral serum, and the clinical prognosis conditions of the tuberculosis patients can be disclosed by utilizing an Rv0733 specific immune response contrast experiment in the process of tracking treatment of the tuberculosis sufferer.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Immune complex vaccine
InactiveCN112933221AImprove immunityLow costSsRNA viruses negative-senseSsRNA viruses positive-senseImmune complex depositionTGE VACCINE
The invention relates to the technical field of biological medicines, in particular to an immune complex vaccine. The vaccine can be prepared by adding a homologous specific monoclonal antibody in a conventional vaccine preparation process, but not limited to the conventional vaccine preparation process. The vaccine is characterized in that any live virus does not need to be added, and the vaccine contains an immune complex formed by mutual combination of a protein antigen and a specific homologous monoclonal antibody thereof. The immune complex can improve the level of a vaccine-induced specific antibody and improve vaccine-induced specific cellular immunity. The vaccine has the advantages of being good in safety, excellent in immune protection effect, suitable for preventing various virus diseases and the like.
Owner:陈继明
A kind of immunoprotective combination protein and immune vaccine thereof
ActiveCN108727505BGood immune protectionHave synergistic effectAntibody mimetics/scaffoldsAntiparasitic agentsDiseaseBiochemistry
The invention relates to the technical field of biology, and discloses immunoprotection combined protein and an immune vaccine thereof. The immunoprotection combined protein includes Tm16 protein andTm-GST protein, or is Tm16 and Tm-GST fusion protein. The Tm16 protein and the Tm-GST protein are combined to be used as an immunogen immune animal; the immunoprotection effect by aiming at optimum cysticercus cerebralis is generated on animals; the synergistic effect is achieved; higher specific antibody level can be induced; a novel measure for preventing and controlling cysticercus cerebralis diseases is formed.
Owner:SICHUAN AGRI UNIV
Homogeneous immunoassays for multiple allergens
A homogeneous immunoassay method and system for quantitative determination of total immunoglobulin E and specific antibody levels to a plurality of allergens, in which a relatively small sampling of blood is required. The method utilizes relatively small microparticles in aqueous suspension. The immunoassay procedure is an immunometric sandwich procedure preferably utilizing biotin-streptavidin signal amplification techniques and R-phycoerytherin fluorescent labels.
