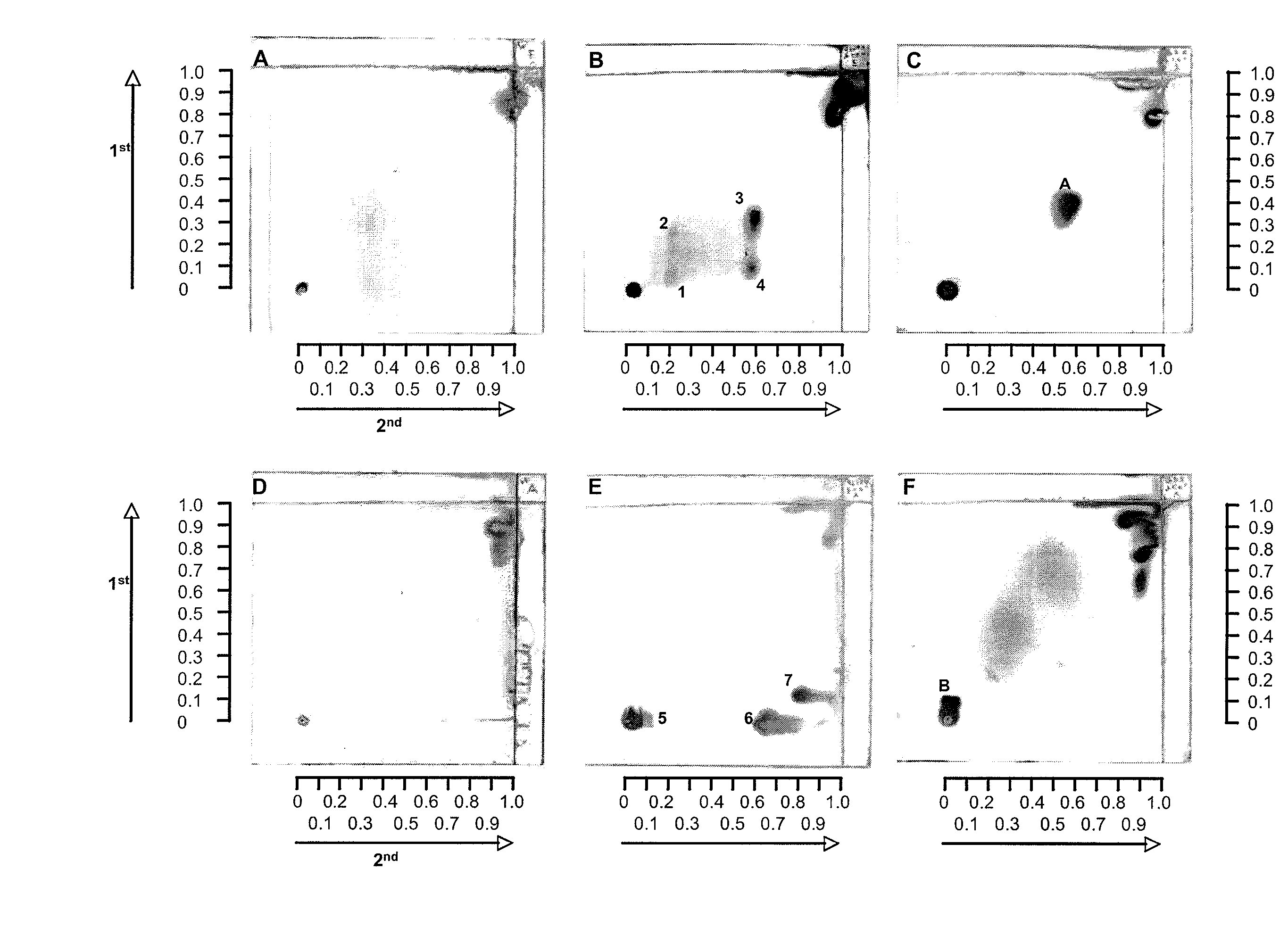
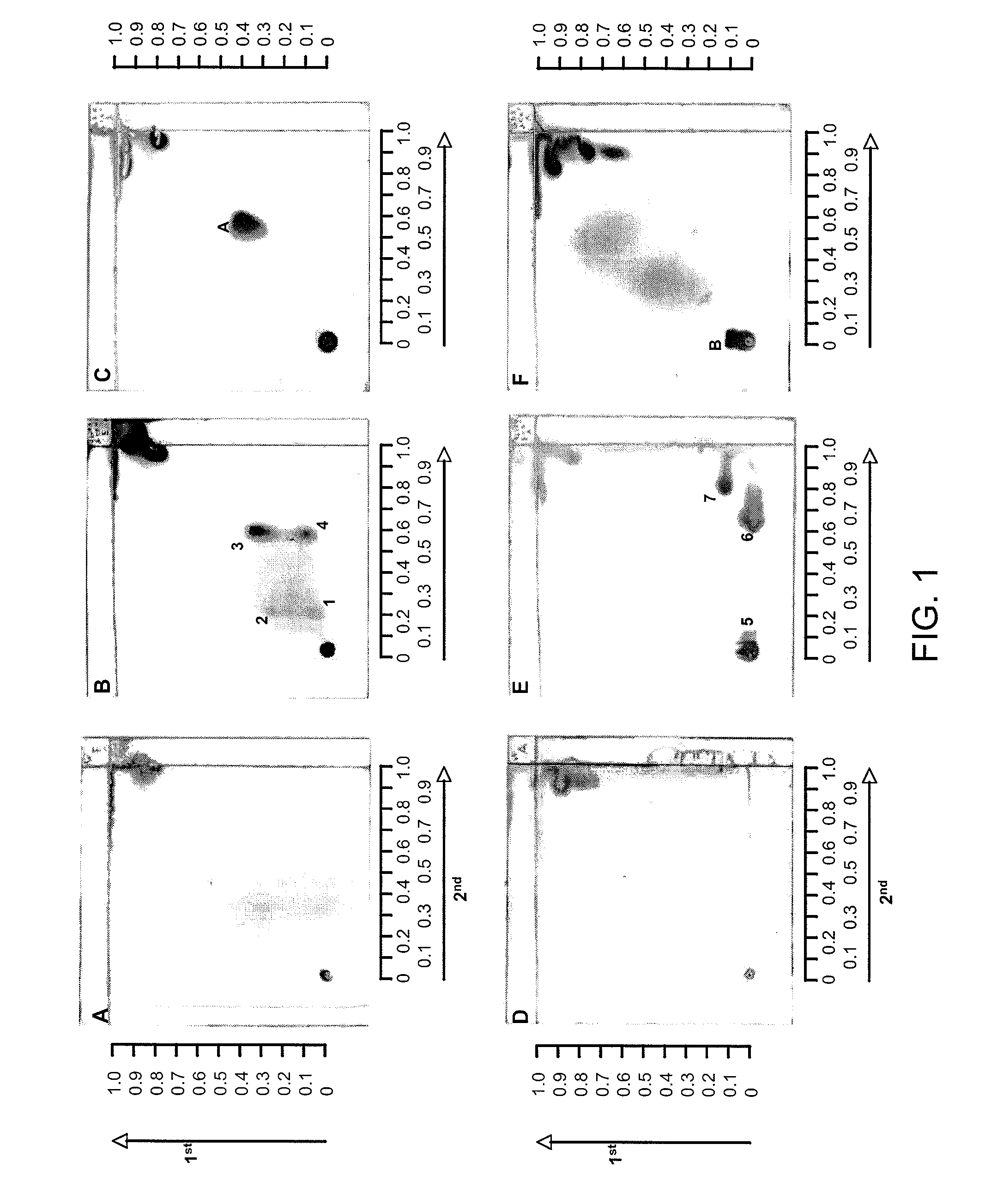
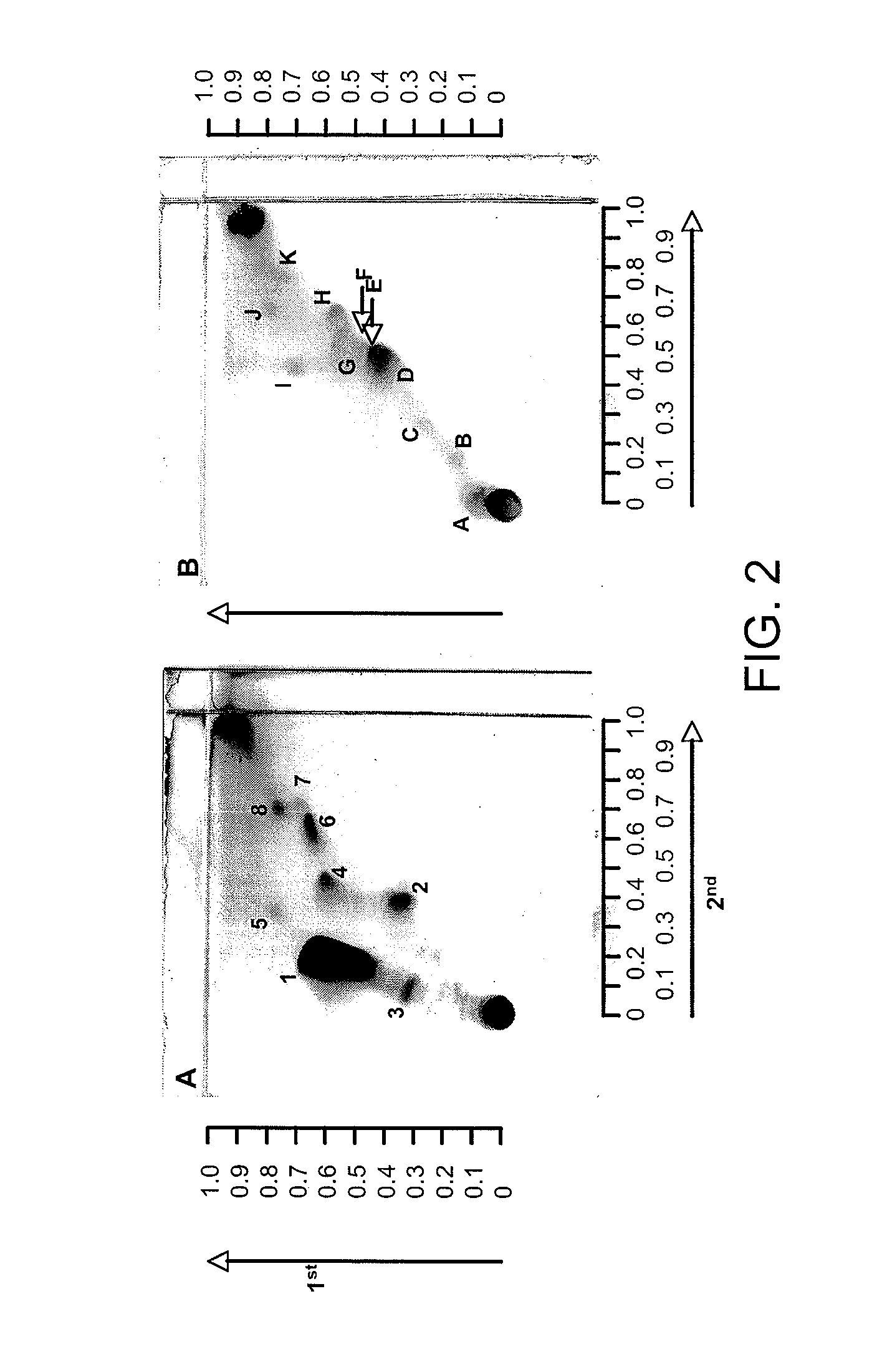
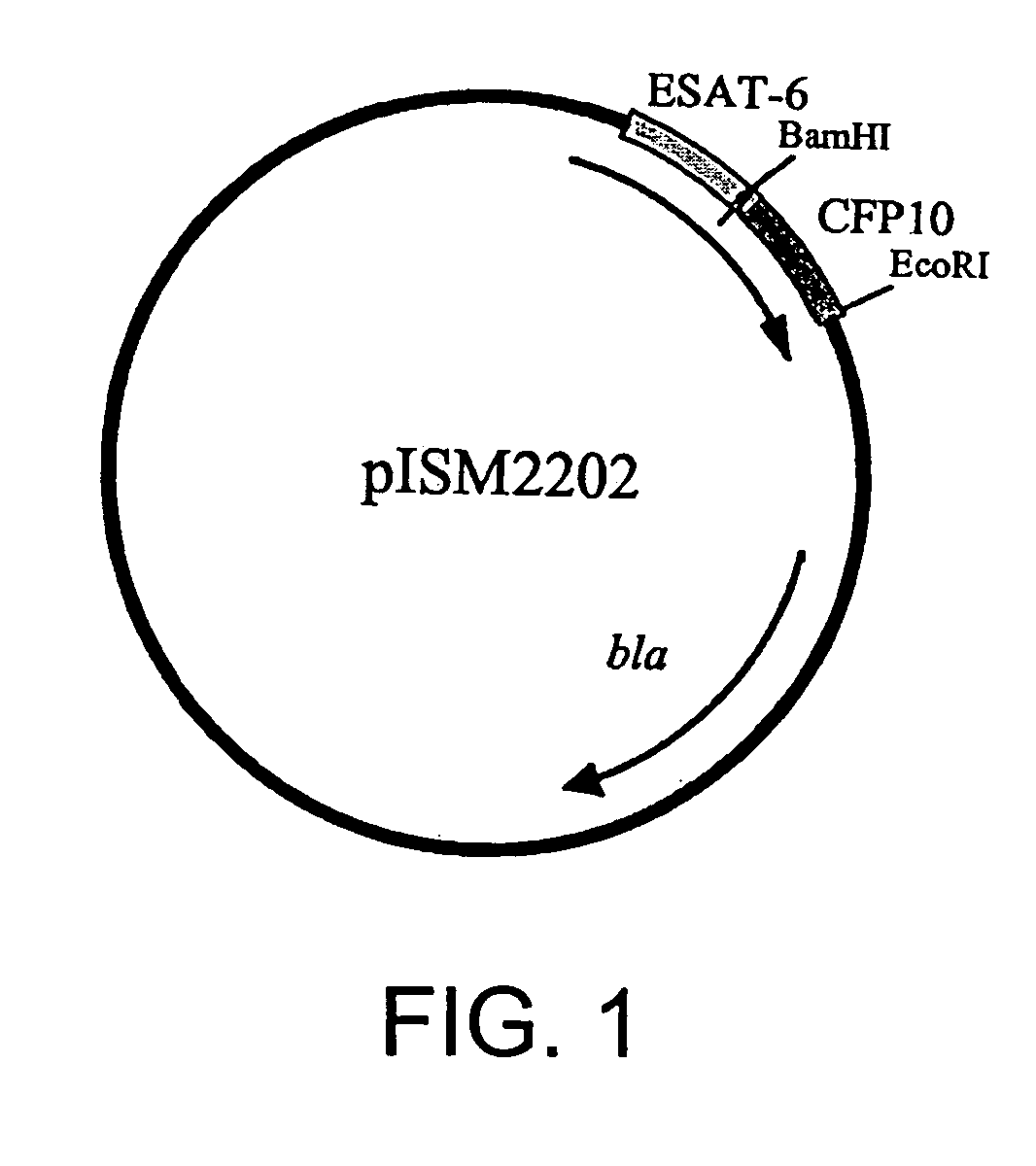
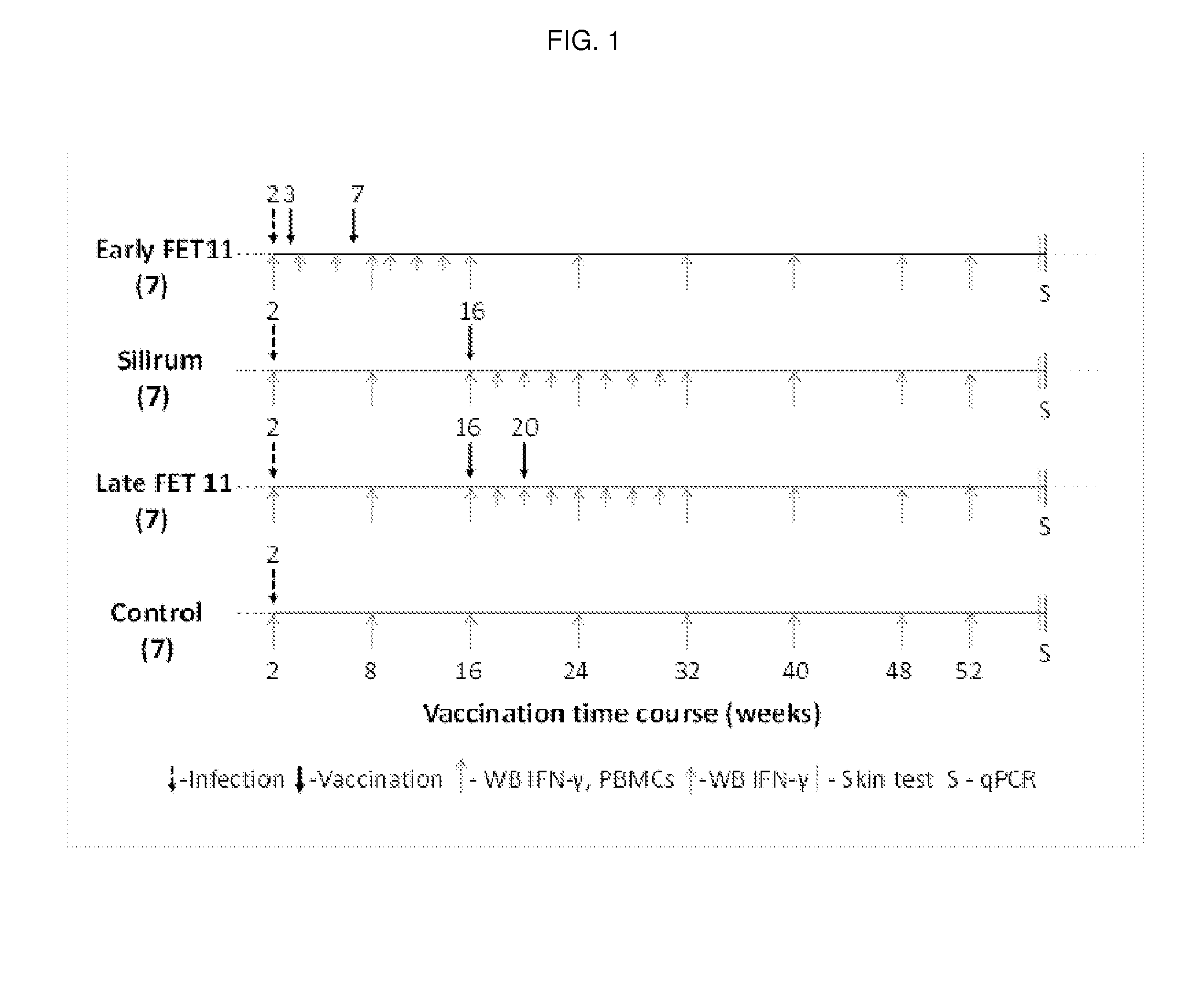
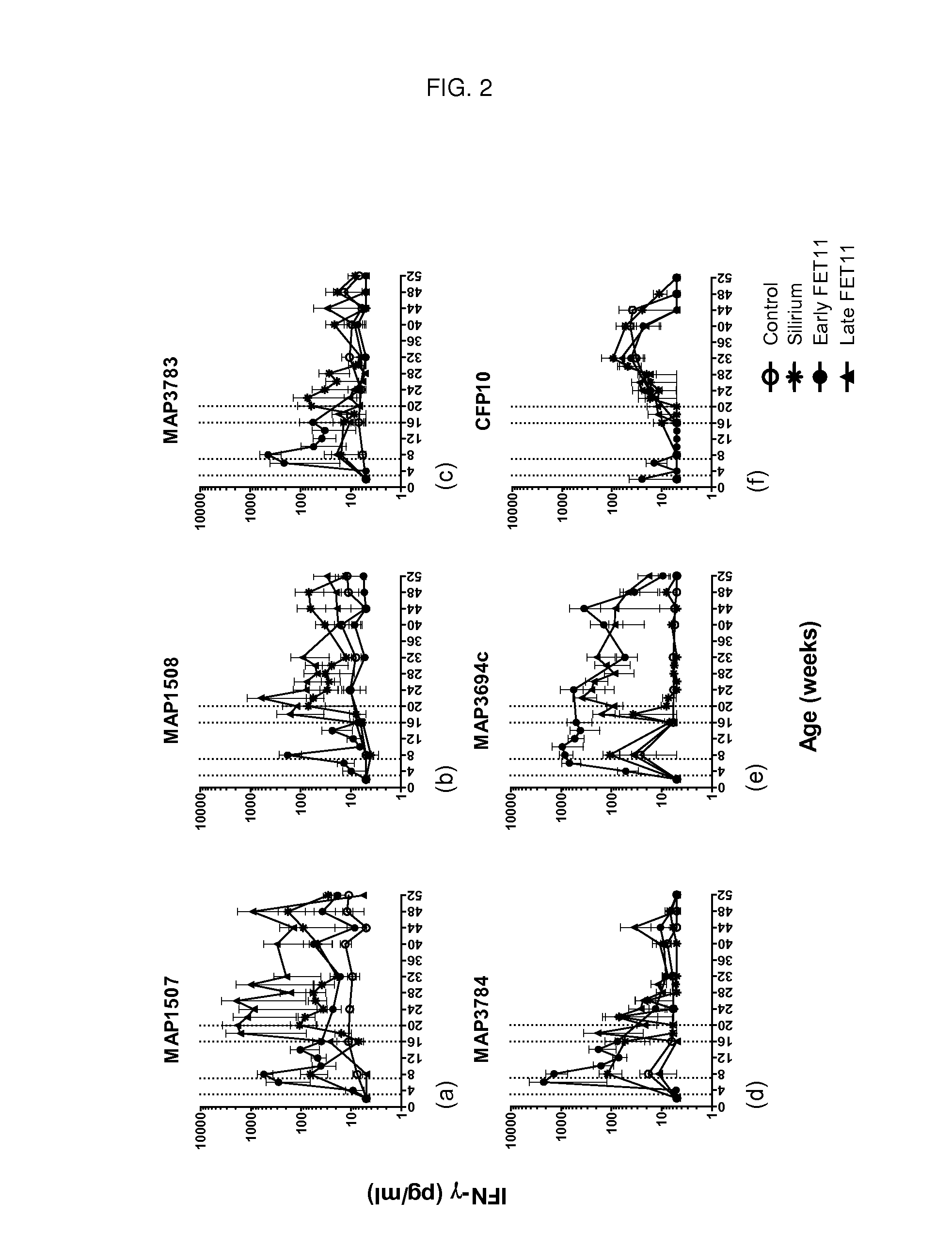
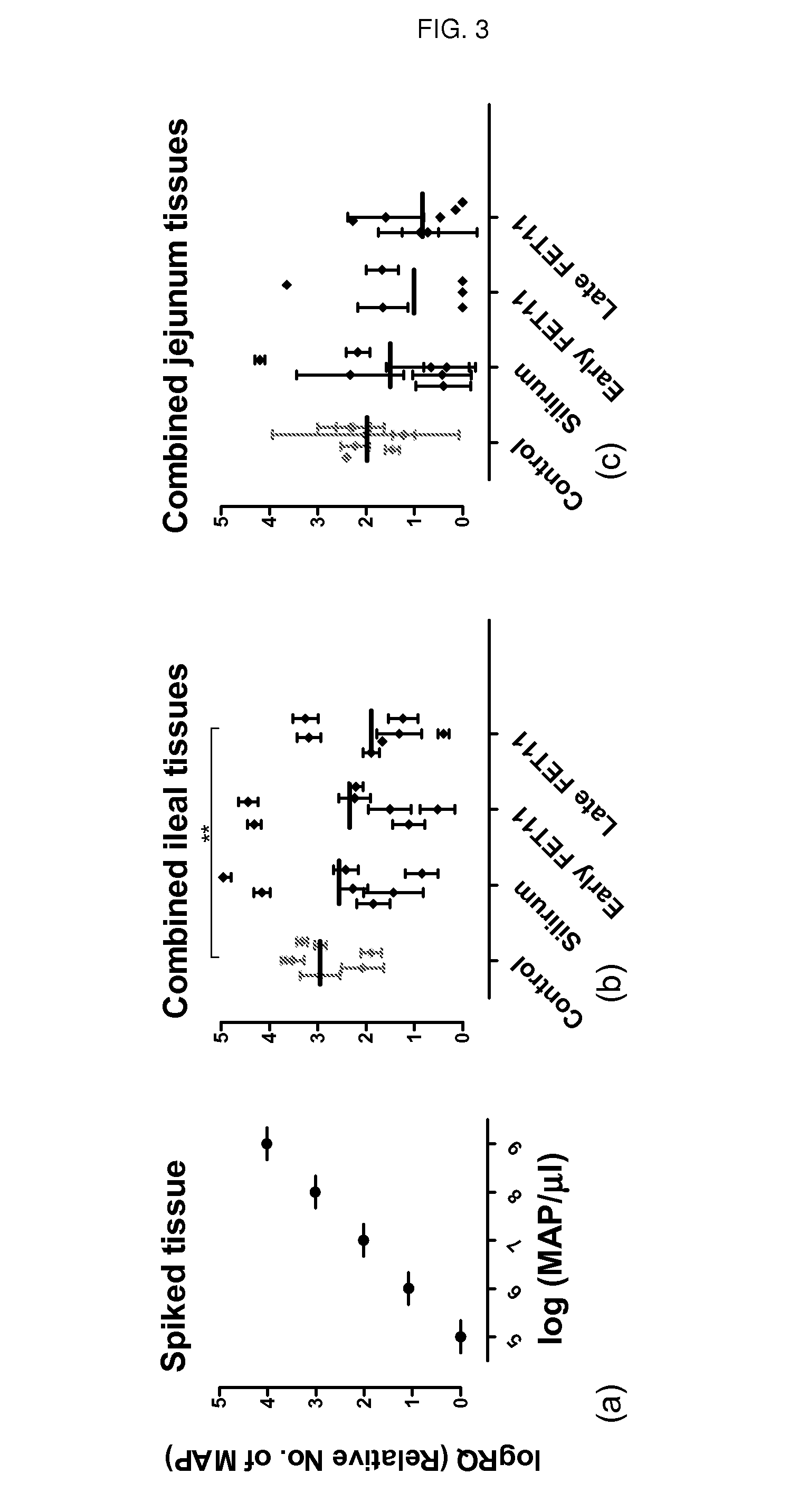
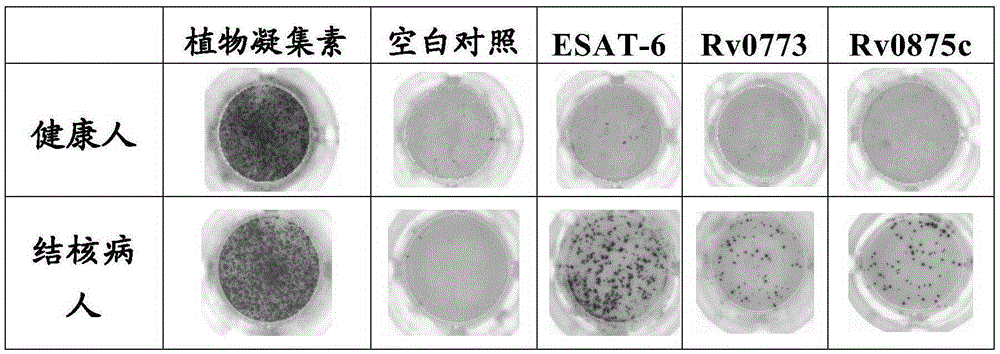
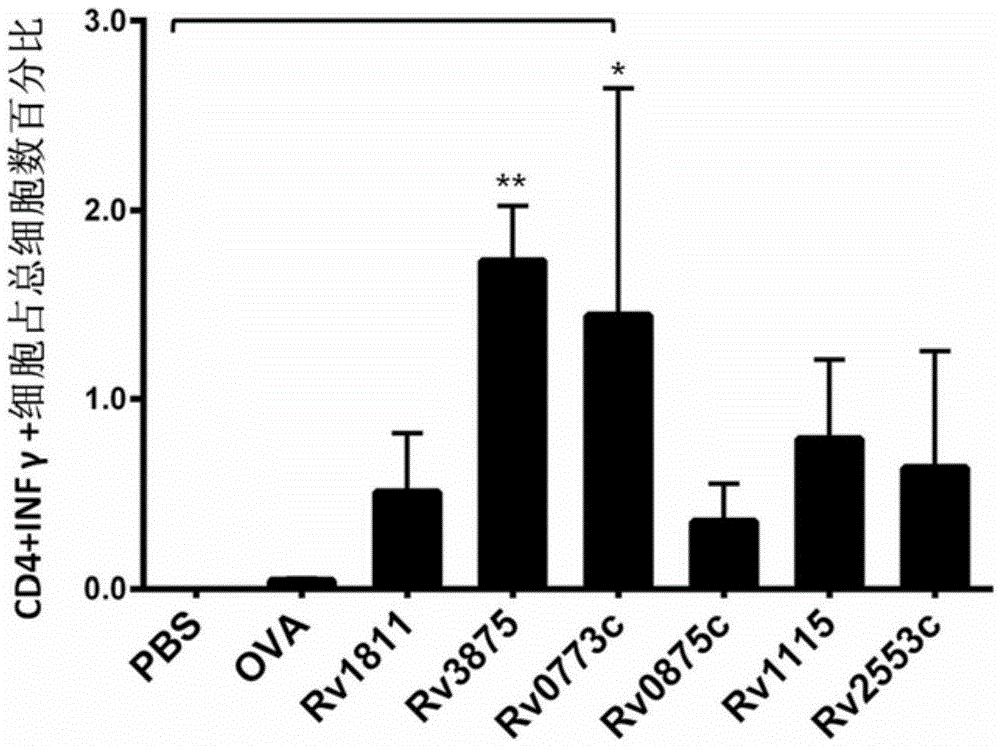
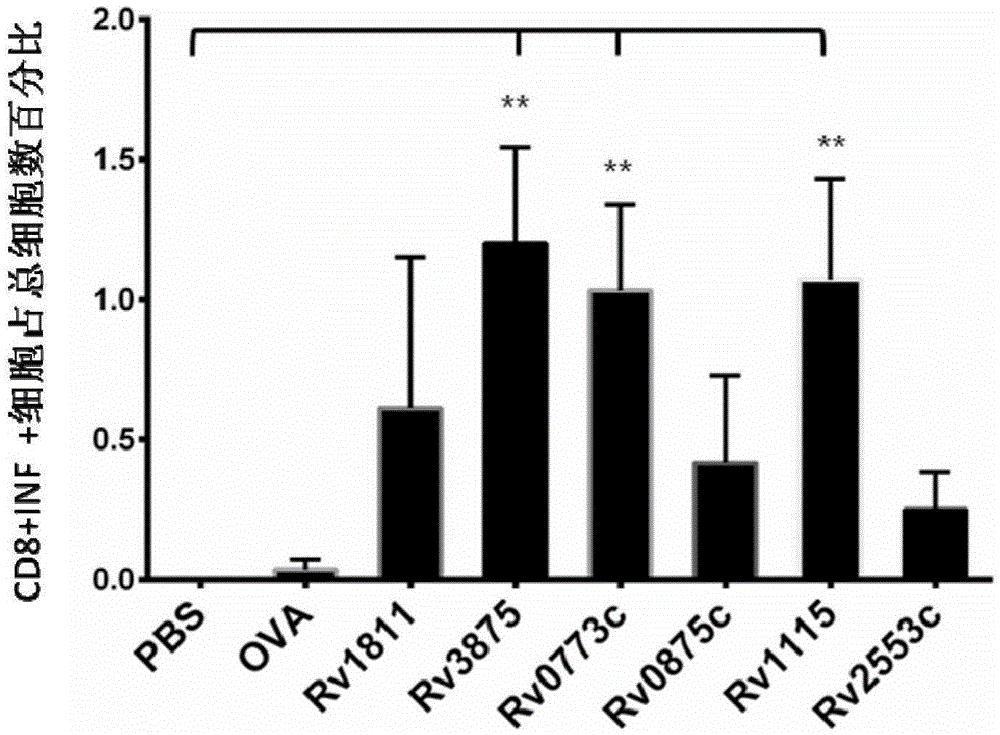
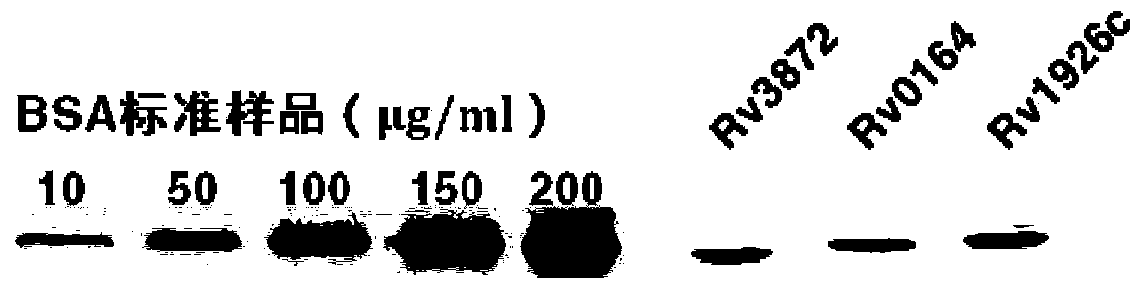Patents
Literature
77 results about "Paratuberculosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Paratuberculosis is a contagious, chronic and sometimes fatal infection that primarily affects the small intestine of ruminants. It is caused by the bacterium Mycobacterium avium subspecies paratuberculosis. Infections normally affect ruminants (mammals that have four compartments of their stomachs, of which the rumen is one), but have also been seen in a variety of nonruminant species, including rabbits, foxes, and birds. Horses, dogs, and nonhuman primates have been infected experimentally. Paratuberculosis is found worldwide, with some states in Australia (where it is usually called bovine Johne's disease or BJD) being the only areas proven to be free of the disease.
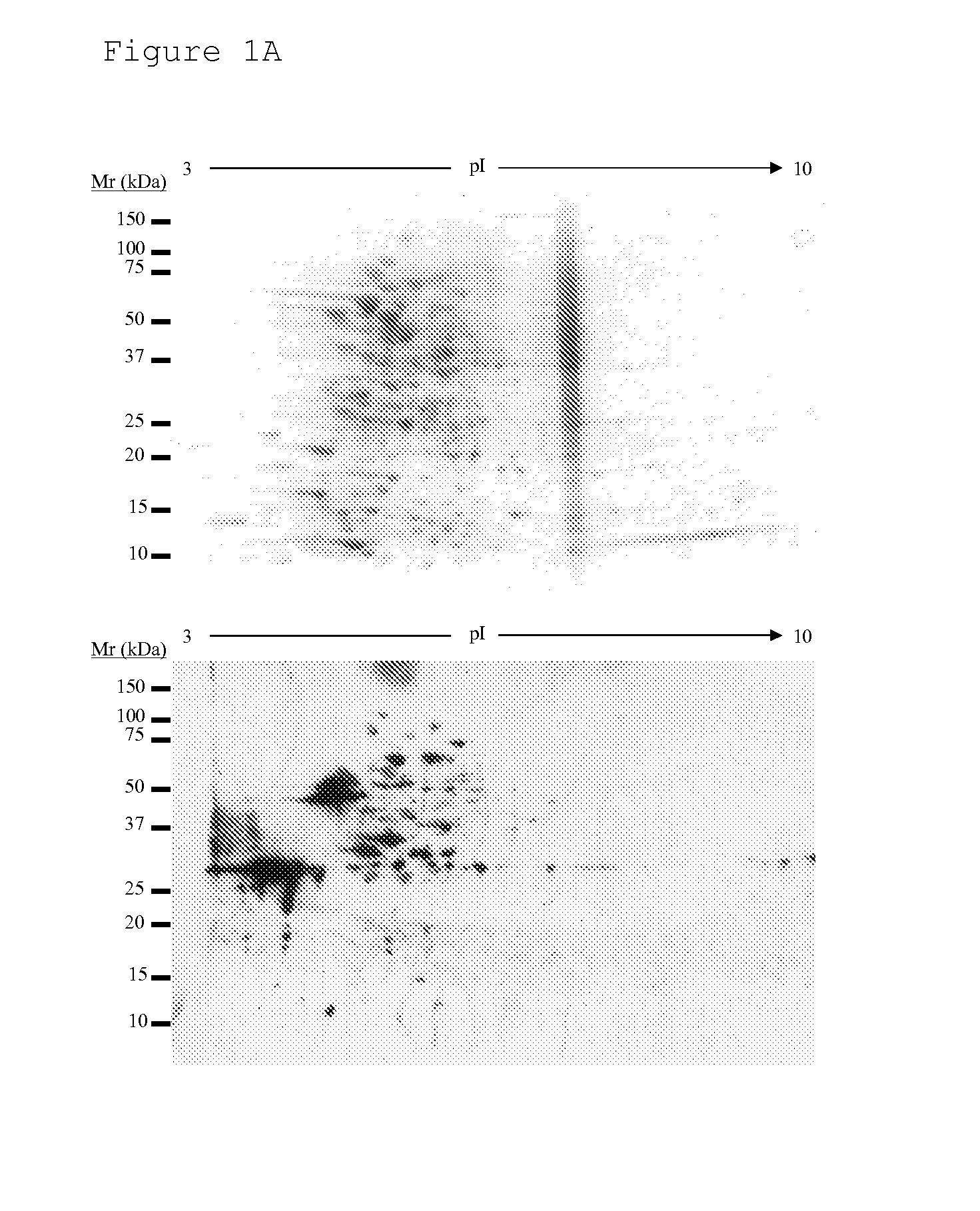
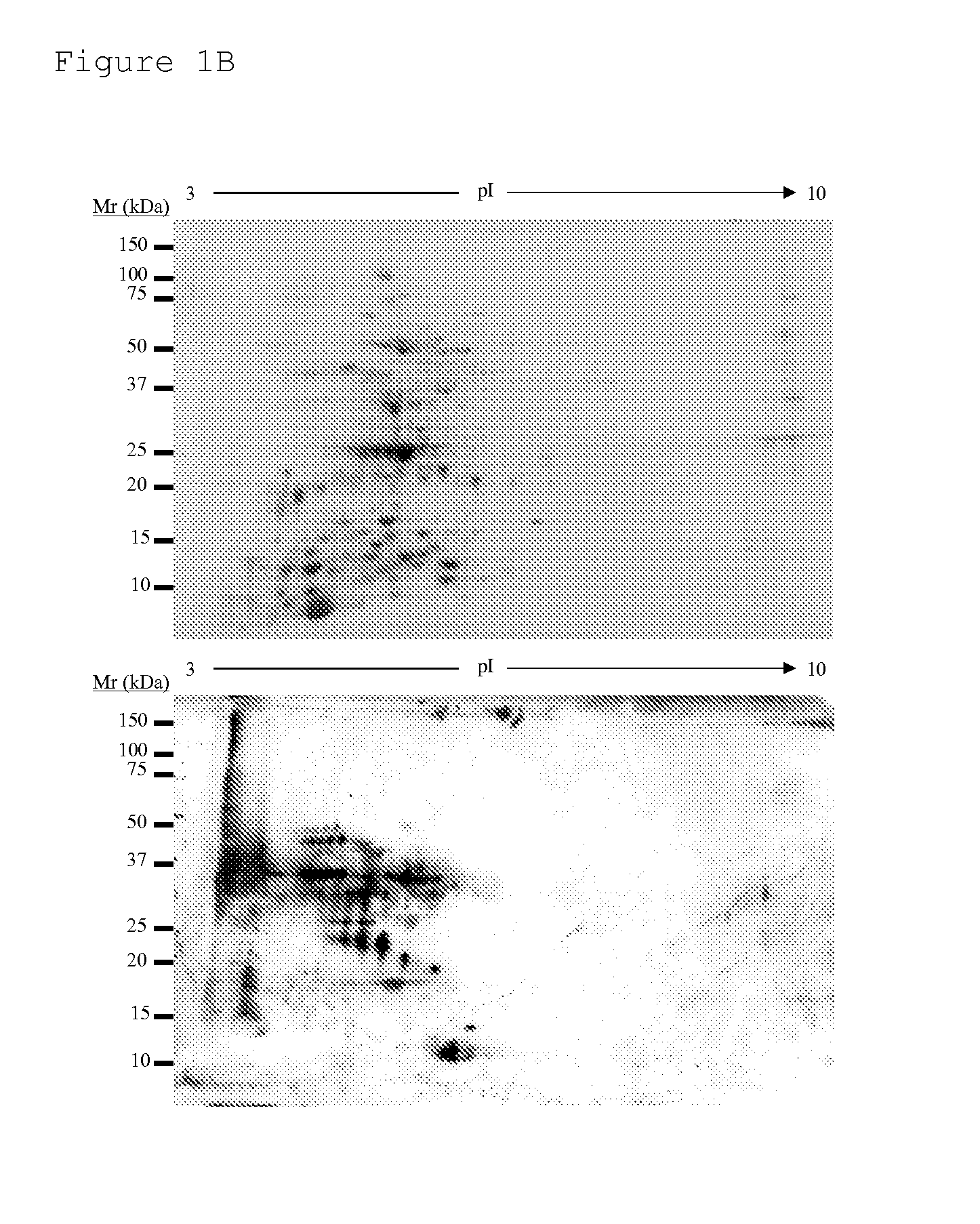
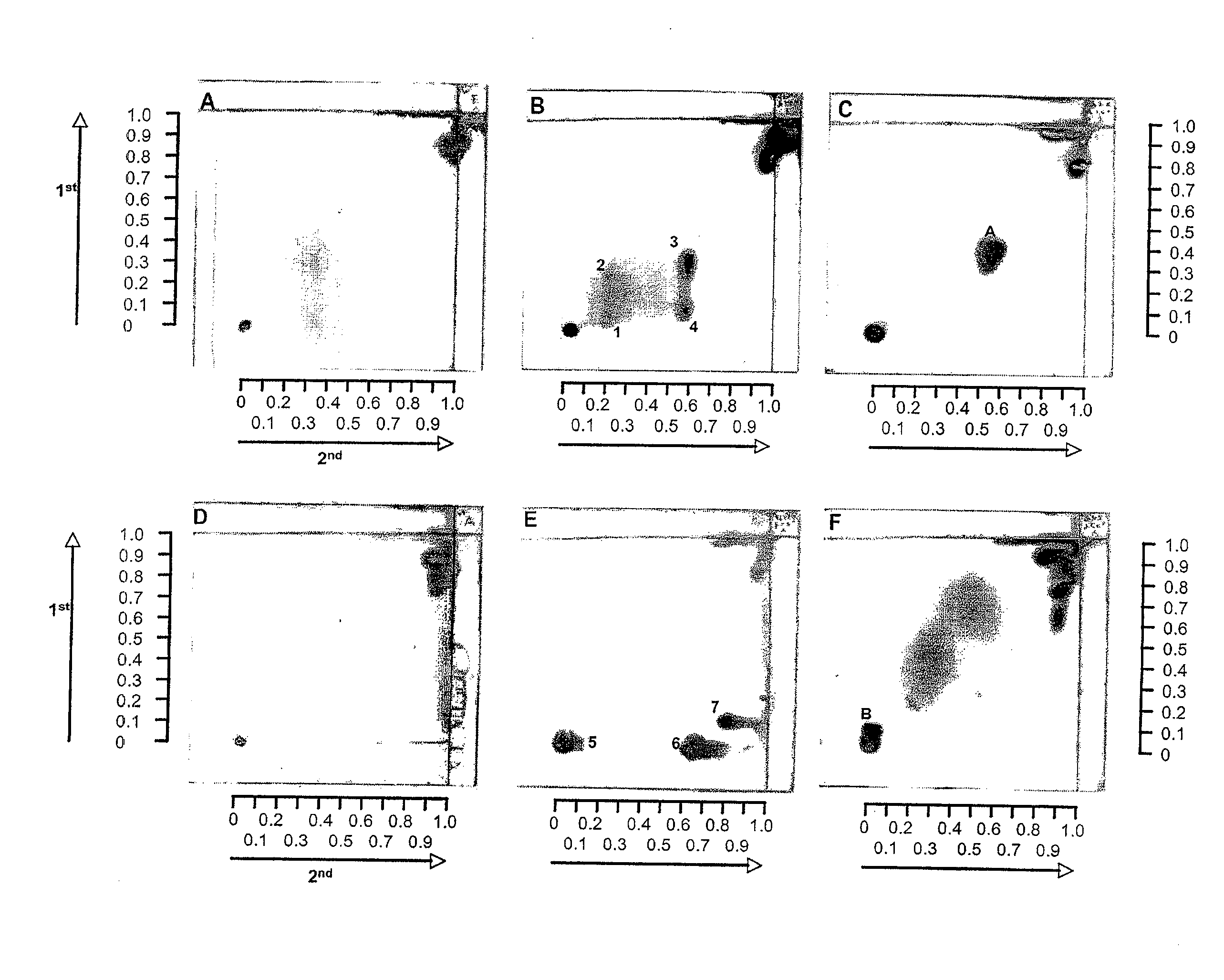
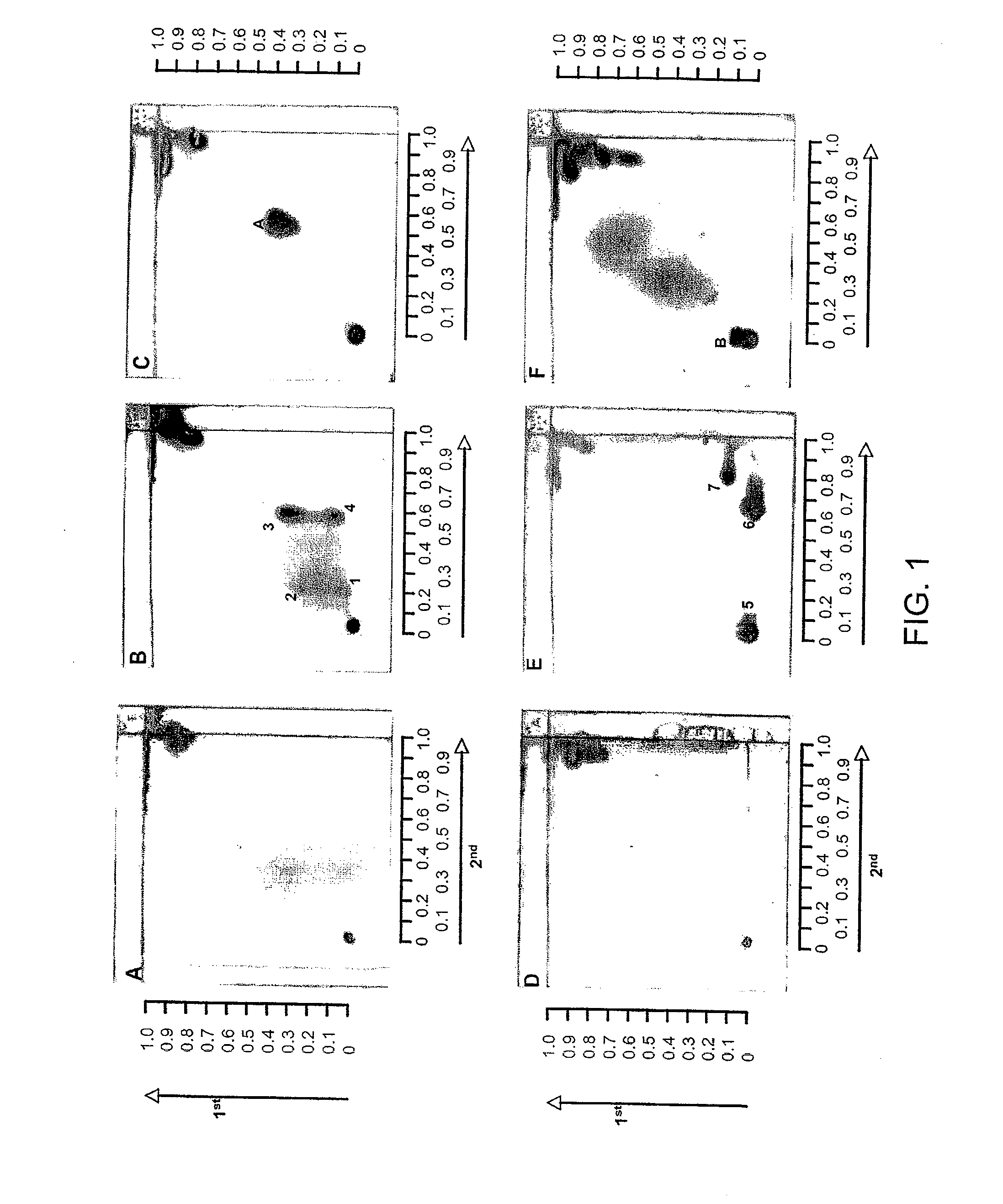
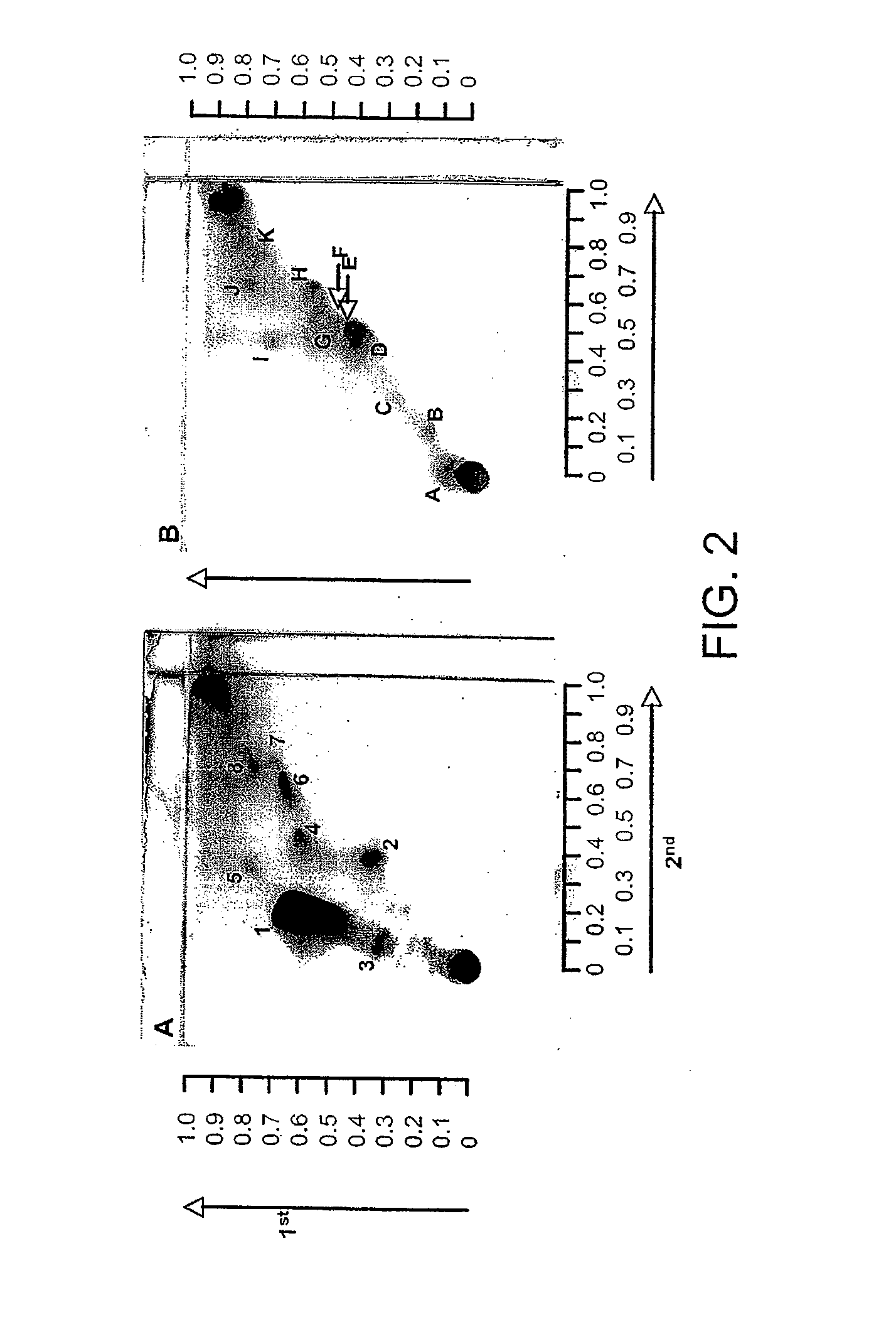
Identification of bacterial species and subspecies using lipids
InactiveUS20080108104A1Reduce in quantityMicrobiological testing/measurementBiological material analysisLipid formationParatuberculosis
The use of free, extractable lipids found in bacteria for identification of bacterial species and subspecies is described. Bacteria have been found to differ sufficiently in their extracted lipid compositions to effect identification using thin layer chromatographic techniques. Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia mallei have been distinguished in this manner. Lipopeptides specific to Mycobacterium avium subspecies paratuberculosis, but not to the closely related bacterium Mycobacterium avium subspecies avium have also been used as a basis for bacterial subspecies identification using mass spectrometry and seroreactivity. Mass spectrometric analysis of total bacterial lipids of Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia mallei, and mass spectrometric analysis of total bacterial lipids for Mycobacterium avium subspecies paratuberculosis and Mycobacterium avium subspecies avium, without further lipid separation, has shown that species and subspecies of bacteria may be identified using such analysis.
Owner:COLORADO STATE UNIVERSITY
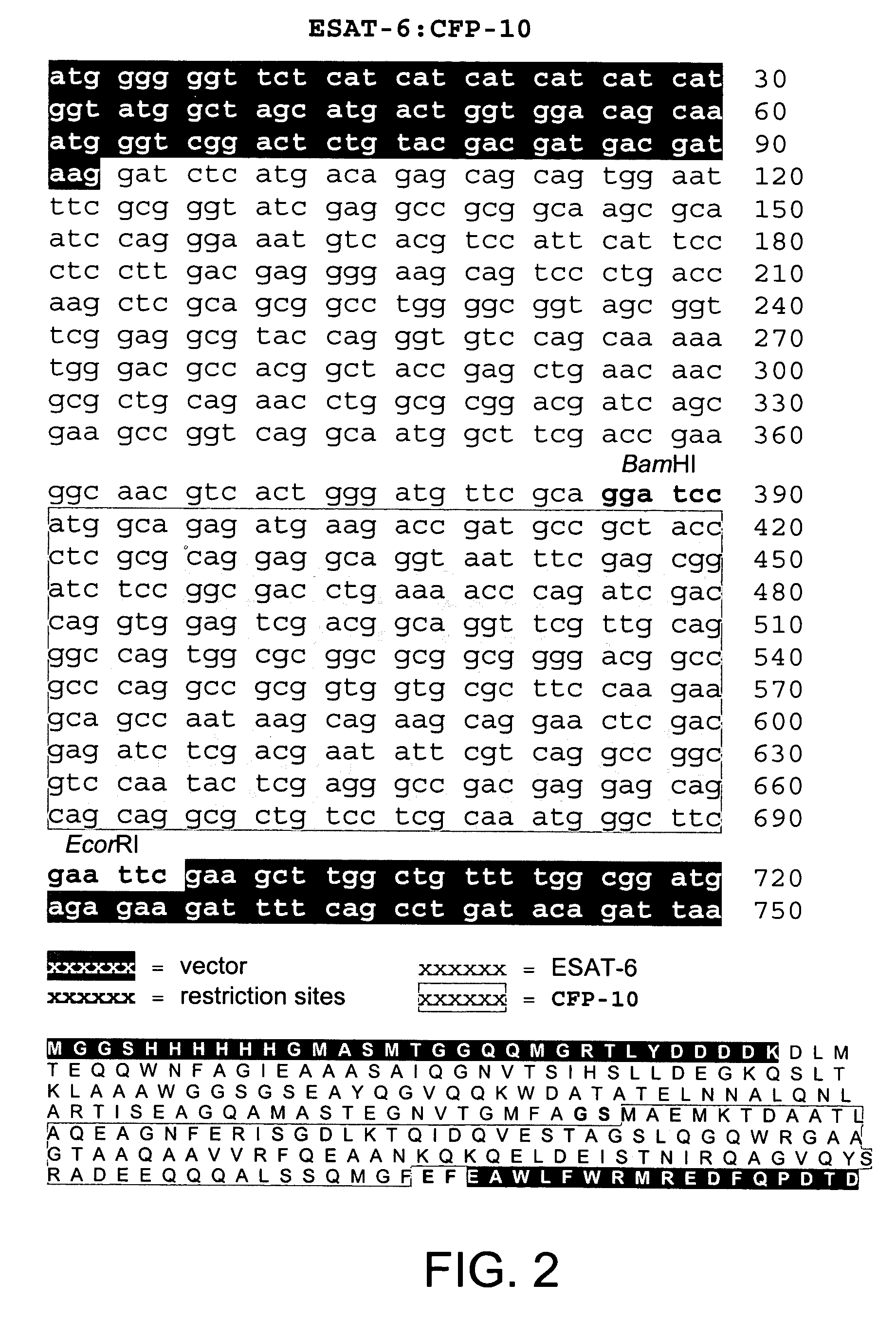
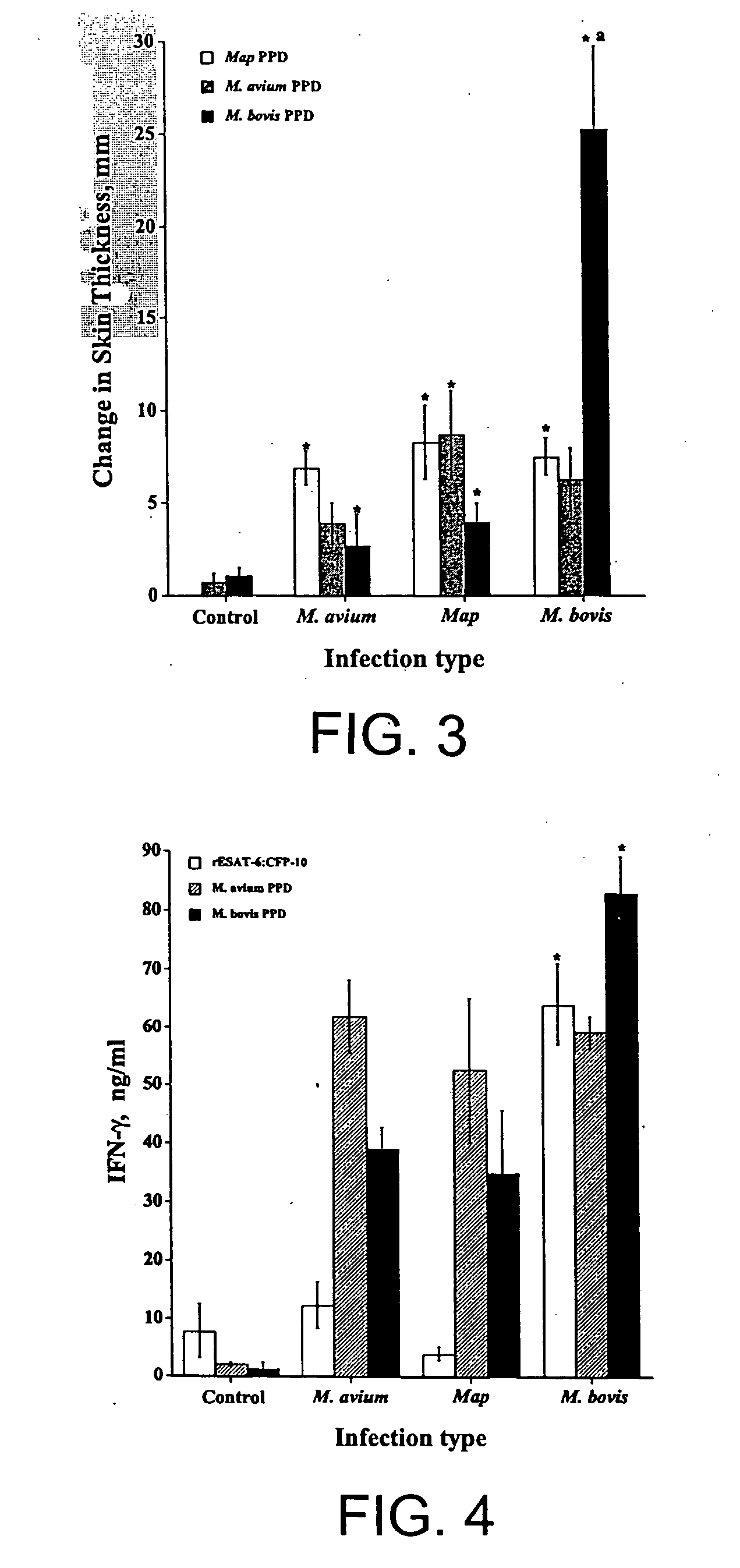
Recombinant ESAT-6:CFP-10 fusion protein useful for specific diagnosis of tuberculosis
InactiveUS20060024332A1Avoid unnecessary slaughterEasy to testBacterial antigen ingredientsAntibody mimetics/scaffoldsDiseaseNitric oxide
The fusion protein rESAT-6:CFP-10 is useful for differentiating infection of an animal with Mycobacterium bovis from exposure of the animal to other species of Mycobacteria, especially M. avium and M. avium subspecies paratuberculosis (Map). Cells stimulated with the fusion protein are capable of eliciting a variety of in vivo and in vitro responses (e.g. hypersensitivity skin response, IFN-γ, nitric oxide, and TNF-α) indicative of M. bovis infection. This invention will facilitate diagnosis of tuberculosis in cattle, reindeer and other susceptible animal species, thereby preventing unnecessary slaughter of uninfected animals suspected of having of the disease.
Owner:UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF AGRI THE
Serodiagnosis model establishing method for active tuberculosis disease
InactiveCN101424661AEasy to set upCreate method quicklyMaterial analysis by electric/magnetic meansVenous bloodData acquisition
The invention relates to an establishing method of a serum diagnosing model of active tuberculosis, which has the following steps: step 1 is a serum source taking and preparing step which comprises an experiment group and a control group, the venous blood of a checked person is collected, serum and the rest are separately stored after serum separation at the temperature of -80 DEG C in a refrigerator for spare use; step 2 is the step of the mass spectrometric analysis of the serum and comprises sample treatment, protein plate check, spectrometric analysis and data acquisition; step 3 is the step of statistic analysis and the difference protein screening; step 4 is the step of establishing the diagnosing model, and Biomarker Pattern 5.0 software is used for establishing the model of diagnosing active tuberculosis. When the model is used for identifying the active tuberculosis and the control group, the sensitivity is 96.9 percent, the specificity is 97.8 percent, the positive predicating value and the negative predicating value are 97.7 percent and 97.1 percent, and the total accurate rate is 97.3 percent.