Owner:IMMUNETECH
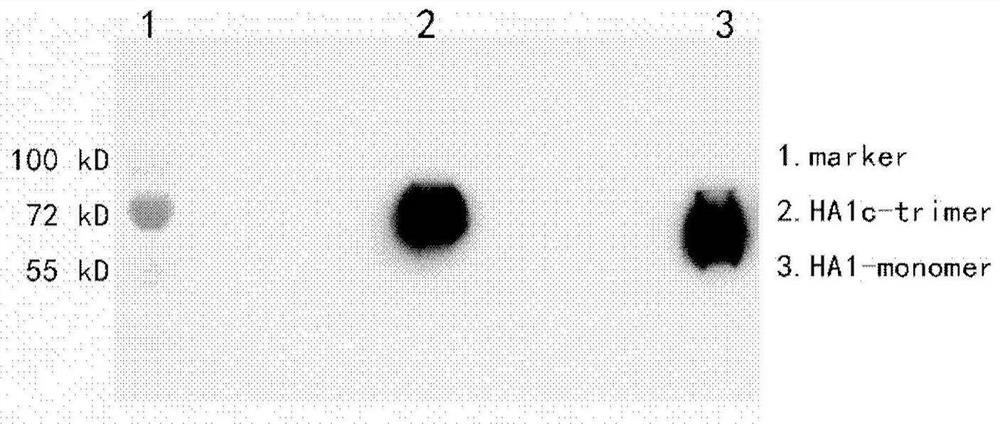
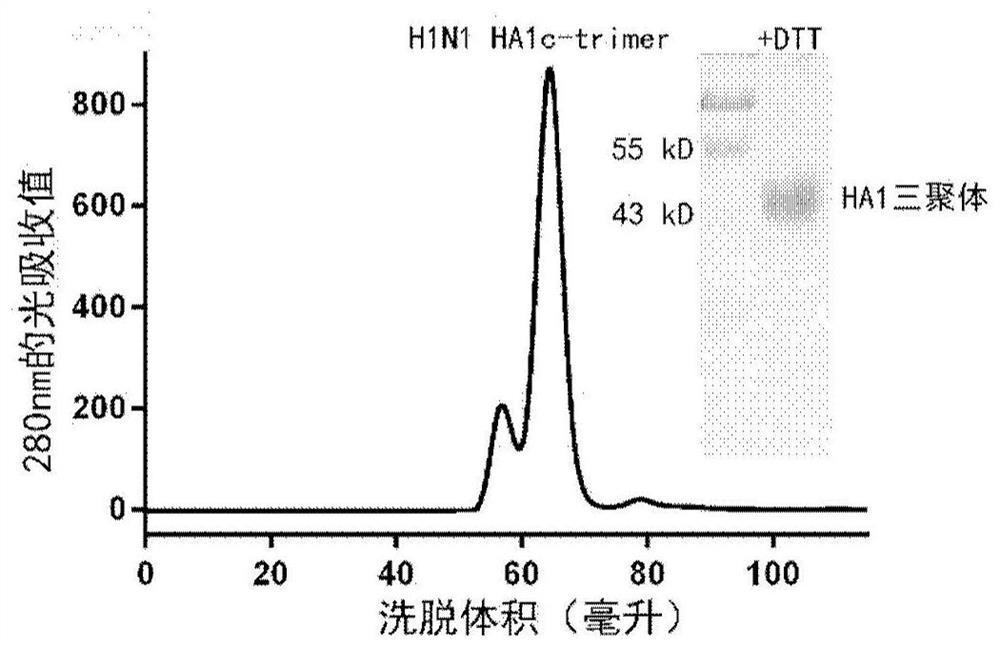
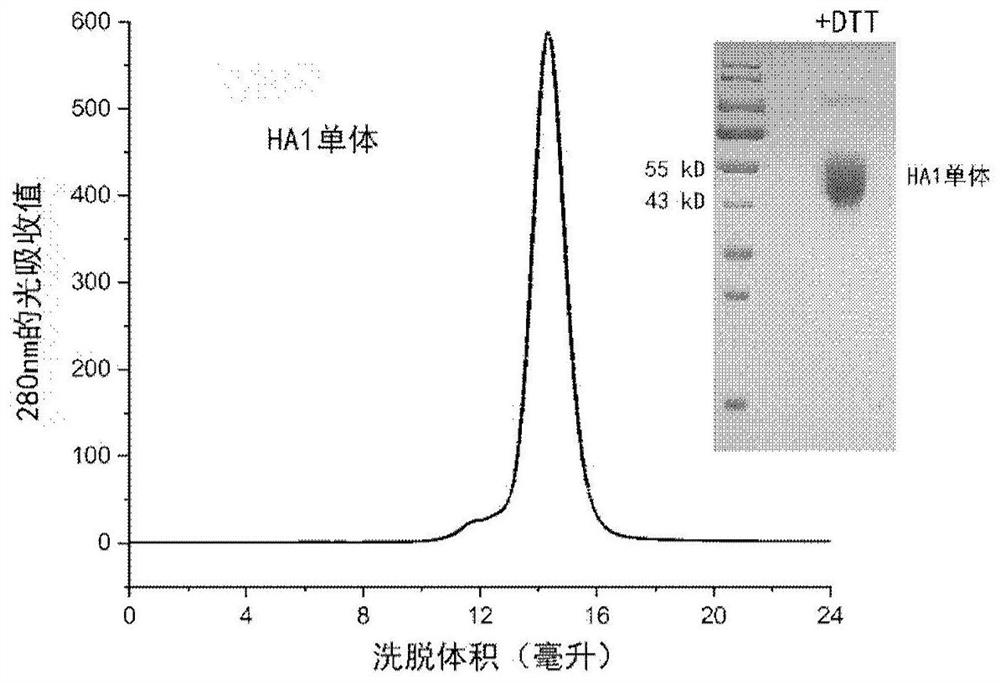
Influenza virus trimer subunit vaccine and application thereof
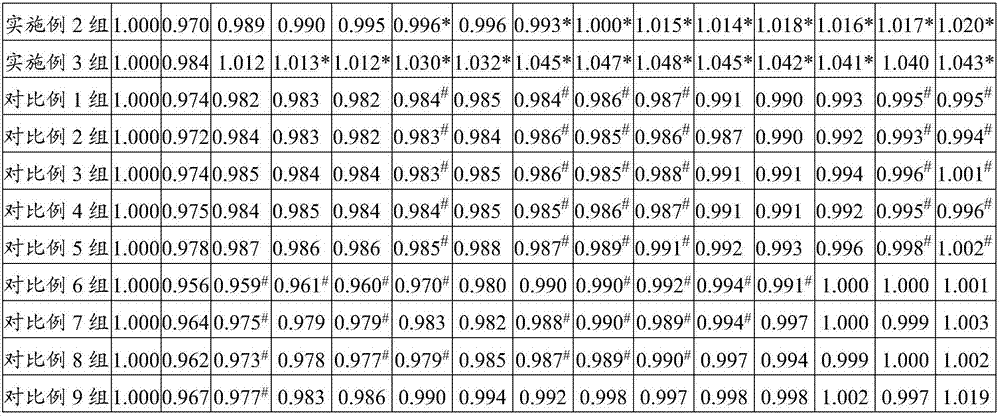
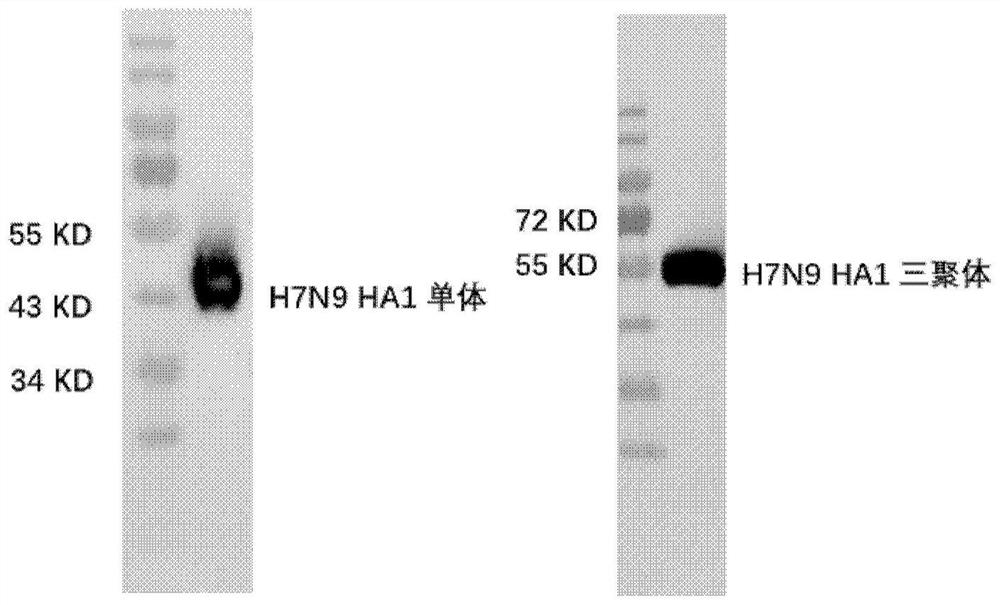
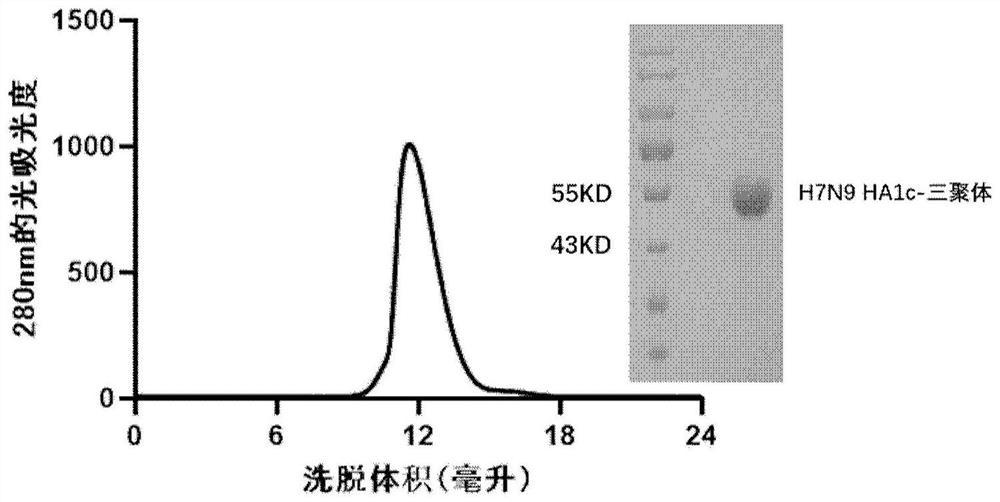
PendingCN114409803ASsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininProtein trimer
The invention discloses a subunit influenza virus vaccine based on trimerization HA (hemagglutinin). Insect cells are used for expressing influenza virus H1N1 HA protein trimer in vitro to form fusion trimer proteins H1N1 HA1c-trimer, H3N2 HA1c-trimer or B HA1c-trimer with LAH at the N terminal and H1N1 HA1, H3N2 HA1 or B-type HA1 protein at the C terminal, compared with respective HA1 monomer proteins, the trimer proteins have better immune effect, and mice can generate a higher-titer specific antibody aiming at the influenza virus HA1. The trimer form HA1 provided by the invention overcomes the defect of insufficient immunogenicity of an HA1 monomer, and improves the level of a specific antibody generated by mice and aiming at the influenza virus HA1; the design can be used for improving the immunogenicity of the influenza virus HA1, so that the influenza virus HA1 can be used as a more effective vaccine.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Traditional Chinese medicine composition and application thereof in preparation of products for preventing H7 subtype avian influenza virus
InactiveCN106913851AGood antiviral effectGood treatment effectAntiviralsImmunological disordersAvian influenza virusCurative effect
The invention discloses a formula, which contains a water extract of mushroom, poria cocos, ginger, and dried orange peel and can prevent H7 subtype avian influenza virus, and an application thereof. The formula is mainly composed of mushroom, poria cocos, ginger, and dried orange peel. The anti-virus experiment results show that the formula can prominently enhance the immunity of mice infected by H7 subtype avian influenza virus; the pneumonia lesion degree of the mice is improved; the specific antibody level in the serum of the mice is obviously improved; the number of viruses in the lung of the mice is prominently reduced; the lung cell factor IL-2 level is improved; and the in-vitro cell test results show that the traditional Chinese medicine composition has a prominent antivirus performance and good curative effect. The formula has a good effect on preventing H7 subtype avian influenza virus.