Owner:中国人民解放军总医院第二附属医院 +1
Method of identification of animals resistant or susceptible to disease such as ruminant brucellosis, tuberculosis, paratuberculosis and salmonellosis
InactiveUS6114118AInexpensiveSimple technologyMicrobiological testing/measurementFermentationParatuberculosisRuminant animal
The present invention relates to materials and methods for identifying animals that are resistant or susceptible to diseases associated with intracellular parasites such as brucellosis, tuberculosis, paratuberculosis and salmonellosis. More particularly, the present invention relates to the identification of a gene, called NRAMP1, which is associated with the susceptibility or resistance of an animal, such as an artiodactyla to diseases such as brucellosis, tuberculosis, paratuberculosis and salmonellosis. Still more particularly, the present invention relates to the identification of specific sequences of bovine NRAMP1 which associate with resistance or susceptibility to ruminant brucellosis, tuberculosis, paratuberculosis and salmonellosis, and to the method of identifying said sequences to identify animals who are susceptible or resistant to disease.
Owner:MCGILL UNIV +1
A single or multistage mycobacterium avium subsp. paratuberculosis subunit vaccine
InactiveUS20160228528A1Improved protective immunityImprove immunityAntibacterial agentsBacterial antigen ingredientsParatuberculosisMycobacterium avium avium
The present invention provides one or more immunogenic polypeptides for use in a preventive or therapeutic vaccine against latent or active infection in a human or animal caused by a Mycobacterium species, e.g. Mycobacterium avium subsp. paratuberculosis. Furthermore a single or multi-phase vaccine comprising the one or more immunogenic polypeptides is provided for administration for the prevention or treatment of infection with a Mycobacterium species, e.g. Mycobacterium avium subsp. paratuberculosis. Additionally, nucleic acid vaccines, capable of in vivo expression of the multi-phase vaccine comprising the one or more immunogenic polypeptides, is provided for prevention or treatment of infection with a Mycobacterium species, e.g. Mycobacterium avium subsp. paratuberculosis.
Owner:DANMARKS TEKNISKE UNIV +1
Specific marker of mycobacterium tuberculosis and application thereof
InactiveCN103063843AStrong specificityHigh activityMicroorganism based processesBiological testingParatuberculosisB cell
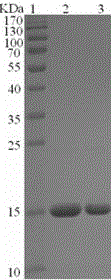
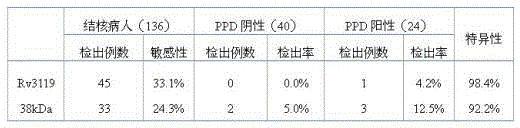
The invention discloses a specific marker (Rv3119 recombinant protein) of mycobacterium tuberculosis and discloses an application of the Rv3119 recombinant protein in preparation a tuberculosis diagnostic reagent and a tuberculosis diagnostic kit. The Rv3119 specific antigen provided by the invention has high sensitivity in detecting phthisis, is a B cell target antigen with relatively strong activity, can be applied in preparation of rapid detection kit for the tuberculosis, and is safe and reliable.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
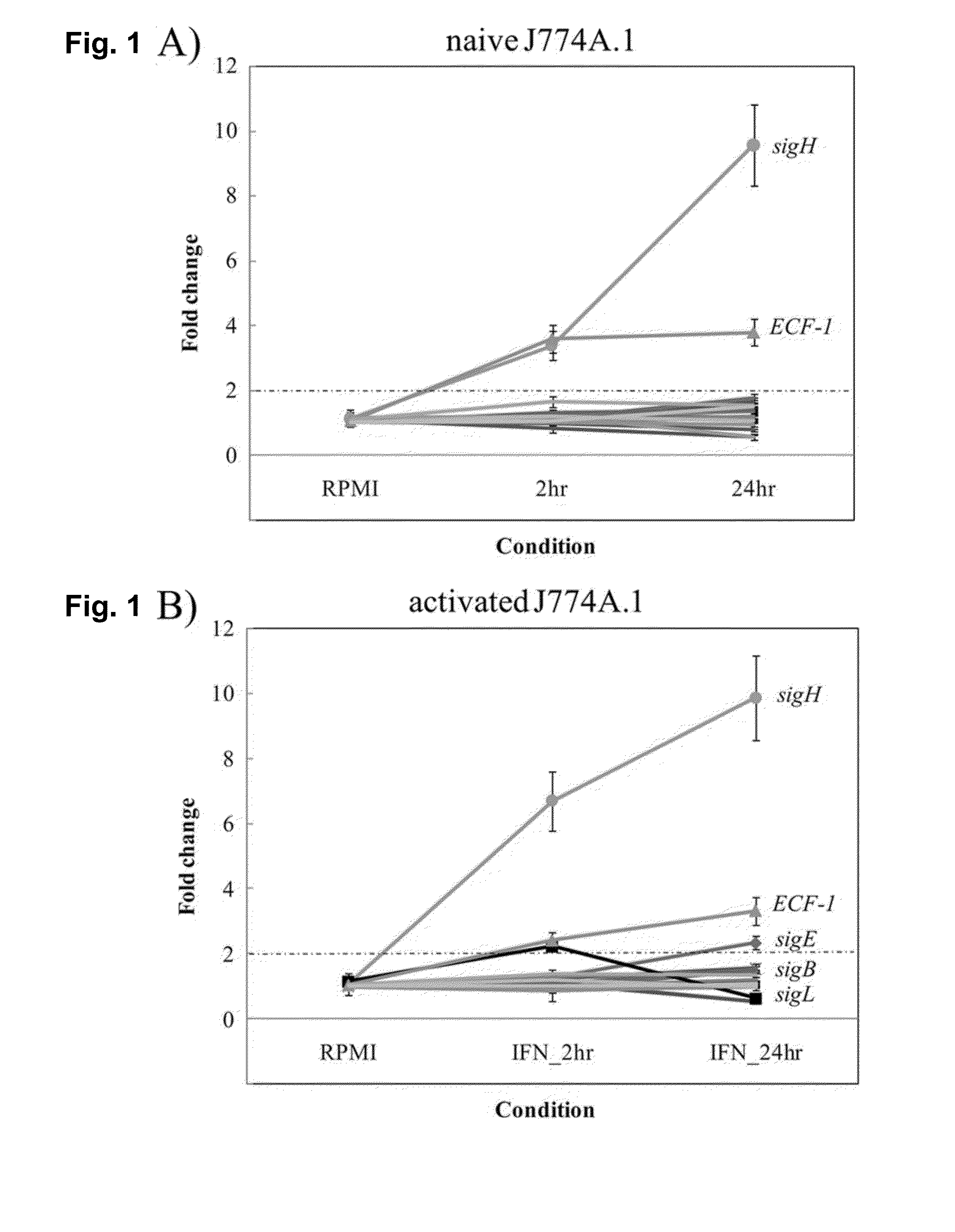
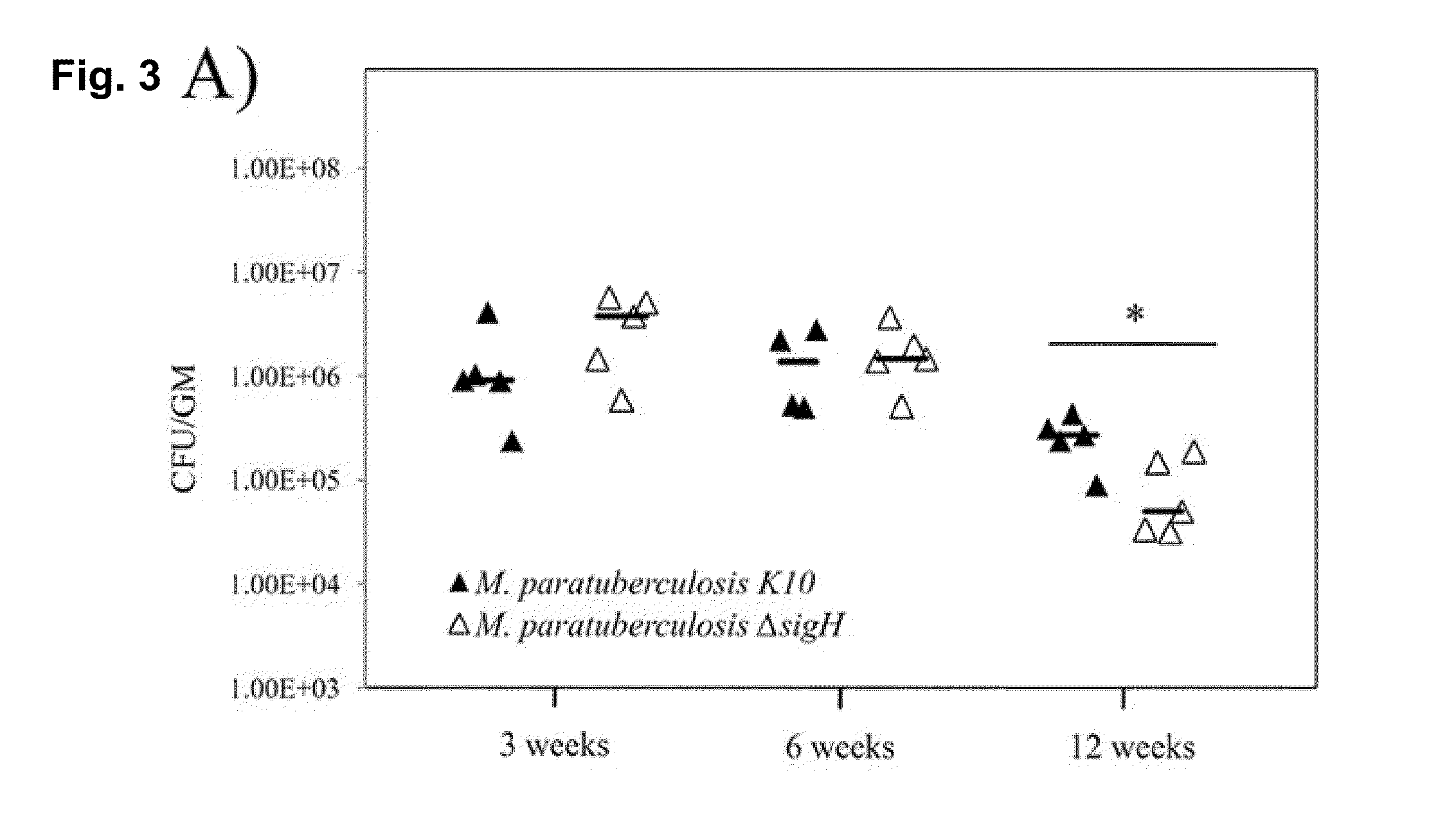
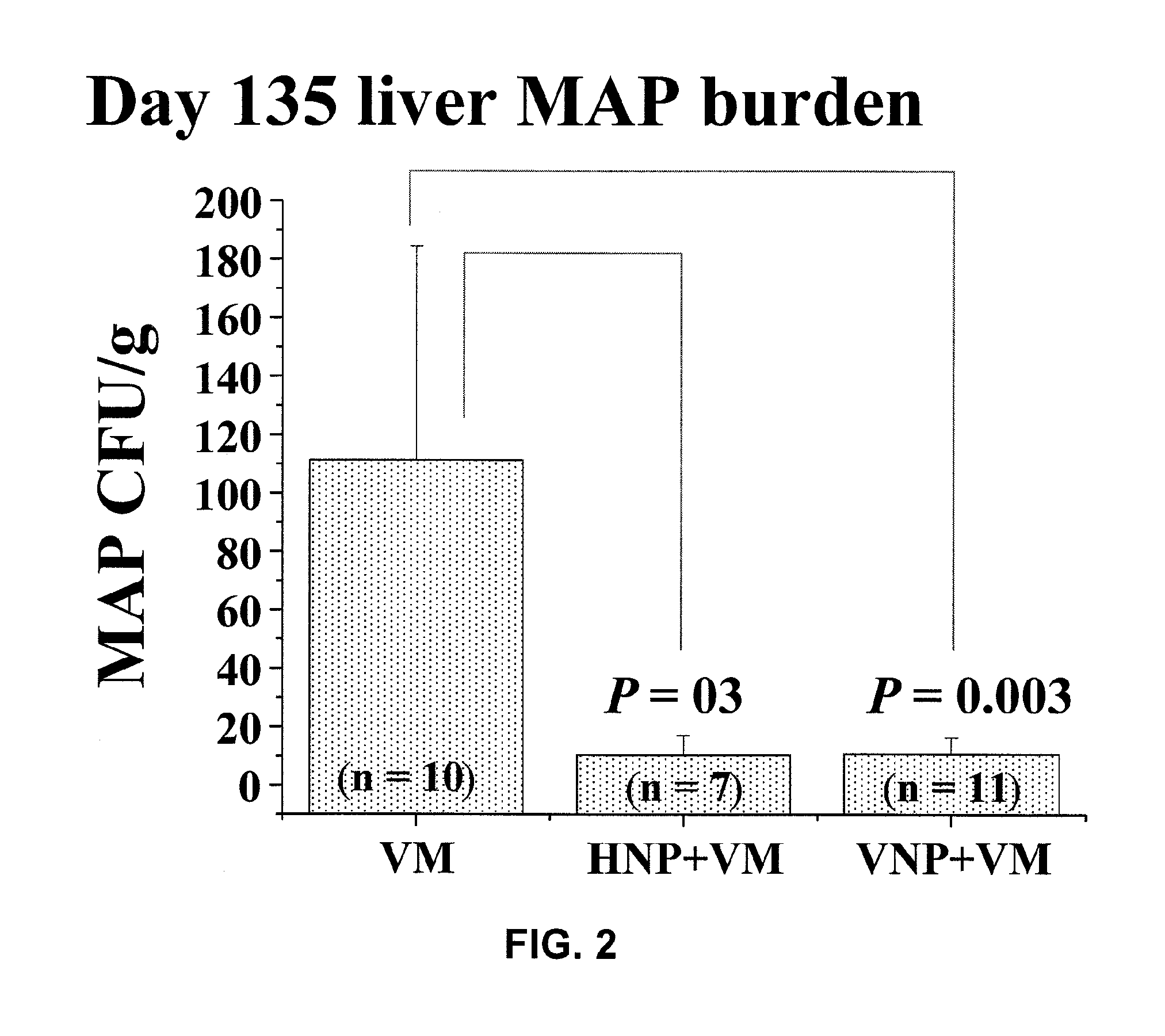
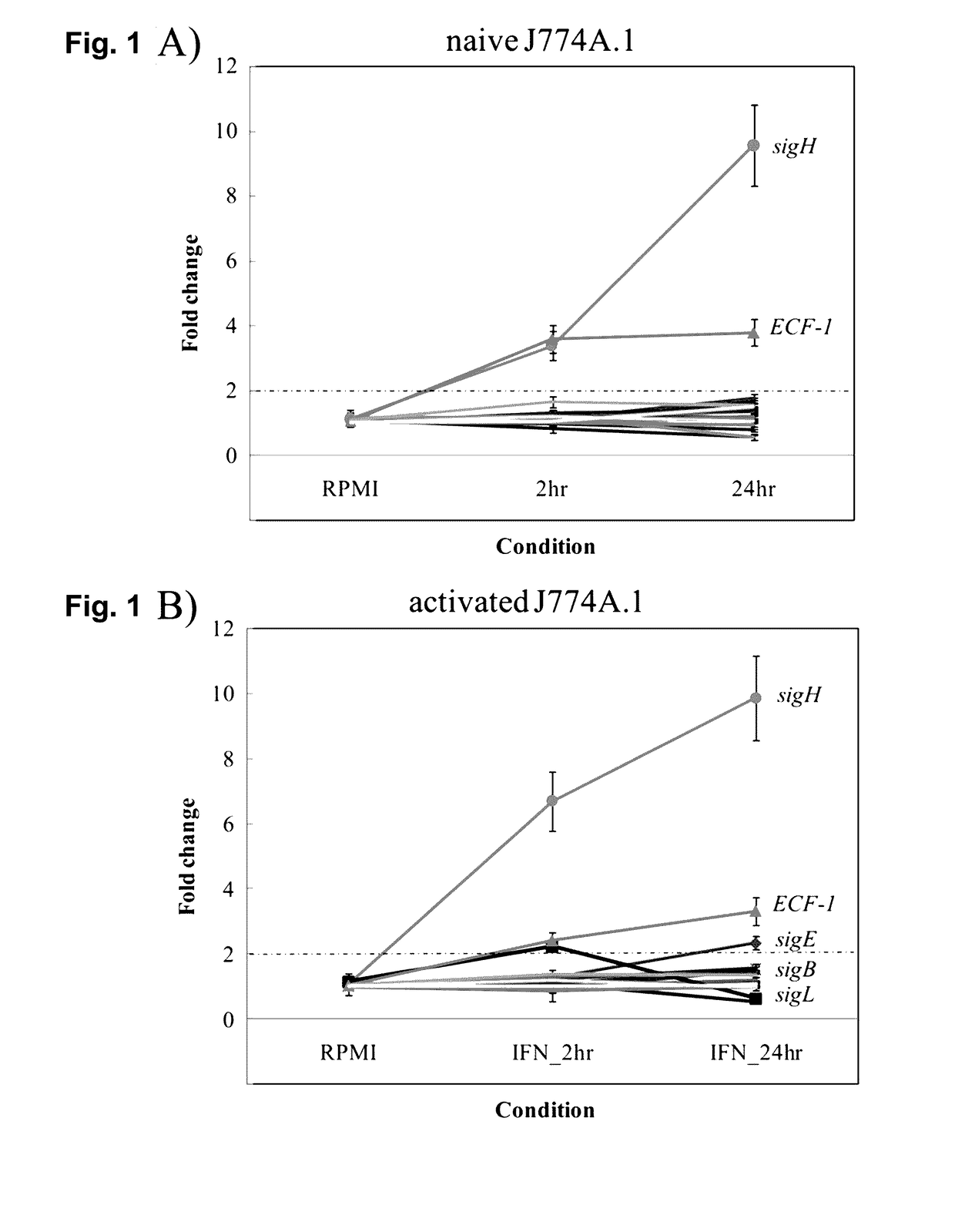
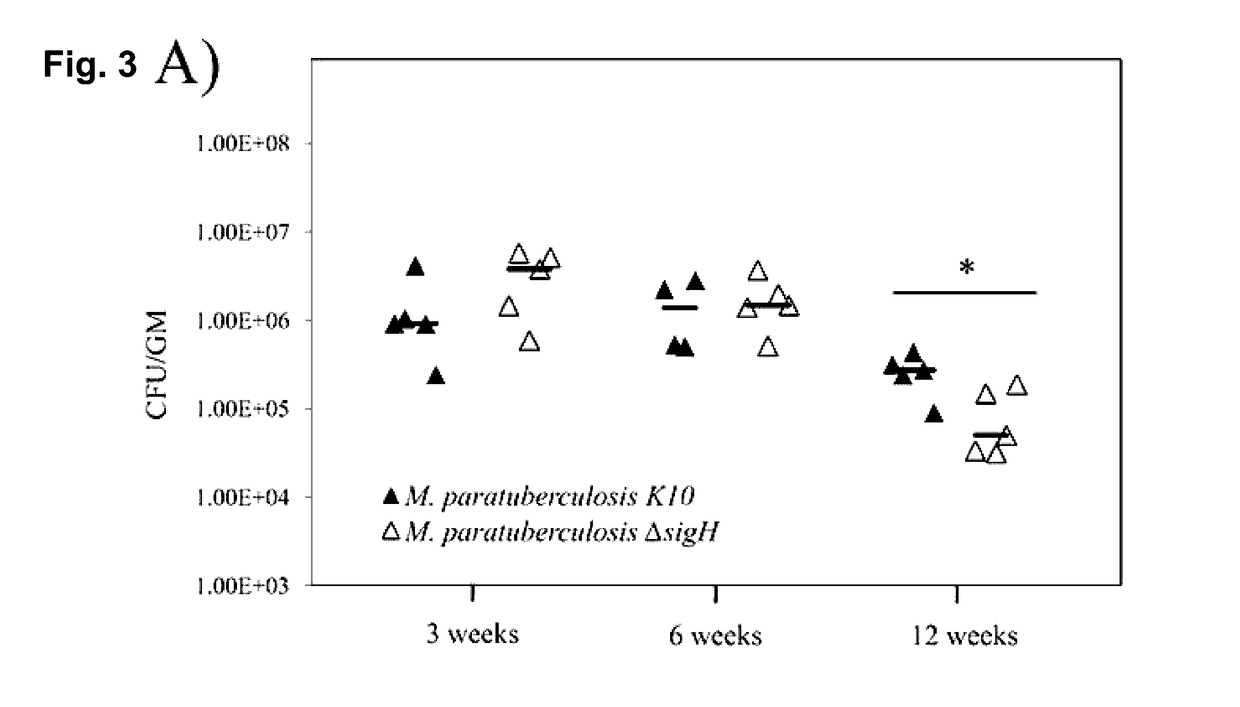
Global Gene Regulators (GGR) As Vaccine Candidates Against Paratuberculosis
Described herein is a mycobacterium mutant, comprising at least one mutation in at least one gene sequence encoding global gene regulators (GGRs) selected from the group consisting of sigH, sigL, sigE, ECF-1, and mixtures thereof, wherein the GGR gene is at least partially inactivated. Described herein also is a vaccine based on the mutant and a method of differentiating between subjects that have been infected with mycobacterium and subjects that have not been infected with mycobacterium or have been vaccinated with a mycobacterium vaccine.
Owner:WISCONSIN ALUMNI RES FOUND
miRNA marker for assistant diagnosis of tuberculosis and application thereof
The invention discloses a miRNA marker for assistant diagnosis of tuberculosis and application thereof. The invention requires the protection of the application of a substance for detecting miRNAs inserum exosomes in the preparation of products used for diagnosing or assisting the diagnosis of tuberculosis patients or for diagnosing or assisting the diagnosis of active tuberculosis patients, wherein each miRNA refers to one or a combination of the following five kinds of miRNAs: miR-28-3p, miR-193b-5p, miR-370-3p, miR-1246 and miR-2110. The invention also requires the application of the substance for detecting miRNAs in serum exosomes in the preparation of products used for diagnosing or assisting the diagnosis of patients with latent tuberculosis infections, wherein each miRNA refers toone or a combination of the following three kinds of miRNAs: let-7e-5p, let-7d-5p and miR-140-5p. A foundation is laid for further development of the early rapid diagnosis technology and method for tuberculosis.