Owner:INFINITUS (CHINA) CO LTD
Avian influenza virus trimer subunit vaccine and application thereof
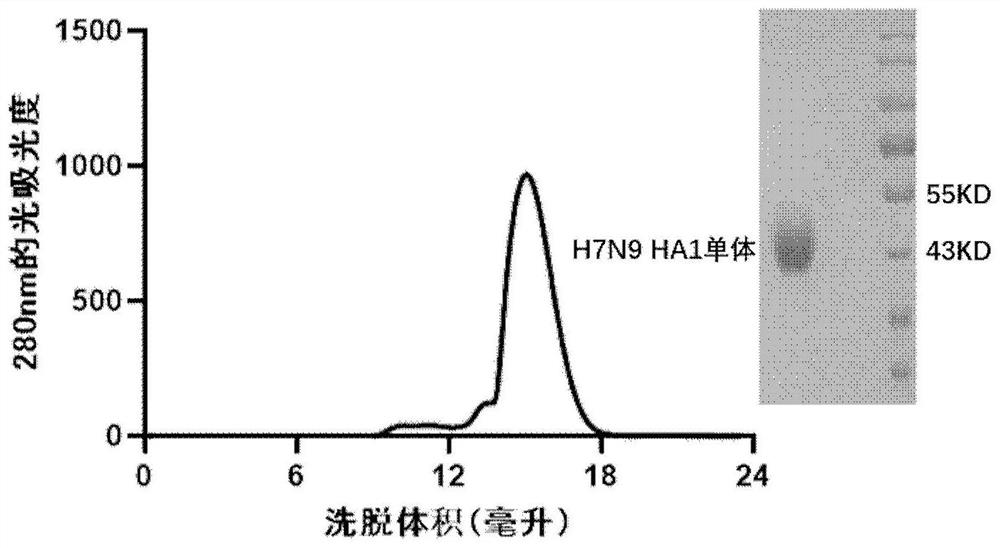
The invention discloses a subunit avian influenza virus vaccine based on trimerization HA (hemagglutinin). Insect cells are used for in-vitro expression of influenza virus H1N1 HA protein trimer to form fusion trimer proteins H7N9 HA1c-trimer and H5N1 HA1c-trimer in a region LAH at the N terminal and H7N9 HA1 or H5N1 HA1 protein at the C terminal, and compared with respective HA1 monomer proteins, the trimer proteins have better immune effect, and a mouse can generate a higher-titer specific antibody aiming at the influenza virus HA1. The trimer form HA1 provided by the invention overcomes the defect of insufficient immunogenicity of an HA1 monomer, and improves the level of a specific antibody generated by mice and aiming at the influenza virus HA1; the design can be used for improving the immunogenicity of the influenza virus HA1, so that the influenza virus HA1 can be used as a more effective vaccine.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
A subunit vaccine of Necrobacterium bovis and preparation method thereof
ActiveCN110613842BImprove the level ofGood immune protectionAntibacterial agentsBacterial antigen ingredientsWhite blood cellTGE VACCINE
The invention relates to a subunit vaccine of Necroptosis bovis, which comprises three proteins, an outer membrane protein 43kDa OMP of Necroptosis bovis, a truncated protein PL-4 of necroptosis bacillus leukotoxin and a truncated protein H2 of necrolysin. The protein dosage ratio is 1:1:1. The present invention also relates to a preparation method of the above-mentioned subunit vaccine of Necroptosis bovis. The subunit vaccine of Necroptosis bovis of the present invention comprises three kinds of proteins of Necroptosis bovis outer membrane protein 43kDa OMP, Bacillus necroptosis leukotoxin truncated protein PL-4 and necrolysin truncated protein H2, combined application, after immunization The level of specific antibodies produced is high, which can well stimulate the cellular immunity and humoral immunity of mice, and effectively protect mice against the attack of necrotic bacillus.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Recombinant salmonella containing porcine epidemic diarrhea virus S499-789 gene
InactiveCN104673807AImprove protectionImprove stabilityBacteriaMicroorganism based processesDiseaseEpidemic diarrhea
The invention discloses a nucleotide sequence shown in SEQ ID NO:1, and discloses a recombinant carrier and recombinant bacteria containing the nucleotide sequence. A specific antibody generated by immunization of the recombinant salmonella containing the S499-789 gene for the porcine epidemic diarrhea virus is high in level; and the recombinant salmonella has a good protecting effect on a porcine epidemic diarrhea disease and is excellent in clinical application prospect.