Owner:BEIJING CHEST HOSPITAL CAPITAL MEDICAL UNIV
Curative effect evaluation reagent kit of phthisis and application of reagent kit
ActiveCN104808003AIncreased sensitivityImprove featuresDisease diagnosisBiological testingParatuberculosisTreatment effect
Owner:李继承
Paratuberculosis fluorescence PCR rapid diagnosis kit
InactiveCN101168783AQuick monitoringQuick solveMicrobiological testing/measurementParatuberculosisFluorescence
The invention discloses a paratuberculosis fluorescence PCR quick diagnosis reagent box, in particular relates to a method and a reagent box for quick detection of the paratuberculosis original bacterium-paratuberculosis mycobacteria, and belongs to the biological technology field. The invention is a group of nucleotide sequences for detecting paratuberculosis virus, and the invention is characterized in that the reagent box is the nucleotide sequences shown from the sequence table SEQ ID No.1 to the sequence table SEQ ID No.4. The invention has the advantages that: firstly, the specificity is good, the template is discriminated through a real-time fluorescence PCR technology and the specificity hybridization of a primer or a probe, the invention has very high accuracy and the false positive is low; secondly, the sensitivity is high, and a sensitive fluorescence detection system is adopted to perform real-time monitoring to the fluorescence signal; thirdly, the operation is simple, the automation degree is high, and the step of the electrophoresis detection of the product of the traditional PCR amplification is not required; fourthly, the detection is not easy to cause pollution, a closed pipe is amplified, a cover opening is not required, and the opportunity of the cross contamination and the environment opportunity is less.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
Tuberculosis immunodiagnosis molecular marker and vaccine use thereof
ActiveCN105388300AHigh strengthAntibacterial agentsBacterial antigen ingredientsParatuberculosisTuberculosis immunization
The invention belongs to the fields of immunology and cytobiology, relates to a tuberculosis immunodiagnosis molecular marker and vaccine use thereof, and in particular relates to use of any one or more of proteins, selected from amino acid sequences shown as SEQ ID NO: 1-4, in preparing a tuberculosis diagnostic agent; preferably, the tuberculosis is phthisis. The protein shown by any sequence in SEQ ID NO: 1-4 can serve as a tuberculosis (such as phthisis) immunodiagnosis molecular marker, and has good coincidence rate and reaction intensity; besides, the tuberculosis immunodiagnosis molecular marker has the potential for being applied to preparation of a tuberculosis vaccine.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Novel Combination of Naturally Occurring Compounds to Assist With Suspected Mycobacterial Infections Related to Autoimmune Conditions
InactiveUS20170173059A1Hydrocarbon active ingredientsDispersion deliverySide effectAutoimmune condition
Owner:MAGRONE JR NICHOLAS
An oligonucleotide gene chip and its application in the detection of various germs
InactiveCN102260738AGood repeatabilityStrong specificityMicrobiological testing/measurementNucleotideOligonucleotide
The invention discloses an oligonucleotide gene chip and application of the oligonucleotide gene chip to the detection of various bacteria. Probes of the oligonucleotide gene chip can be hybridized with polymerase chain reaction (PCR) amplification products of M. tuberculosis, M. bovis, M. avium, M. paratuberculosis and Brucella respectively; and the probes have nucleotide sequences shown as SEQ ID No:25, SEQ ID No:31, SEQ ID No:35, SEQ ID No:37 and SEQ ID No:39 respectively. The gene chip is applied to the detection of the bacteria and has high repeatability, and interassay and intraassay coefficients of variation (CV) are less than 15 percent. The gene chip shows high accuracy on the detection of a pathogenic bacterium sample to be detected, which indicates that the method has high specificity and sensitivity; the high-throughput and parallel detection is realized; detection results are quick and accurate; the whole process from the preparation of nucleic acid to the finishing of the detection only needs 6 to 8h; and the gene chip has broad prospect in preparing reagents and products for pathogen detection, import and export quarantine and epidemiological analysis.
Owner:SOUTH CHINA AGRI UNIV
Combination vaccines against Mycobacterium sp. and methods of using the same
The invention relates to a combination vaccine against Mycobacterium avium subspecies paratuberculosis (MAP) and M. tuberculosis and / or M. bovis for use in methods of immunizing a subject against mycobacterial infection, preventing or treating mycobacterial infection, and preventing a disease associated with mycobacterial infection.
Owner:GREENSTEIN ROBERT J
Mycobacterium tuberculosis antigens, nucleotide sequences and application thereof
InactiveCN105218648AEasy to detectEasy diagnosisAntibacterial agentsBacterial antigen ingredientsParatuberculosisEpitope
The invention relates to 12 mycobacterium tuberculosis antigen polypeptides, and antigen epitope polypeptides having polypeptide sequences having at least 90% of the same as each other; the antigen polypeptides or the antigen epitope polypeptides are matched with an immunologic adjuvant to obtain mycobacterium tuberculosis vaccines; the antigen polypeptides or the antigen epitope polypeptides are used as antigens, and monoclonal antibodies are prepared and used for detecting whether the sample contains mycobacterium tuberculosis; nucleic acid sequences of the 12 mycobacterium tuberculosis antigen polypeptides and polynucleotides having the nucleic acid sequences having at least 90% of the same as each other are encoded; gene vaccines containing the nucleic acids and the polynucleotides are prepared. Beneficial effects comprise that the polypeptides can be used for recombined vaccine polypeptides, or be used for development of antigen diagnostic reagents, so as to be beneficial for detection and diagnosis of pulmonary tuberculosis.
Owner:邓林红 +1
Prevention And Treatment Of Gastrointestinal Infection In Mammals
InactiveUS20130189236A1Reduce in quantityImprove food safetyBiocideBacteria material medical ingredientsMammalEnteropathy
Methods are disclosed for the prevention and / or treatment of certain gastrointestinal (GI) diseases, such as Johne's Diseases (JD) in animals and Crohn's Disease (CD) in human. Administration of certain probiotic bacteria, such as lactic acid producing bacteria, to animals helps inhibit GI infection by Mycobacterium avium subsp. paratuberculosis (MAP). MAP is the primary pathogenic agent suspected of causing various inflammatory bowel diseases in cattle or humans.
Owner:CHR HANSEN AS
Global gene regulators (GGR) as vaccine candidates against paratuberculosis
Described herein is a mycobacterium mutant, comprising at least one mutation in at least one gene sequence encoding global gene regulators (GGRs) selected from the group consisting of sigH, sigL, sigE, ECF-1, and mixtures thereof, wherein the GGR gene is at least partially inactivated. Described herein also is a vaccine based on the mutant and a method of differentiating between subjects that have been infected with mycobacterium and subjects that have not been infected with mycobacterium or have been vaccinated with a mycobacterium vaccine.
Owner:WISCONSIN ALUMNI RES FOUND
Diagnostic targets against Johne's disease
InactiveUS20070134708A1Sugar derivativesMicrobiological testing/measurementGenomic SegmentParatuberculosis
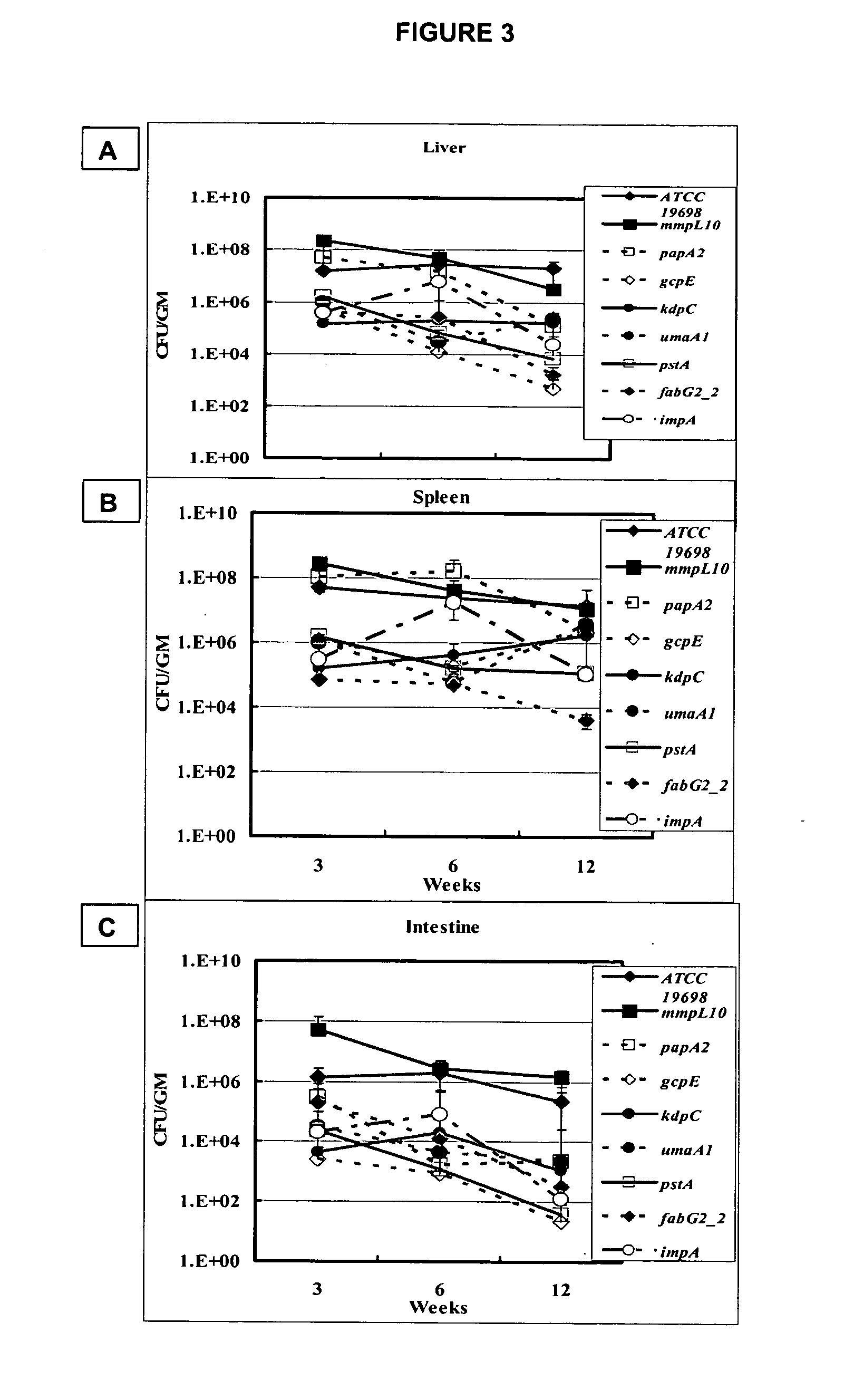
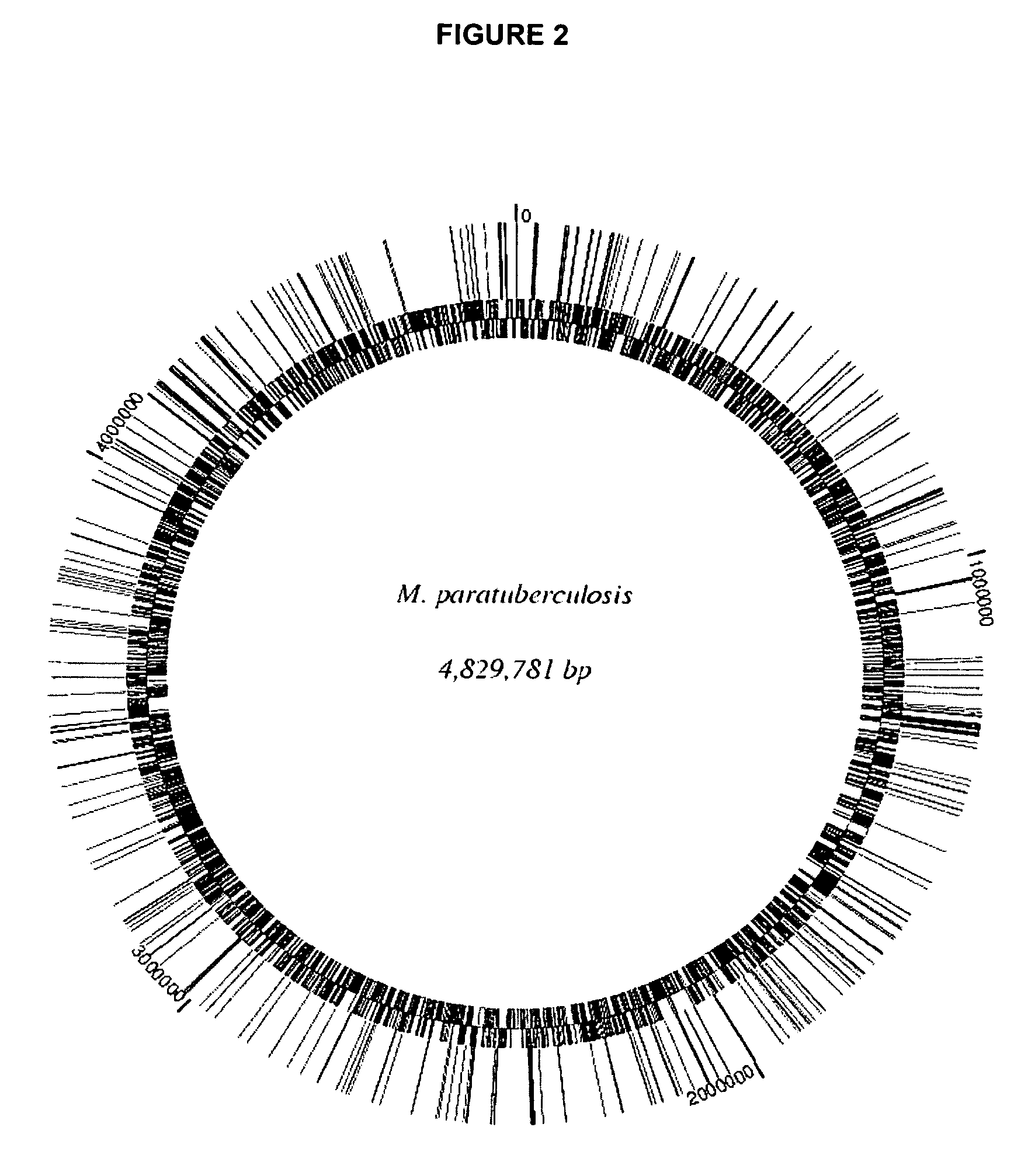
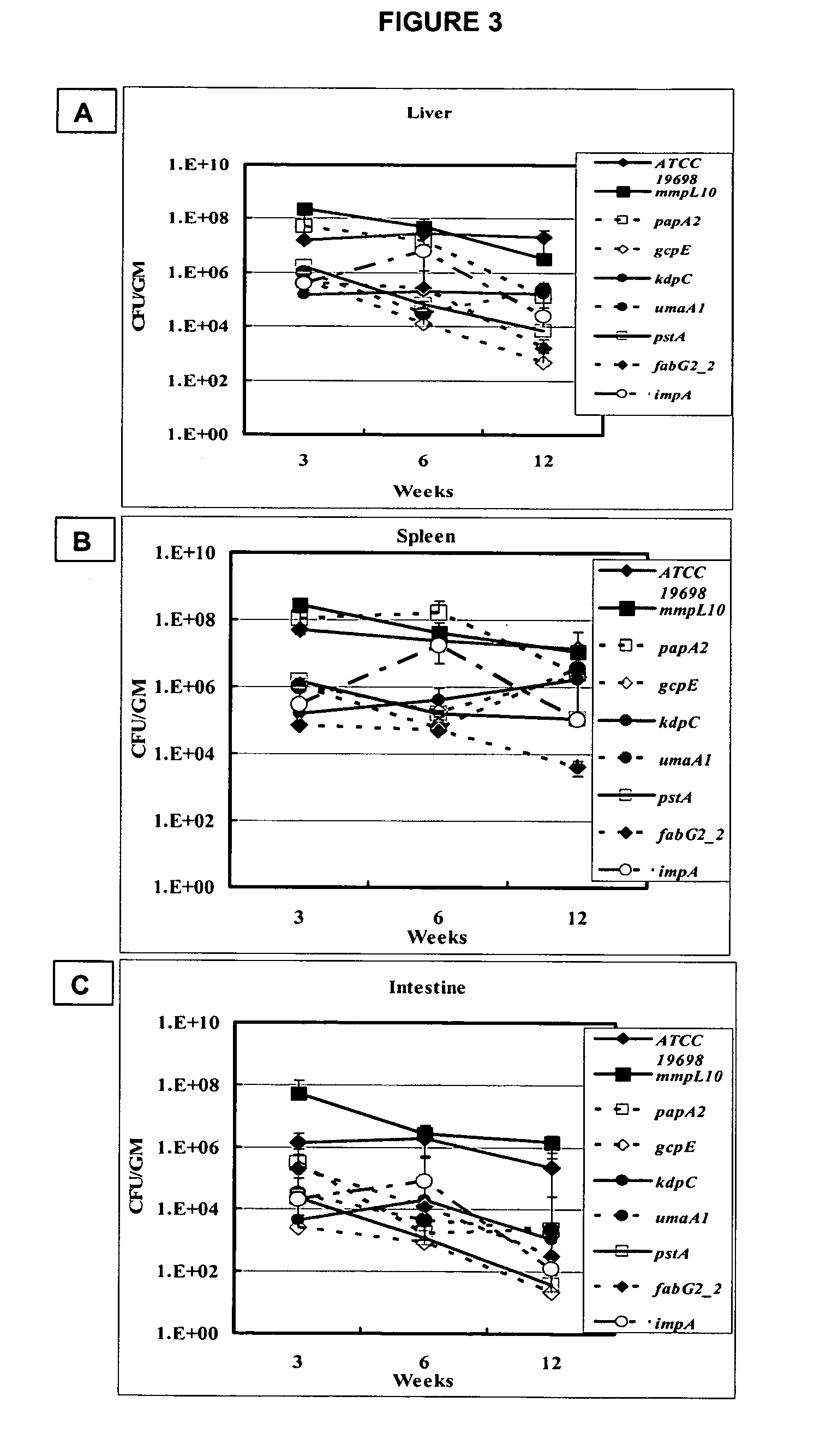
A composition and method for detecting Mycobacterium infection are disclosed. The gcpE, pstA, kdpC, papA2, impA, umaA1, fabG2132, aceAB, mbtH2, lpqP, map0834c, cspB, lipN, and map 1634 genes of M. paratuberculosis are novel virulence determinants for Johne's disease. Eighteen M. paratuberculosis-specific genomic islands were identified. Twenty-four M. avium-specific genomic islands were identified. Inversion of three large genomic fragments (INV) in M. paratuberculosis was also identified. These genomic identifiers represent novel virulence determinants that can be used as diagnostics targets for mycobacterial infection, and could provide suitable targets for vaccine and drug developments against Johne's disease.
Owner:WISCONSIN ALUMNI RES FOUND
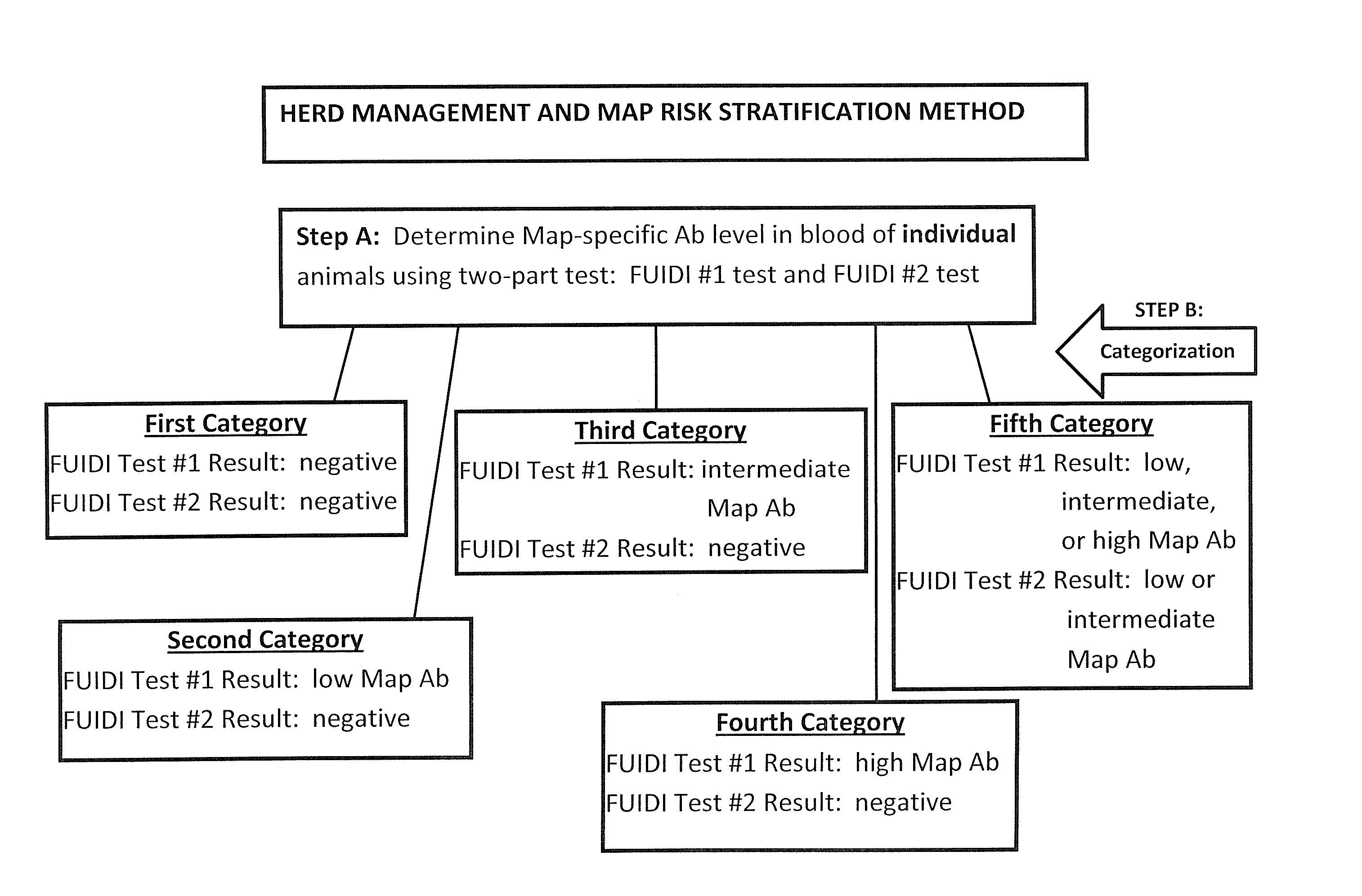
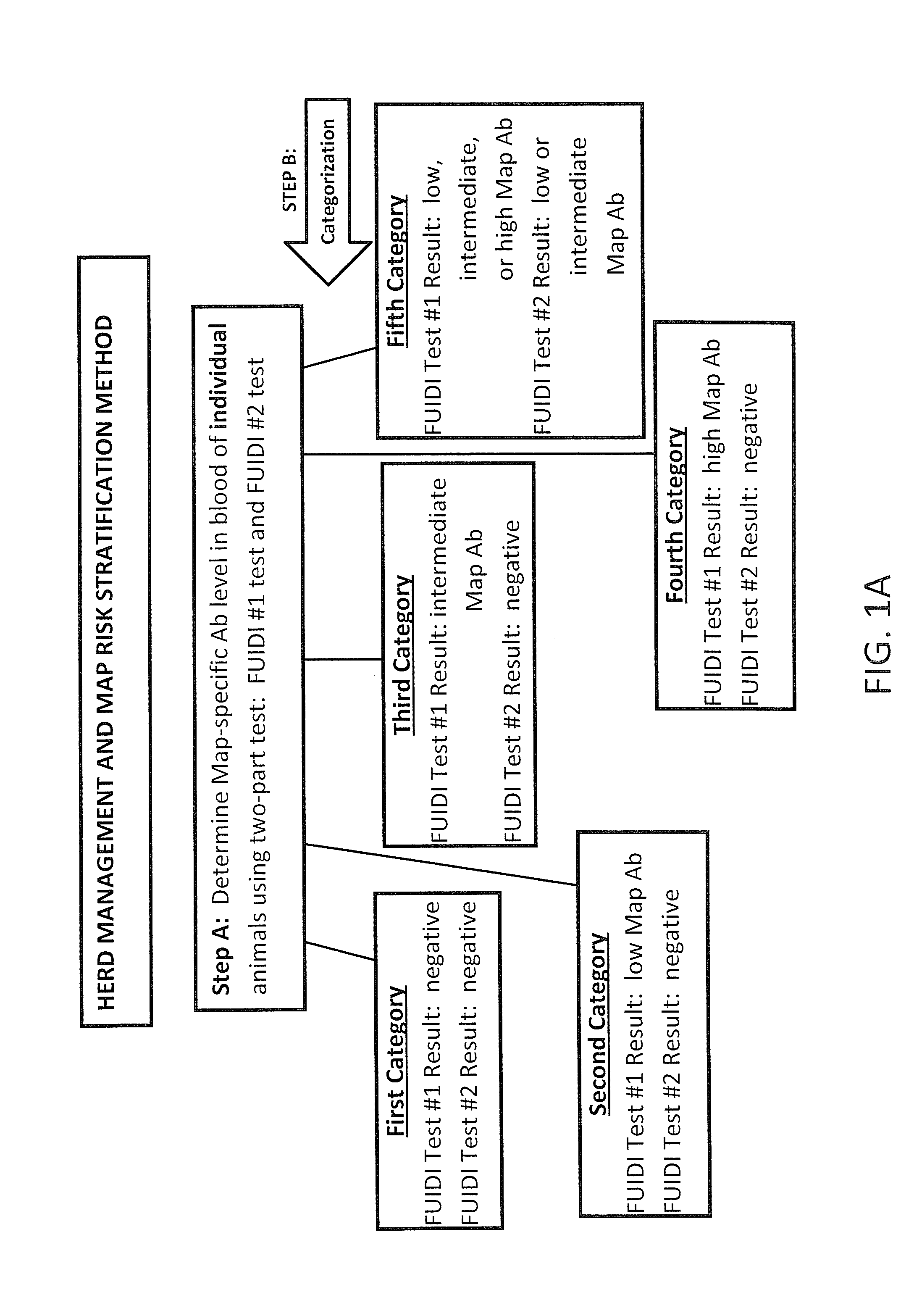
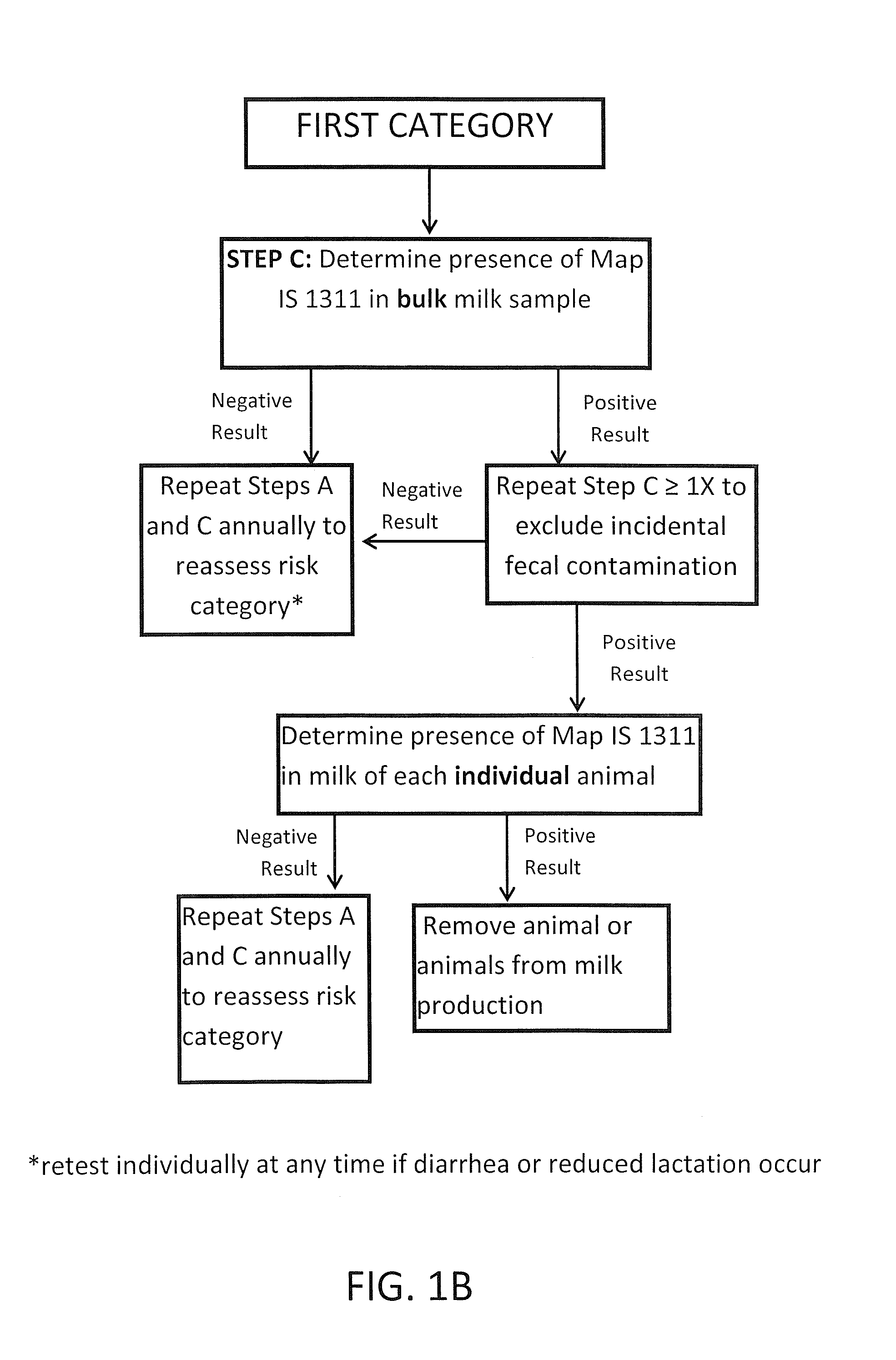
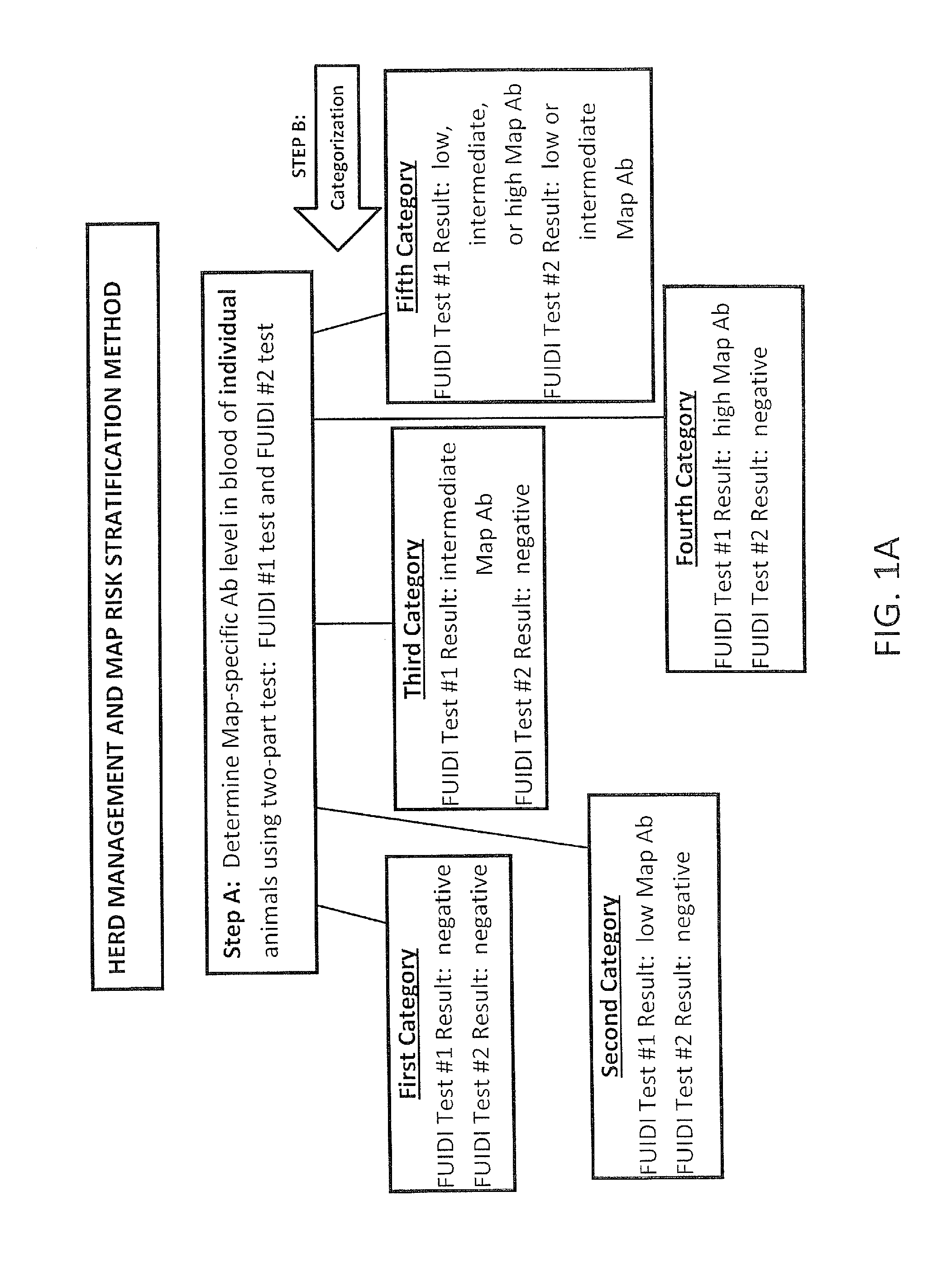
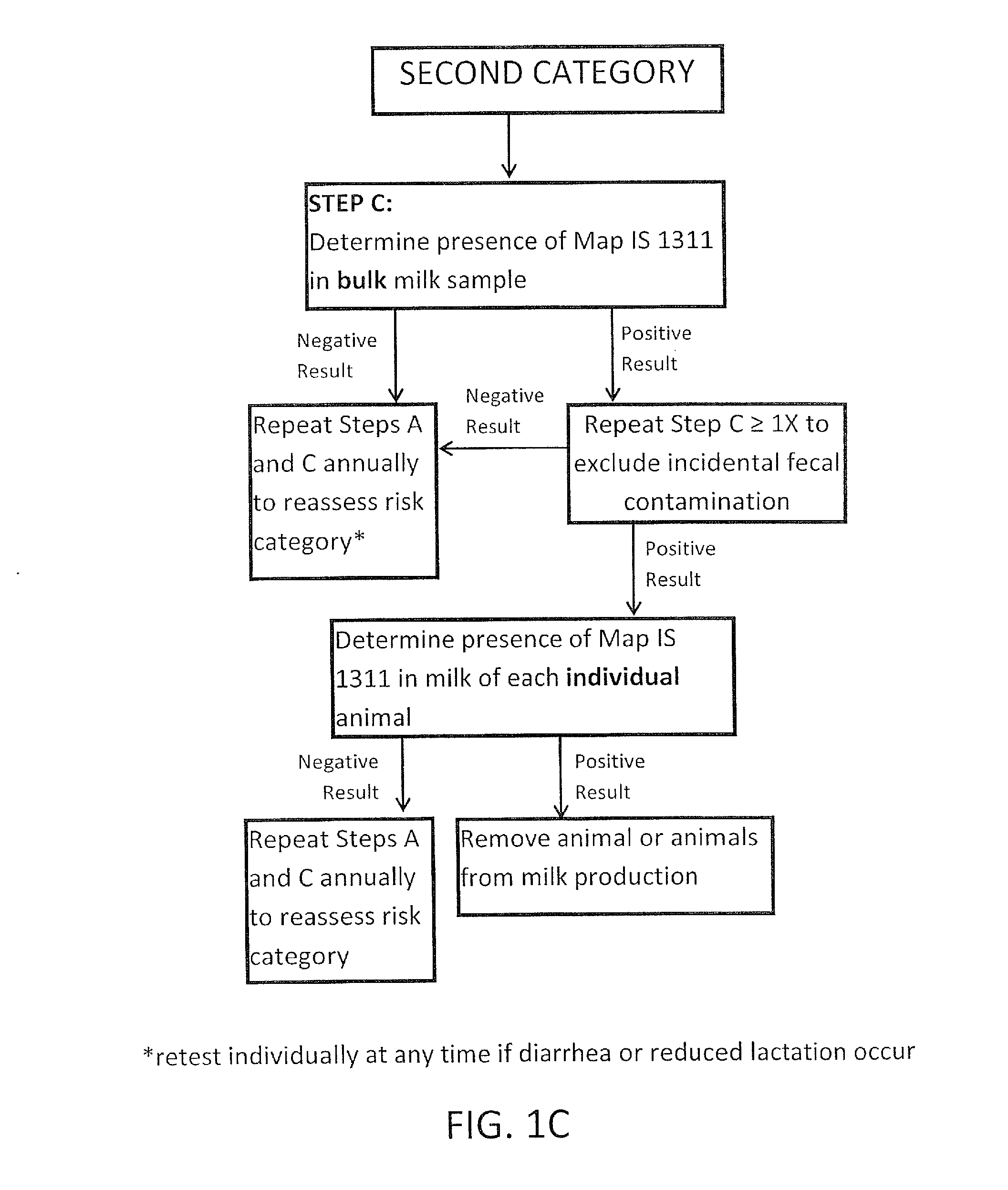
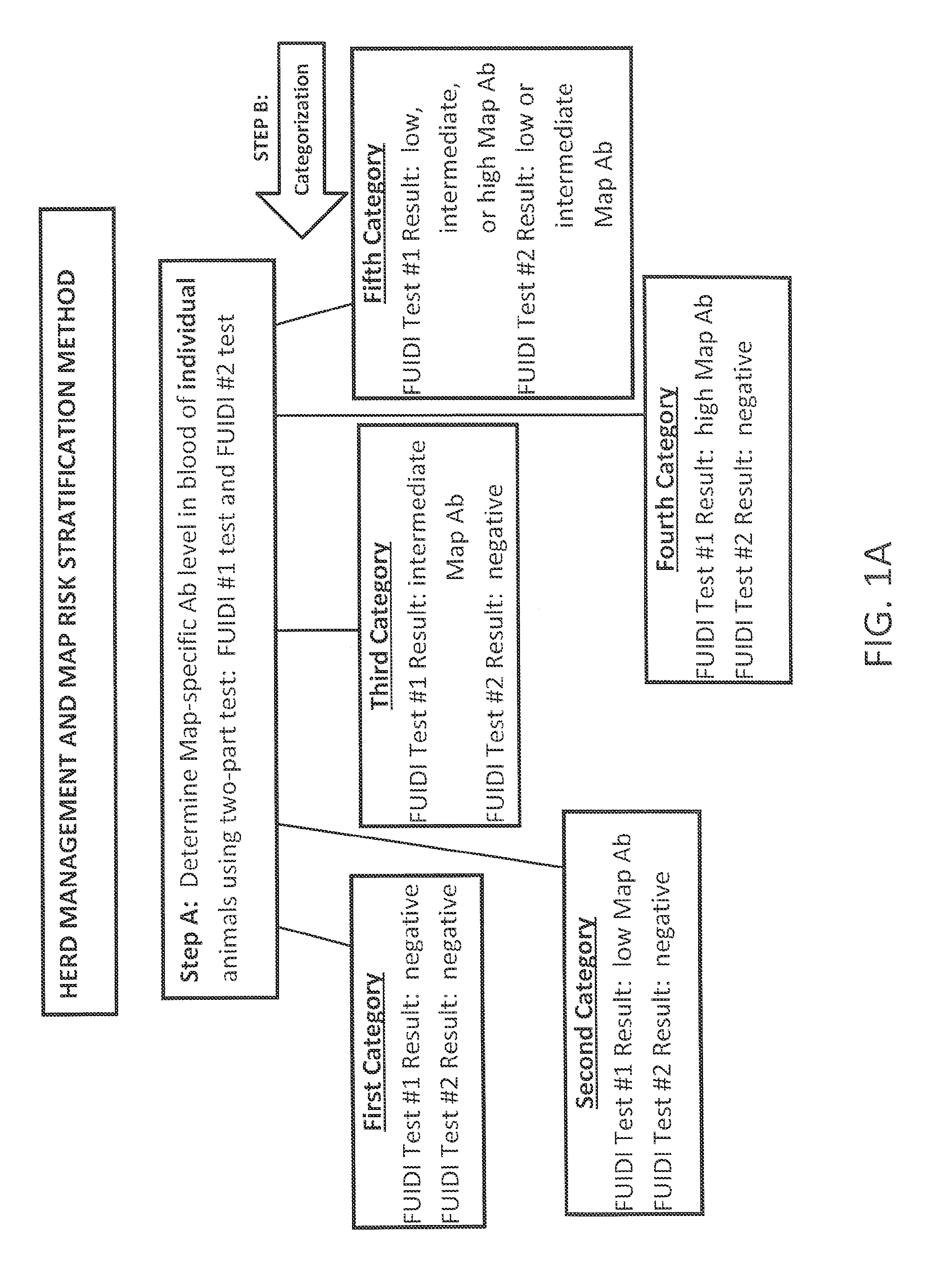
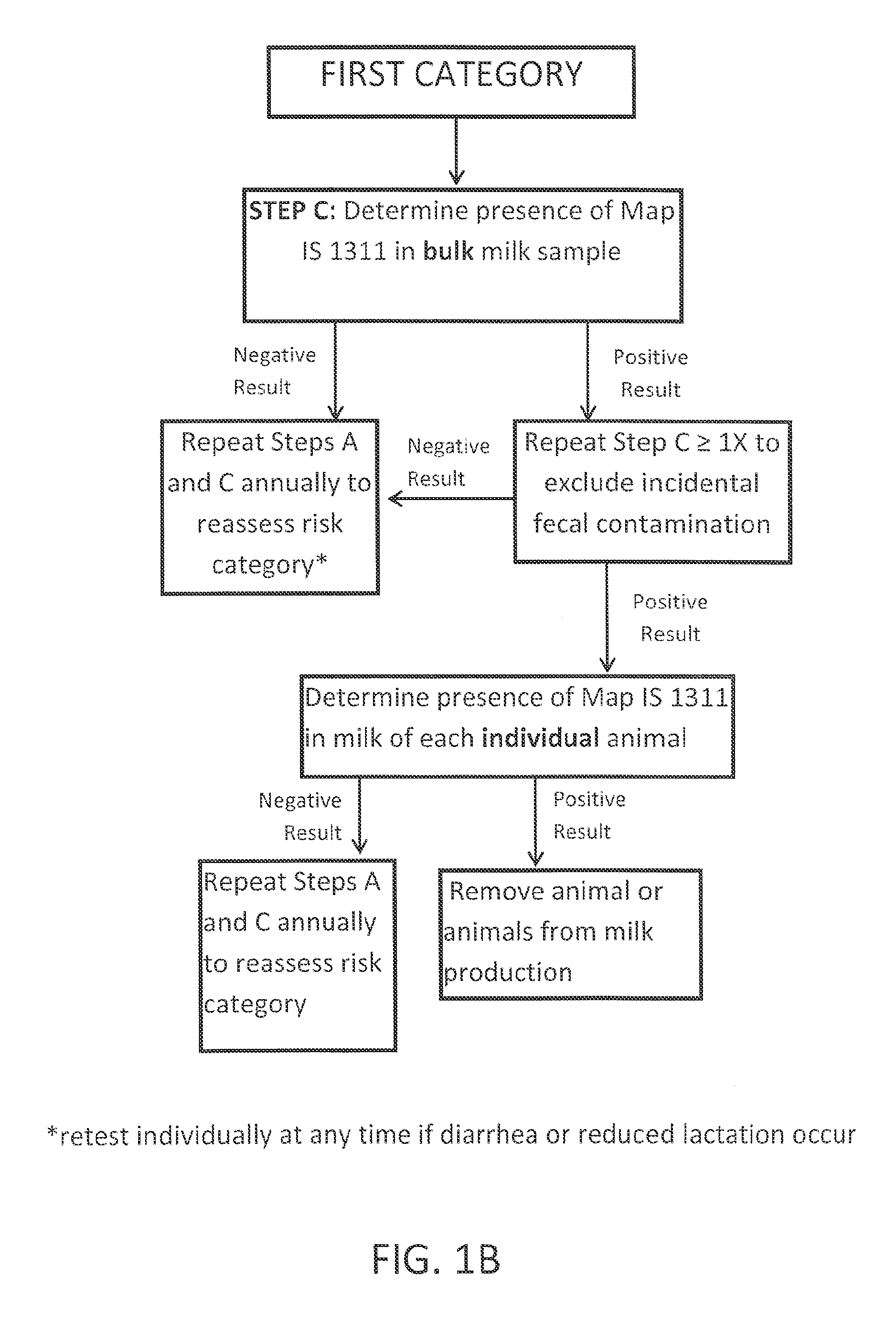
Fuidi herd management and risk stratification methods
InactiveUS20140116352A1Enhanced genetic abilityMicrobiological testing/measurementBiological testingCvd riskBiology
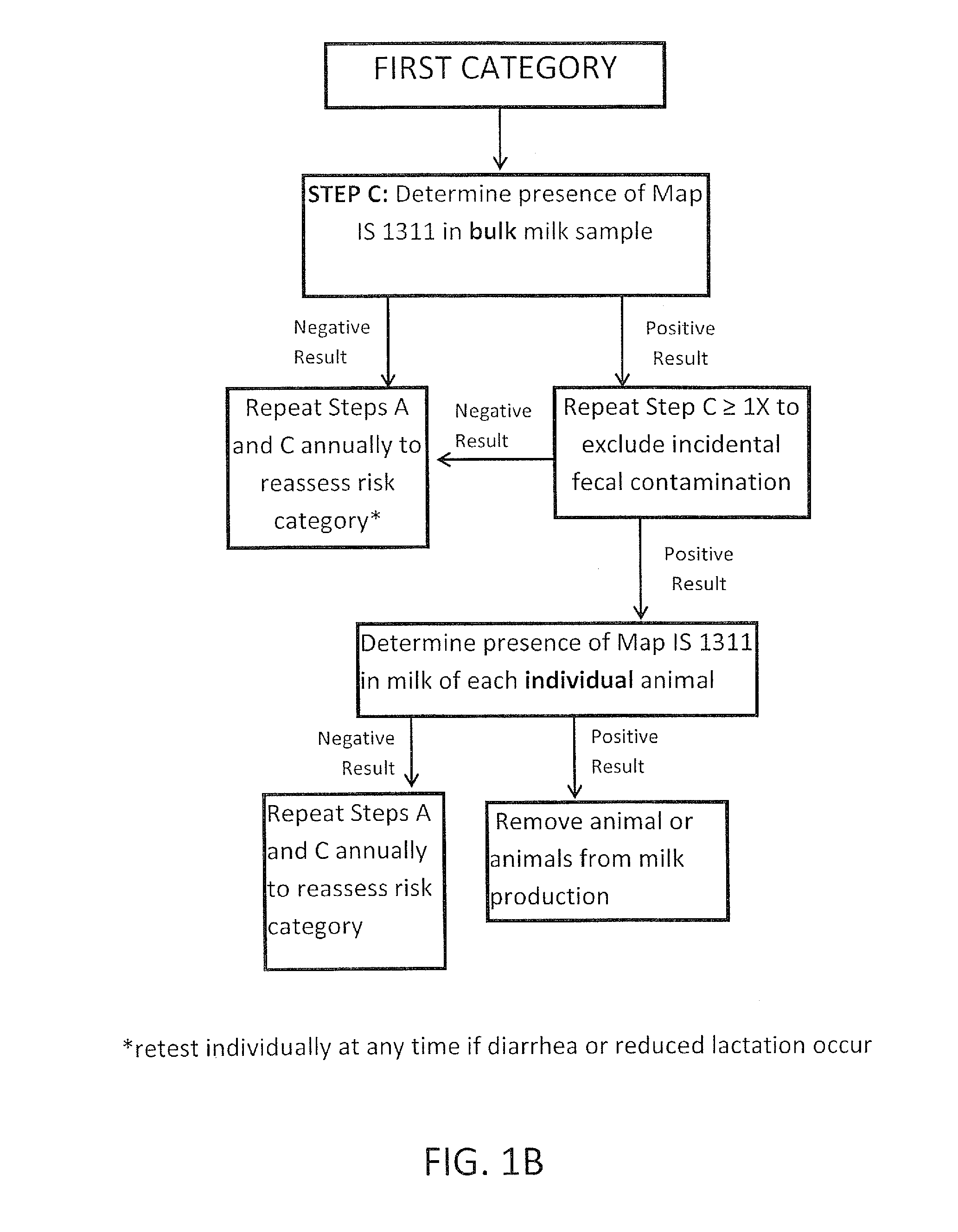
The invention concerns the detection of pathogenic mycobacterium comprising Mycobacterium avium subsp.paratuberculosis (Map) and genomic variants in a bulk milk sample, and more particularly a method for herd management that stratifies the risk of bulk tank milk lots derived from diagnostic-tested subgroups potentially containing DNA from pathogenic mycobacterium including Map. The method involves creating defined risk groups (categories) of milk-producing animals, such as dairy cows, for the presence of Map or related genomic variants in their milk. Another aspect of the invention concerns a method to strengthen the ability of milk-producing animals to resist environmental challenges by Map based on identifying those animals that have and maintain a low antibody level to Map using their female progeny as replacement animals.