Owner:SICHUAN AGRI UNIV
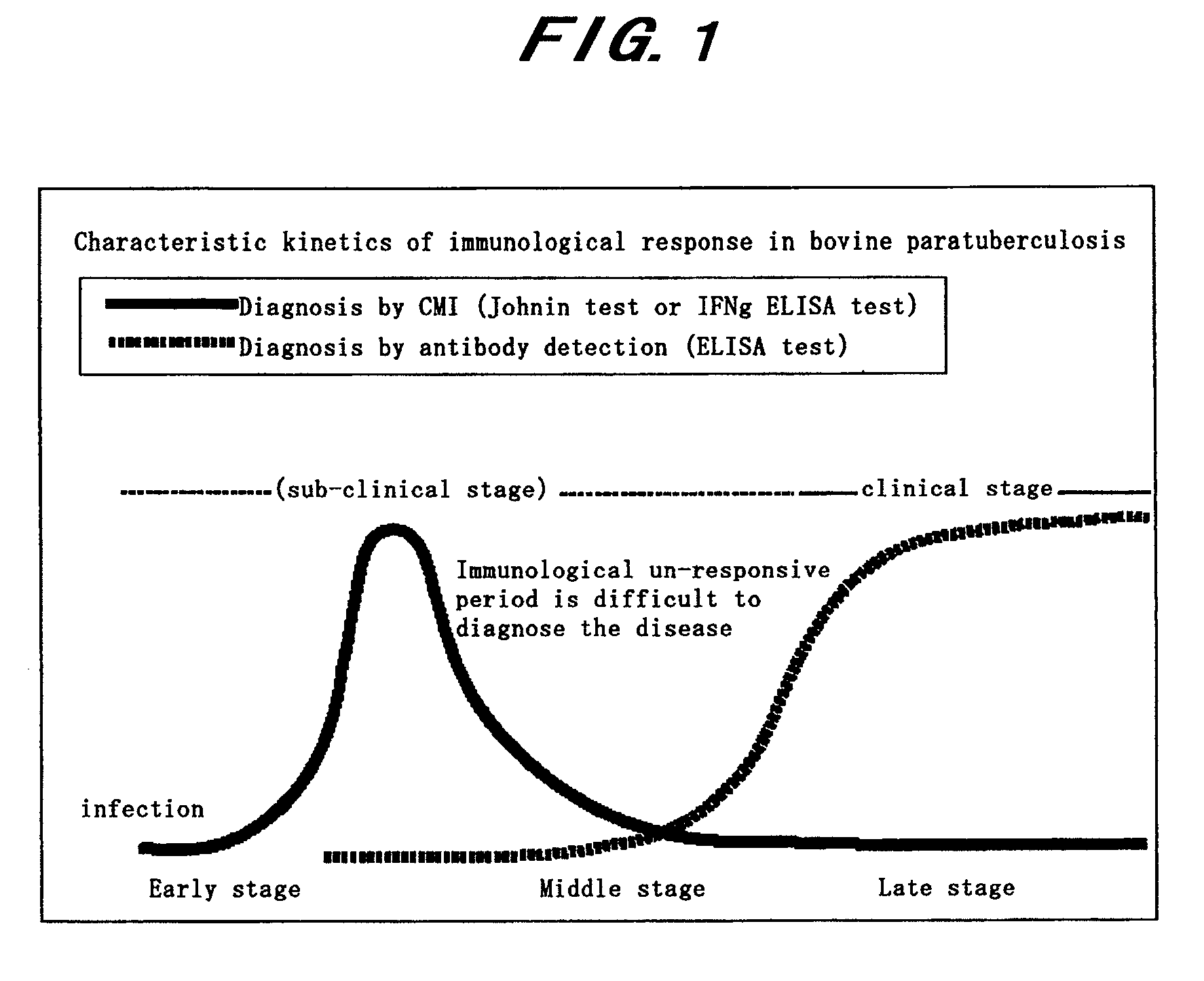
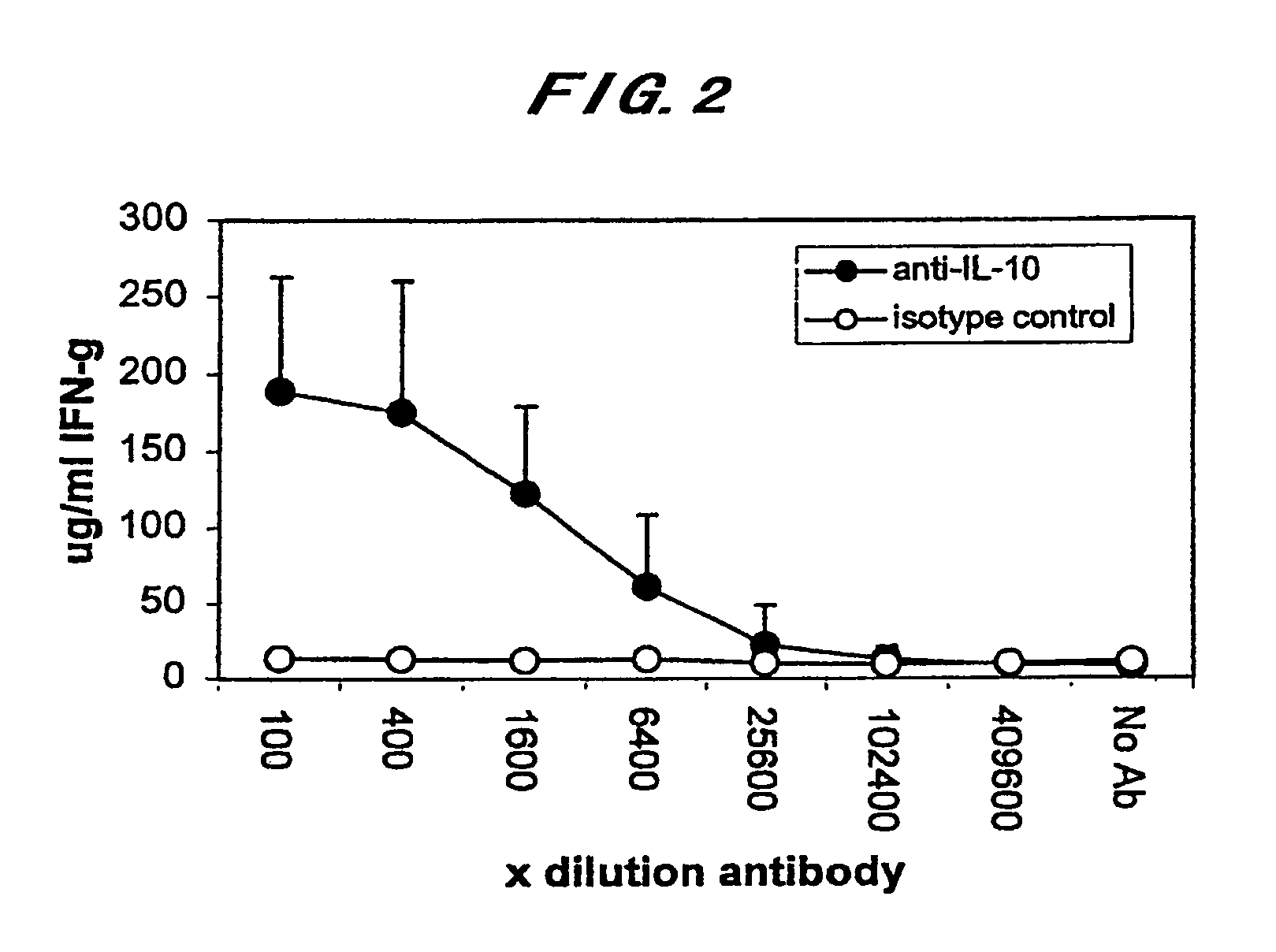
Diagnostic method for paratuberculosis
InactiveUS7731937B2Increase productionImprove responseBacterial antigen ingredientsPeptide/protein ingredientsAntigenMycobacterium Infections
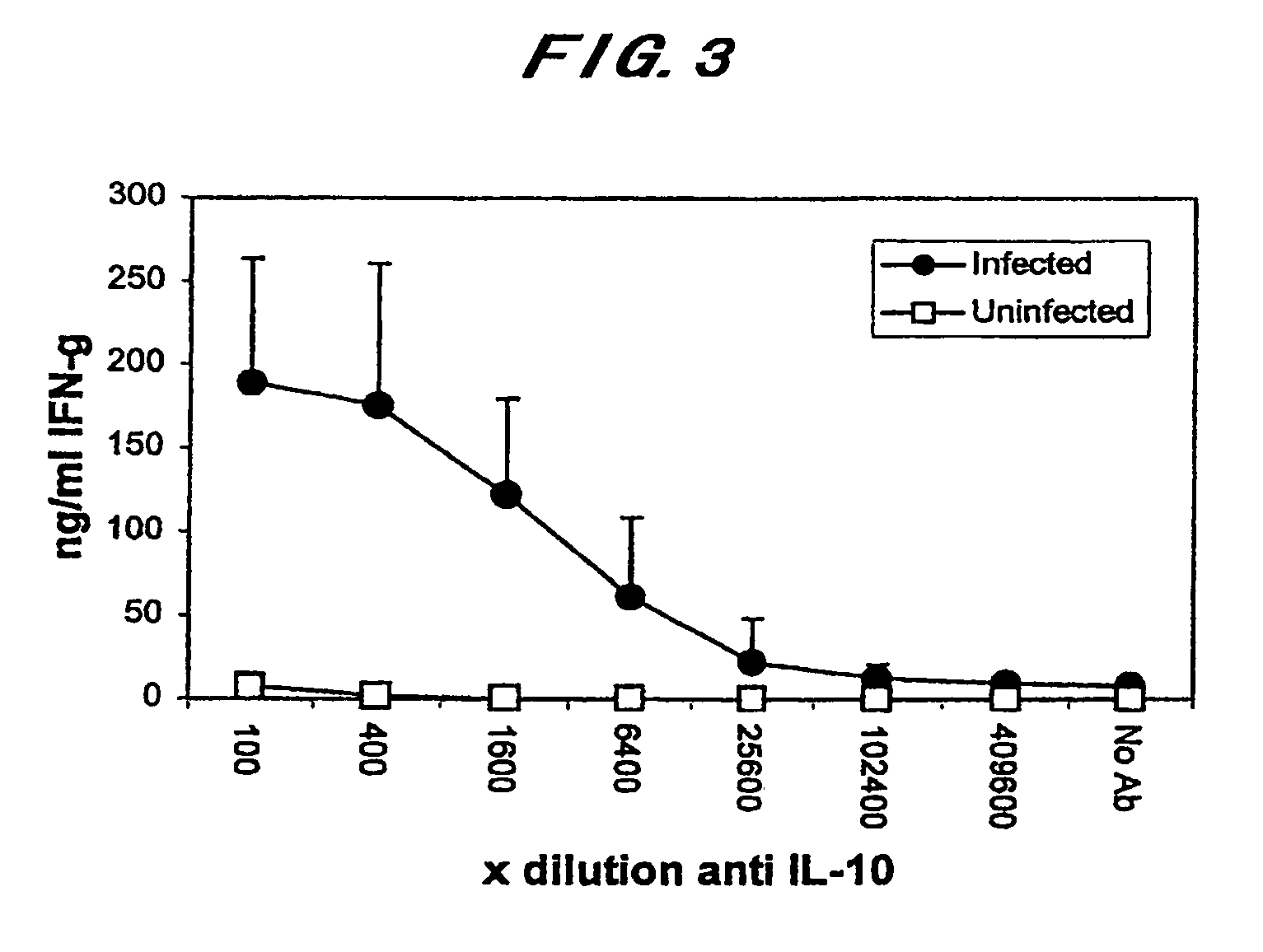
The present invention is intended to provide a diagnostic method for paratuberculosis by which an animal infected with Mycobacterium avium subsp. Paratuberculosis can be diagnosed at a high sensitivity in the sub-clinical infection stage before the specific antibody level begins to increase and a large number of specimens can be treated. The present invention provides: a diagnostic method for paratuberculosis characterized by collecting the blood of a subject animal, adding an anti-IL-10 antibody and a Mycobacterium avium subsp. Paratuberculosis antigen to the collected blood followed by culture, and then measuring the amount of produced IFNγ in the blood; a diagnostic method for paratuberculosis characterized in that the amount of produced IFNγ in blood is measured by the IFNγ ELISA method; and a diagnostic method for mycobacterial infection characterized by collecting the blood of a subject animal, adding an anti-IL-10 antibody and a mycobacterial antigen to the collected blood followed by culture, and then measuring the amount of produced IFNγ in the blood.
Owner:INC ADMINISTRATIVE AGENCY NAT AGRI & BIO ORIENTED RES ORG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com