Owner:MONIF GILLES R G
Vaccine candidates against Johne's disease
A composition and method for immunizing a mammal infected with Mycobacterium are disclosed. The genes gcpE, pstA, kdpC, papA2, impA, umaA1, fabG2_2, aceAB, mbtH2, lpqP, map0834c, cspB, lipN, and map1634 of M. paratuberculosis and the products that they encode are vaccine targets for Johne's and Crohn's disease. Eighteen M. paratuberculosis-specific genomic islands (MAPs) were identified. Three inverted large genomic fragments in M. paratuberculosis (INV) were also identified. These genomic identifiers represent novel virulence determinants that can be used as targets for vaccines and for developments of drugs against Johne's disease. The methods can be used to deliver an immunizing compound to a mammal, to provide an immune response against Johne's or Crohn's disease in the mammal.
Owner:WISCONSIN ALUMNI RES FOUND
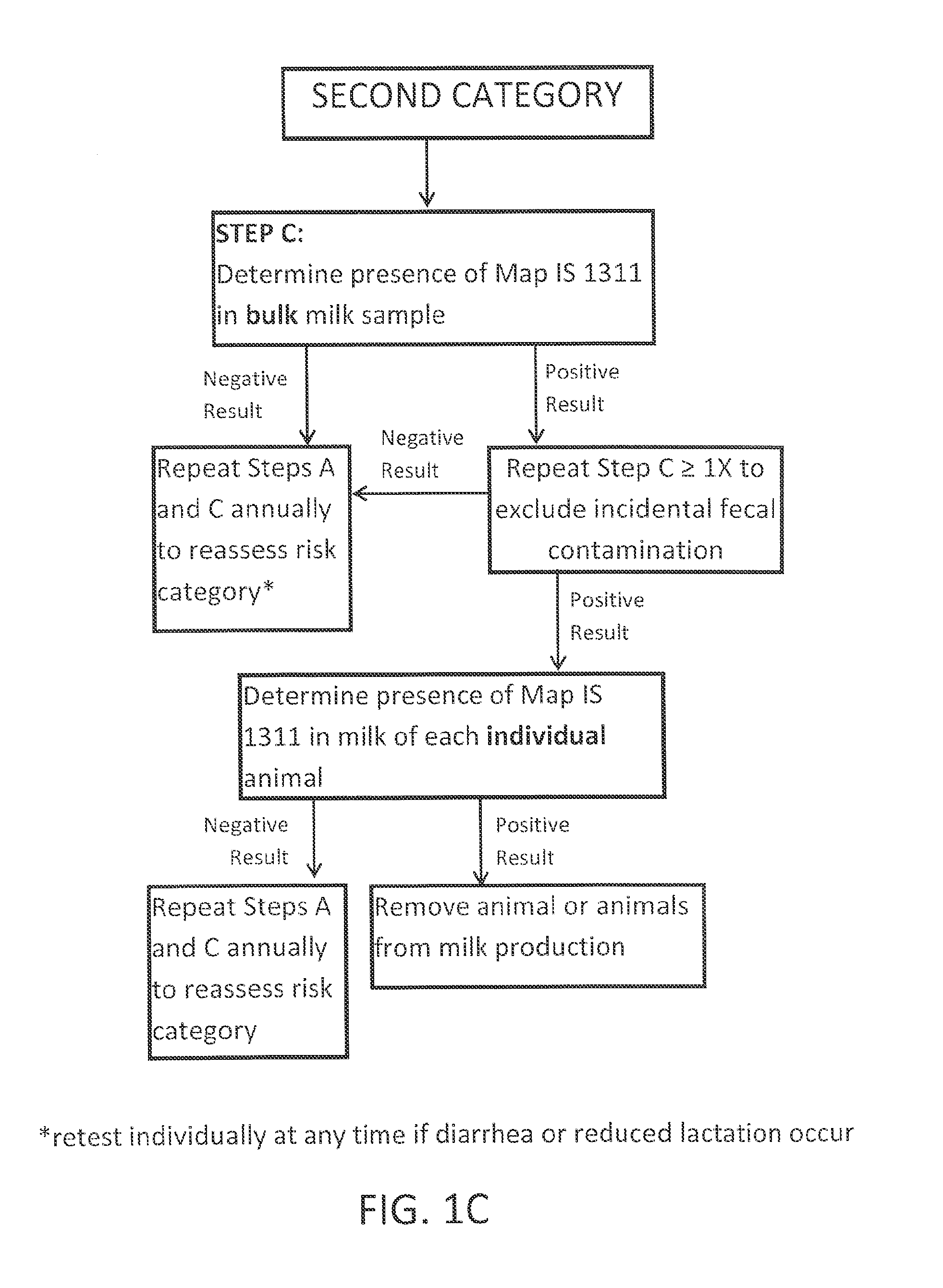
Fuidi herd management and risk stratification methods
InactiveUS20140116353A1Reduce the amount requiredEnhanced genetic abilityMicrobiological testing/measurementBiological testingCvd riskHerd management
The invention concerns the detection of pathogenic mycobacterium comprising Mycobacterium avium subsp. paratuberculosis (Map) and genomic variants in a bulk milk sample, and more particularly a method for herd management that stratifies the risk of bulk tank milk lots derived from diagnostic-tested subgroups potentially containing DNA from pathogenic mycobacterium including Map. The method involves creating defined risk groups (categories) of milk-producing animals, such as dairy cows, for the presence of Map or related genomic variants in their milk. Another aspect of the invention concerns a method to strengthen the ability of milk-producing animals to resist environmental challenges by Map based on identifying those animals that have and maintain a low antibody level to Map using their female progeny as replacement animals.
Owner:MONIF GILLES R G
Medicine used for treating influenza, upper respiratory infection and viral pneumonia
PendingCN105535927AObvious fatigueFatigue has obvious relief effectAntibacterial agentsOrganic active ingredientsTuberculosisInfluenza a
The invention provides a medicine used for treating influenza, upper respiratory infection and viral pneumonia. The medicine is glycoprotein or a mixture of polysaccharide and protein or polypeptide or protein. The glycoprotein provided by the invention has a stabilization effect on body temperatures of endotoxin pyrogenic domestic rabbits, and has an obvious antipyretic effect; the medicine provided by the invention is used for treating the influenza, the upper respiratory infection and the viral pneumonia, and no symptoms of bronchitis, pneumonia and tuberculosis; the complication is 0; the toxin-free concentration of the medicine to Vero-E6 cells is 100 mu g / ml, and has an obvious inhibition effect on SARS viruses. The medicine provided by the invention has obvious pharmacologic actions on seven aspects of in-vitro virus resistance, in-vivo virus resistance, bacterium inhibition, inflammation resistance, antipyretic action, pain alleviation and immunologic function improvement; the medicine has the characteristics of safety, high efficiency and no side effect and has functions of preventing and treating respiratory system diseases, and broad-spectrum anti-virus, anti-inflammatory, anticoagulation, bacteria resisting, antipyretic and immunity adjusting functions.
Owner:SHANDONG ZHONGHAI PHARMA CO LTD
New antigens for paratuberculosis diagnosis and vaccination
InactiveUS20110200603A1Good antigenicityImproving immunogenicityAntibacterial agentsOrganic active ingredientsParatuberculosisEpitope
Antigens of Mycobacterium avium subsp. paratuberculosis, antigenic compositions include at least two of the antigens, as well as epitopes, antibodies or hypervariable fragments thereof and nucleotide sequences coding for them. The antigens are used in diagnosis and / or vaccination against Mycobacterium avium subsp. paratuberculosis, in mammals, and in particular in cattle, but also in sheep and caprines. The antigens have potential application in diagnosis and / or vaccination against Crohn's disease in humans.
Owner:UNIVERSITY OF MONS +2
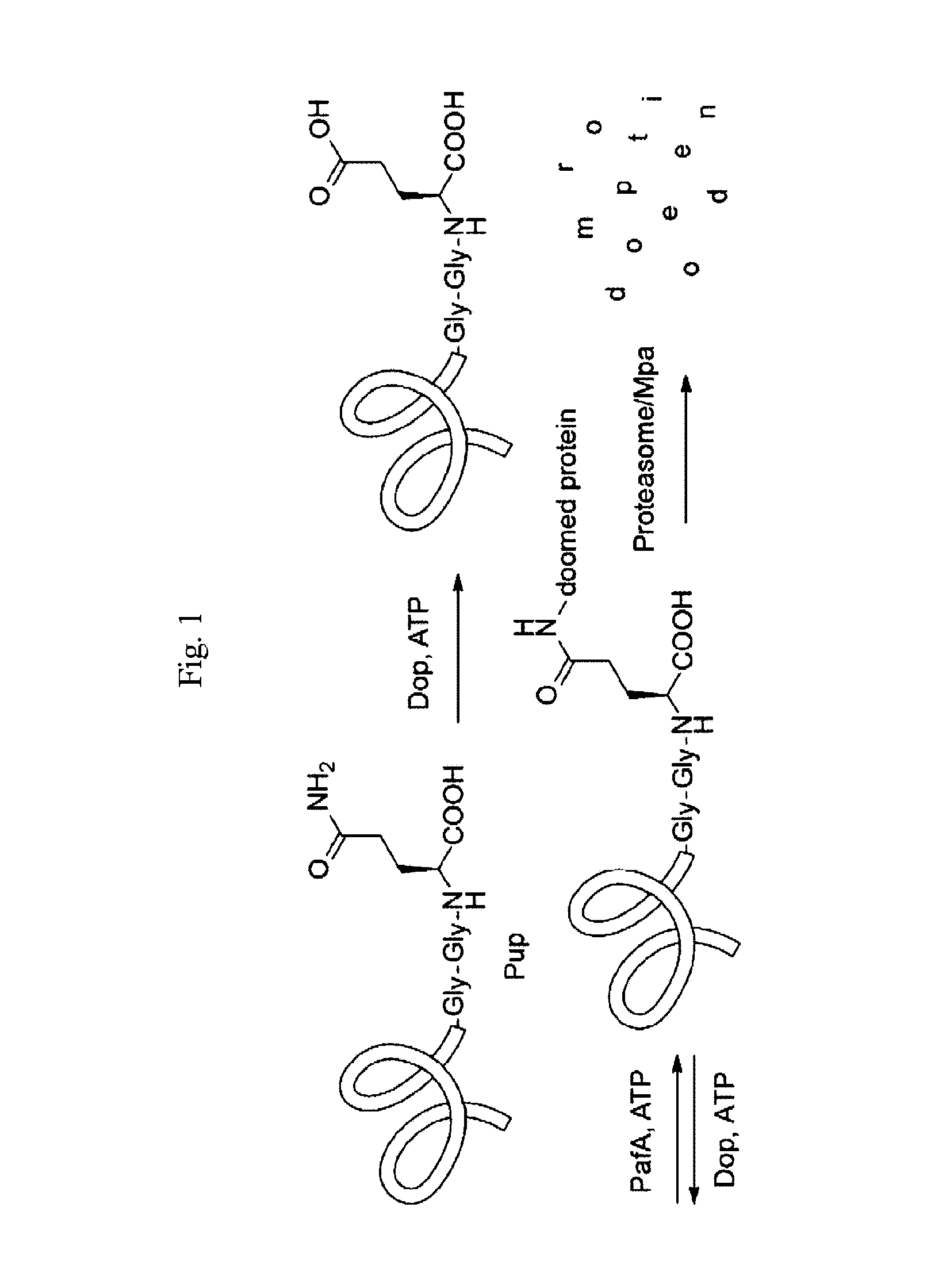
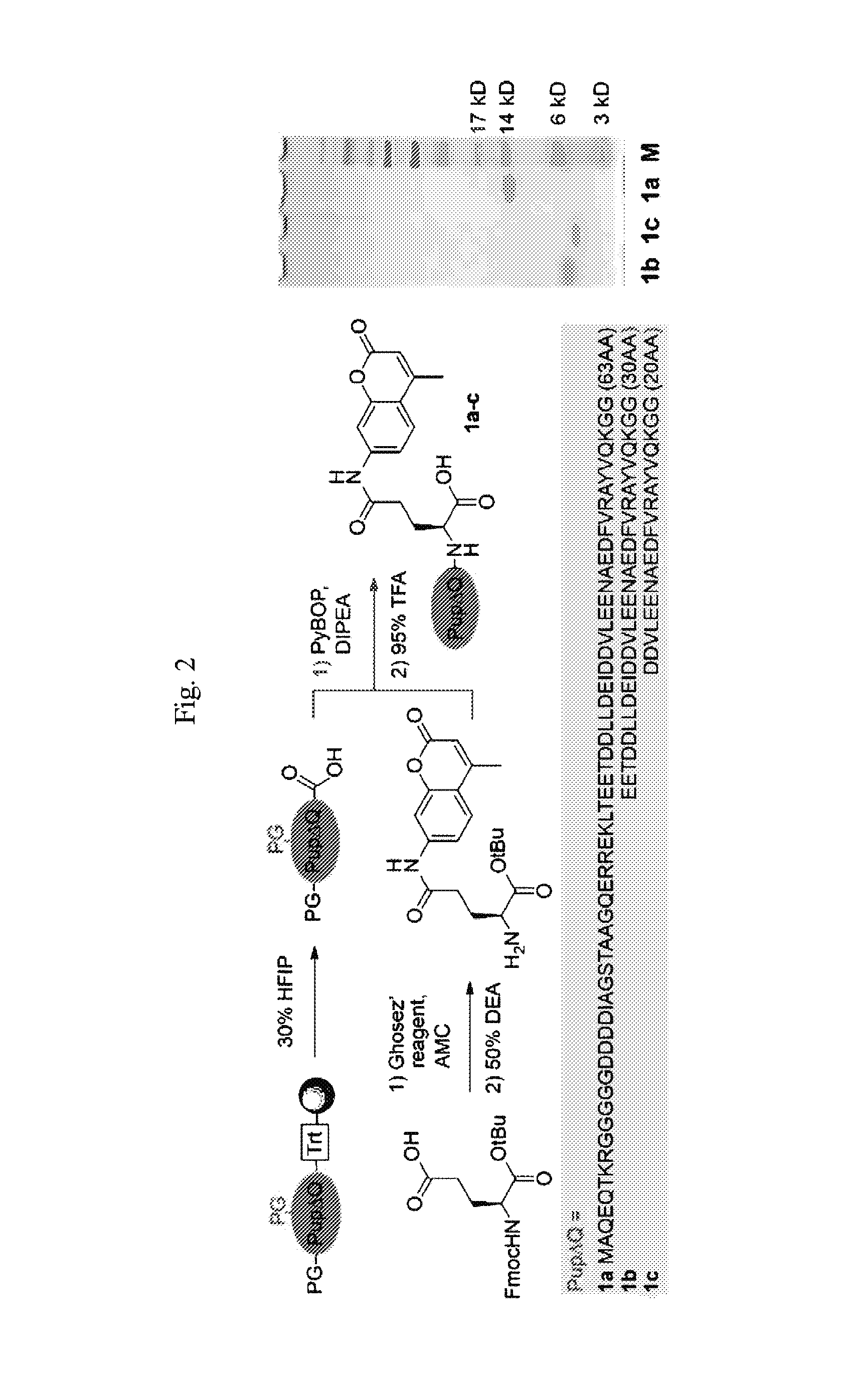
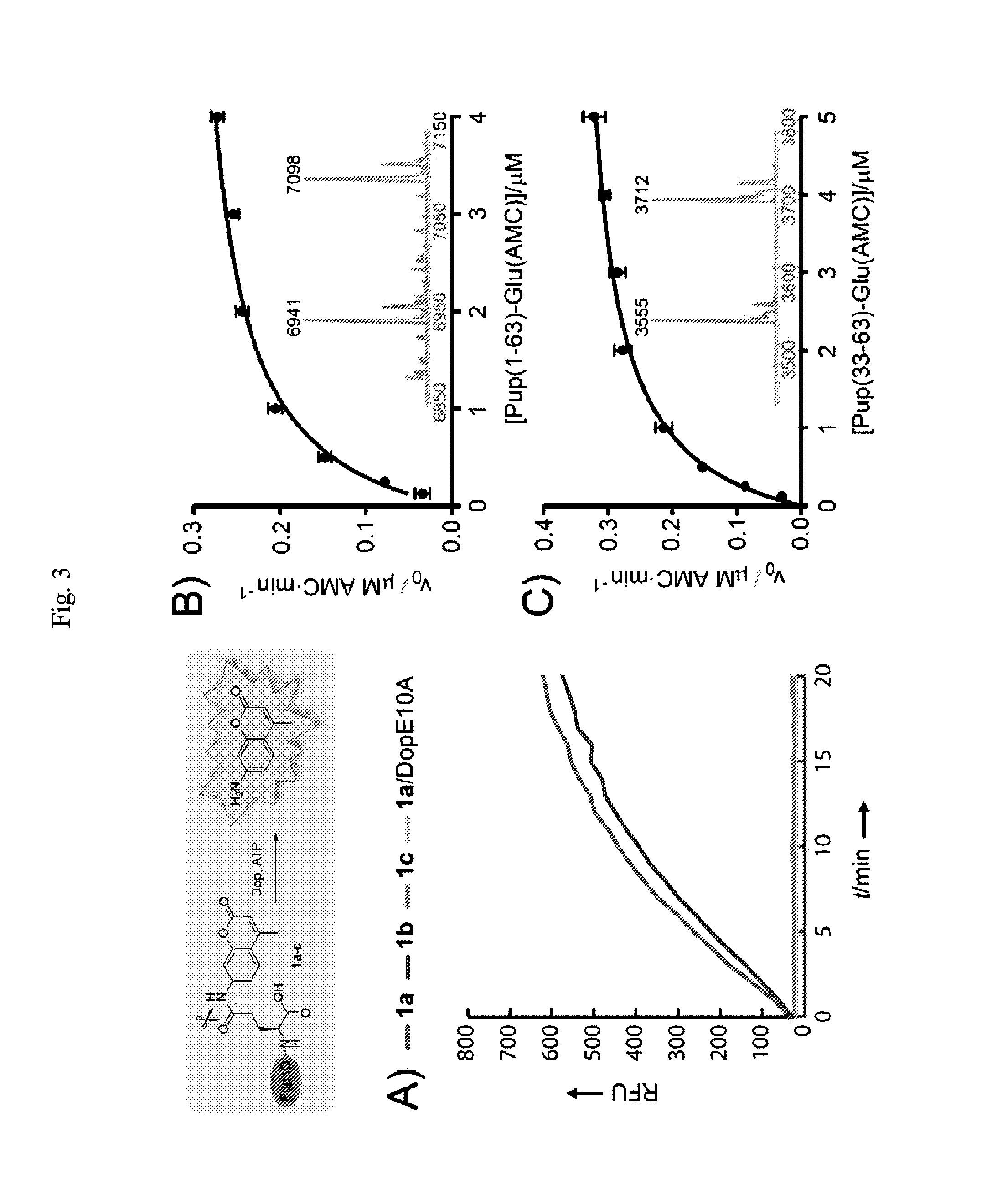
Modified prokaryotic ubiquitin-like protein and methods of use thereof
Methods for making and using substrates of deamidase of prokaryotic ubiquitin-like protein (Dop) are described herein. More particularly, modified prokaryotic ubiquitin-like protein (Pup) and functional fragments thereof that serve as exemplary Dop substrates are described and encompassed herein. Screening methods to identify modulators of Dop and Pup activity and use of modulators identified thereby are also described. Methods of using modulators that are identified as inhibitors of Dop and Pup activity for treating diseases / conditions associated with Mycobacterium tuberculosis (Mtb) infection, such as tuberculosis and leprosy, are also envisioned.
Owner:NEW YORK UNIV
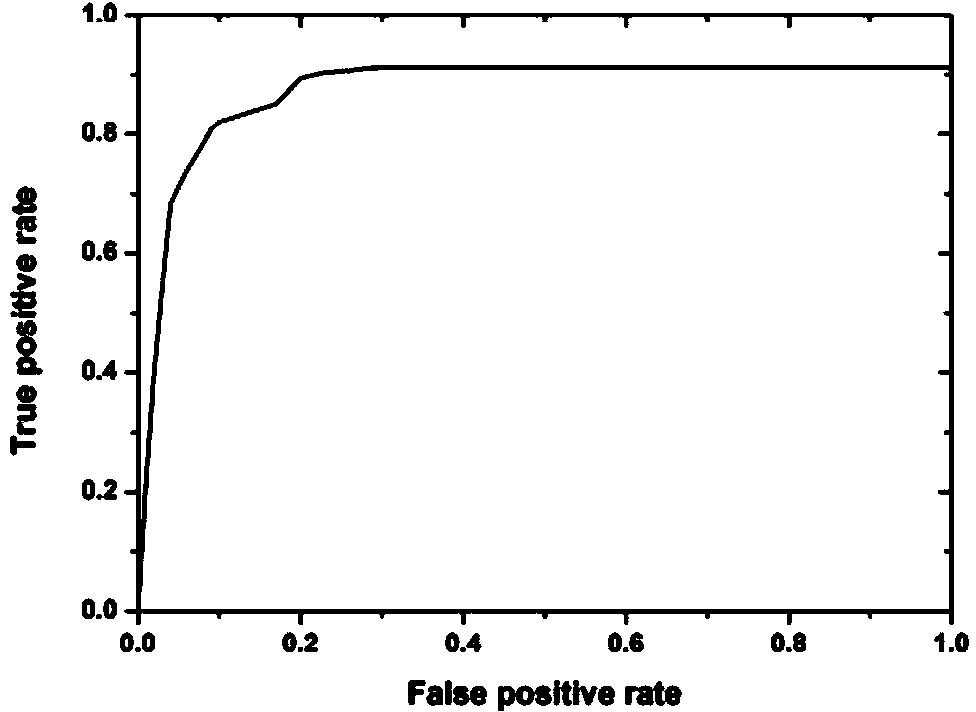
Application of proteins of Rv3872, Rv0164 and/or Rv1926c in developing and/or designing product with functions of identification, diagnosis, auxiliary diagnosis, screening and/or auxiliary screening of active tuberculosis
The invention provides application of proteins of Rv3872, Rv0164 and / or Rv1926c in developing and / or designing a product with functions of identification, diagnosis, auxiliary diagnosis, screening and / or auxiliary screening of the active tuberculosis. The invention further provides a protein chip prepared by the proteins of Rv3872, Rv0164 and / or Rv1926c. According to the invention, the protein chip can be used for detecting the levels of IgG antibodies corresponding to three protein antigens in the blood serum of a patient with active tuberculosis and those in the blood serum of a normal person, conjointly analyzing the testing results of antibodies corresponding to the three groups of proteins and judging whether people to be tested have active tuberculosis or not. Test results show that the specificity and the sensitivity of the optimal operating point of the protein chip for auxiliary diagnosis of the active tuberculosis are 91.1% and 82.4% respectively which are both above the diagnosis indexes of the active tuberculosis in prior art.
Owner:广州博翀生物科技有限公司
Identification of bacterial species and subspecies using lipids
InactiveUS20110086384A1Reduce in quantityMicrobiological testing/measurementBiological material analysisLipid formationParatuberculosis
The use of free, extractable lipids found in bacteria for identification of bacterial species and subspecies is described. Bacteria have been found to differ sufficiently in their extracted lipid compositions to effect identification using thin layer chromatographic techniques. Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia mallei have been distinguished in this manner. Lipopeptides specific to Mycobacterium avium subspecies paratuberculosis, but not to the closely related bacterium Mycobacterium avium subspecies avium have also been used as a basis for bacterial subspecies identification using mass spectrometry and seroreactivity. Mass spectrometric analysis of total bacterial lipids of Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia mallei, and mass spectrometric analysis of total bacterial lipids for Mycobacterium avium subspecies paratuberculosis and Mycobacterium avium subspecies avium, without further lipid separation, has shown that species and subspecies of bacteria may be identified using such analysis.
Owner:COLORADO STATE UNIVERSITY
Reagent and method for detecting Mycobacterium tuberculosis infection in vitro
InactiveCN102305855AValid in vitro assayOvercome the disadvantages of unsatisfactory effectImmunoglobulins against bacteriaFermentationAntigenParatuberculosis
The invention discloses a reagent and a method for detecting Mycobacterium tuberculosis infection in vitro. The reagent comprises a segment of specific T cell reactive polypeptide, namely M232 polypeptide shown as SEQ ID No.1 and screened from early secreting antigen target-6 (ESAT-6) polypeptide; and the M232 polypeptide is contacted with a T cell of a Mycobacterium tuberculosis host to detect cell factors released by the T cell and determine whether the T cell identifies the M232 polypeptide. The invention has the advantages of high sensitivity, no influence of Bacillus Calmette-Guerin (BCG) vaccine and nontuberculosis mycobacterial vaccine, high specificity, capacity of detecting patients with active pulmonary tuberculosis and patients with potential infection, and capacity of detecting healthy Mycobacterium tuberculosis contacts. The invention is particular suitable for detecting tuberculosis and / or potential infection of the tuberculosis for Chinese people. The invention also relates to a kit for detecting Mycobacterium tuberculosis infection in vitro, which comprises two mixed polypeptides, namely M232 and M233, and a tool for detecting the identification of the T cell on protein, or polypeptide or analogs of the polypeptide.
Owner:SUN YAT SEN UNIV
Fuidi herd management and risk stratification methods
InactiveUS20150359198A1Enhanced genetic abilityMicrobiological testing/measurementAvicultureBiotechnologyMilk cow's
The invention concerns the detection of pathogenic mycobacterium comprising Mycobacterium avium subsp. paratuberculosis (Map) and genomic variants in a bulk milk sample, and more particularly a method for herd management that stratifies the risk of bulk tank milk lots derived from diagnostic-tested subgroups potentially containing DNA from pathogenic mycobacterium including Map. The method involves creating defined risk groups (categories) of milk-producing animals, such as dairy cows, for the presence of Map or related genomic variants in their milk. Another aspect of the invention concerns a method to strengthen the ability of milk-producing animals to resist environmental challenges by Map based on identifying those animals that have and maintain a low antibody level to Map using their female progeny as replacement animals.
Owner:MONIF GILLES R G
Diagnosis and treatment of microbacterial infections
InactiveUS20060275848A1Avoid spreadingRaise the possibilityPeptide/protein ingredientsMicrobiological testing/measurementMicroorganismParatuberculosis
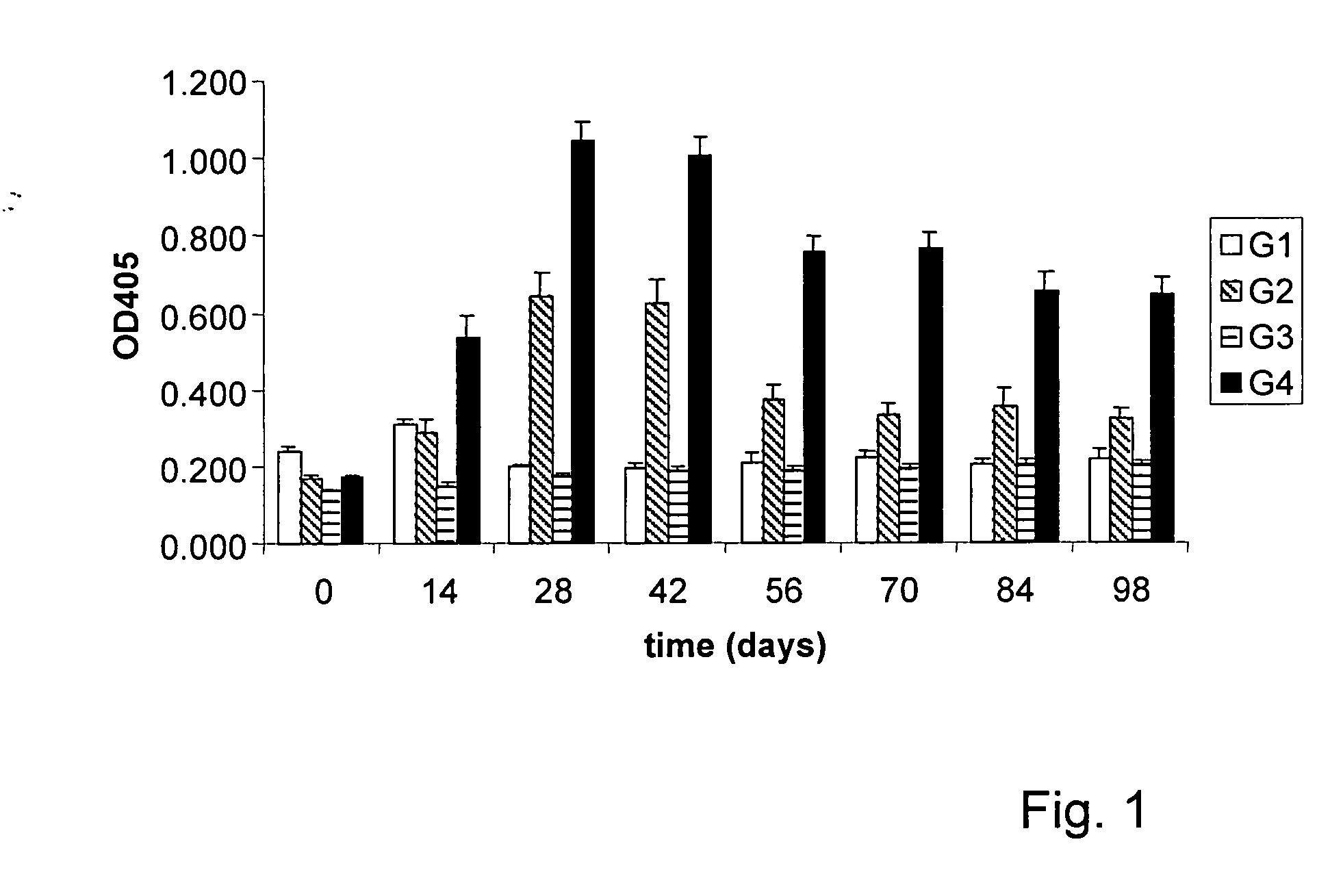
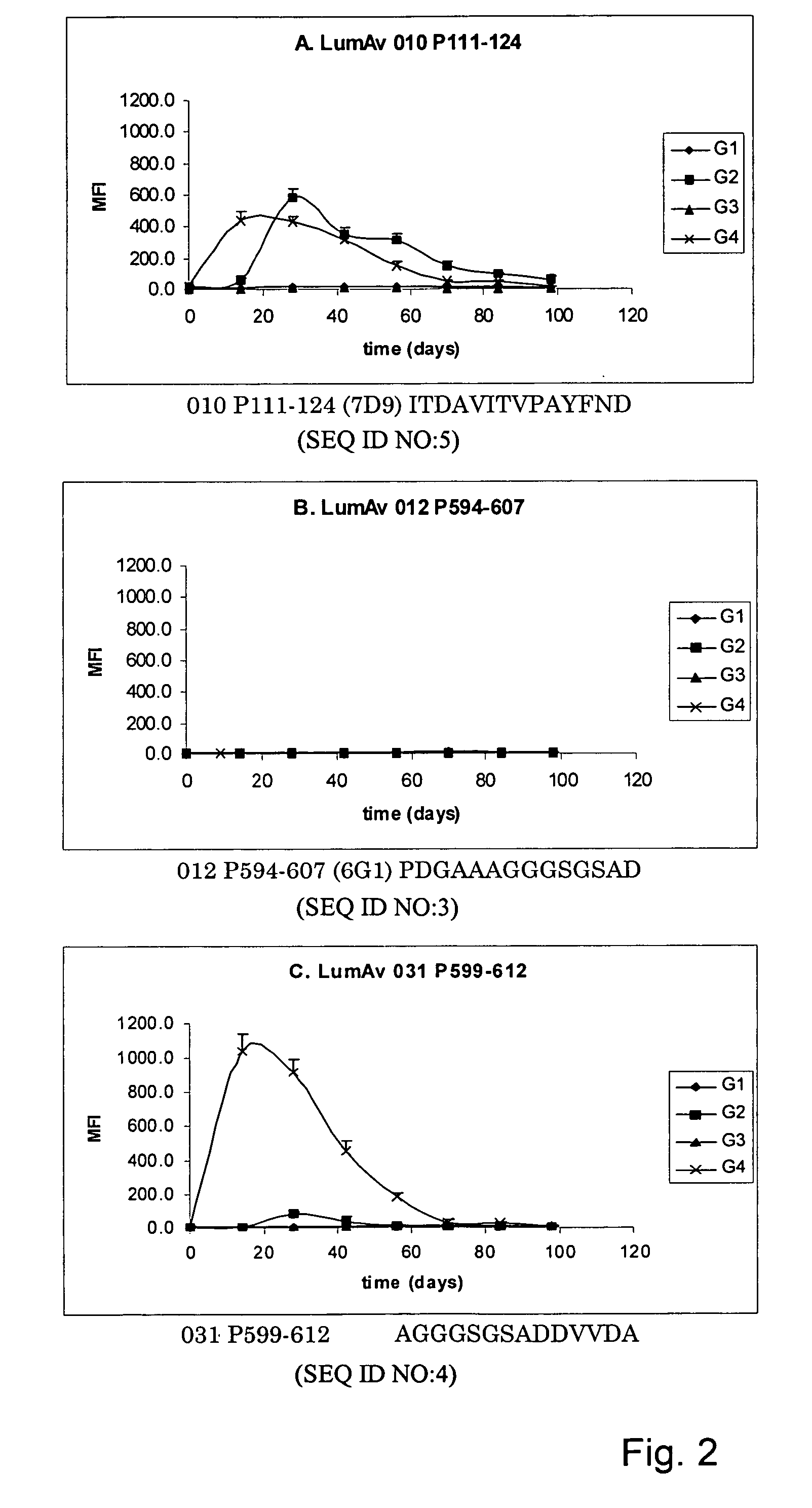
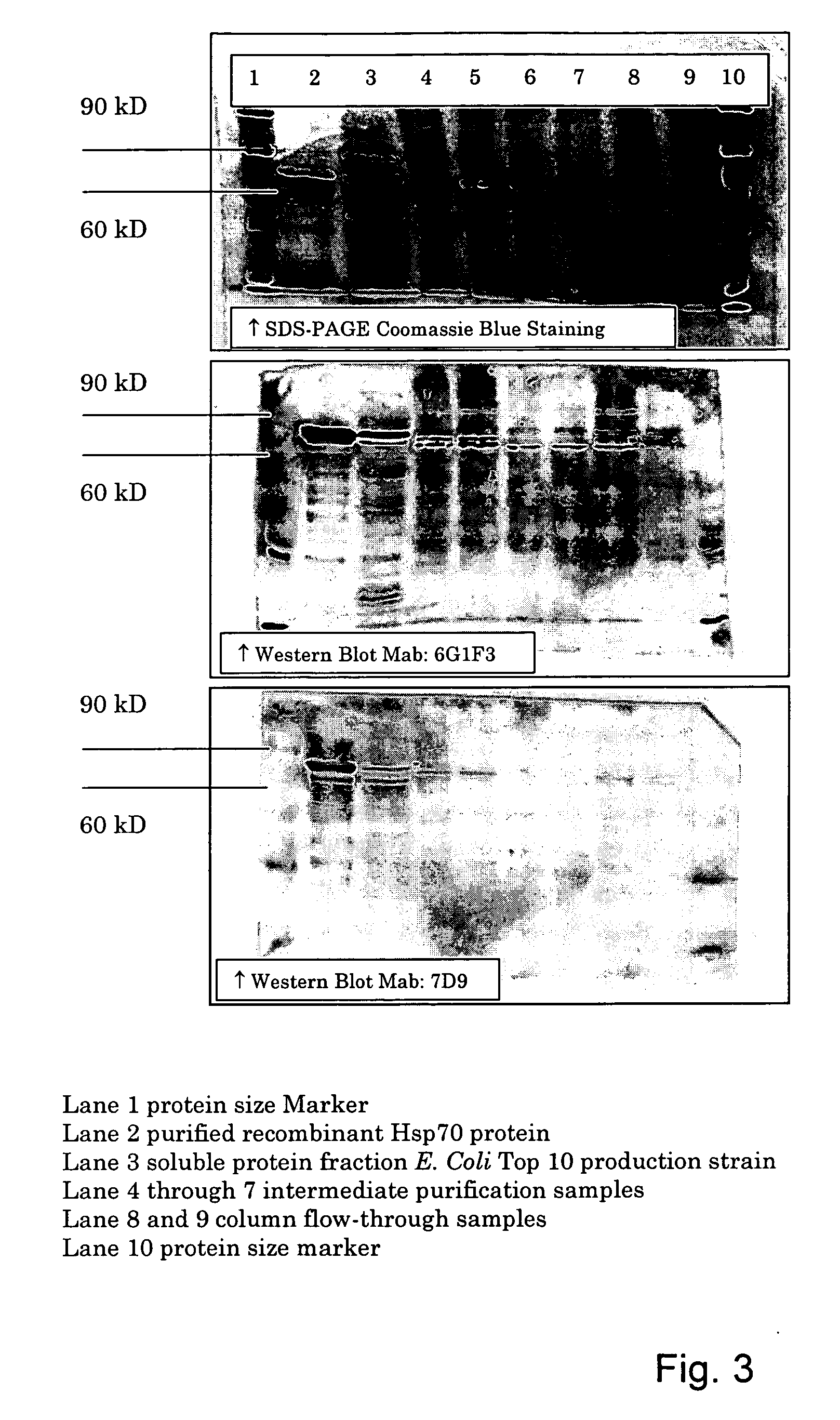
The invention provides a method for detecting infection of an animal with a microorganism that causes a slow progressive disease, comprising providing the animal with a protein of the microorganism, or a functional part, derivative and / or analogue thereof, and measuring an immune response of the animal directed against the microorganism. With a method of the invention, diagnosis during early stages of an infection has become possible. In one aspect, the microorganism comprises Mycobacterium avium subspecies paratuberculosis. Preferably, the protein comprises a surface-associated protein. Peptides comprising B-cell epitopes of M. avium ssp paratuberculosis heat shock protein 70, nucleic acid molecules encoding such peptides and diagnostic kits comprising a peptide and / or nucleic acid molecule of the invention are also herewith provided.
Owner:UTRECHT UNIVERSITY
Mycobacterial infections
InactiveUS20110135578A1Lower Level RequirementsShorten the progressAntibacterial agentsUltrasonic/sonic/infrasonic diagnosticsParatuberculosisDisease
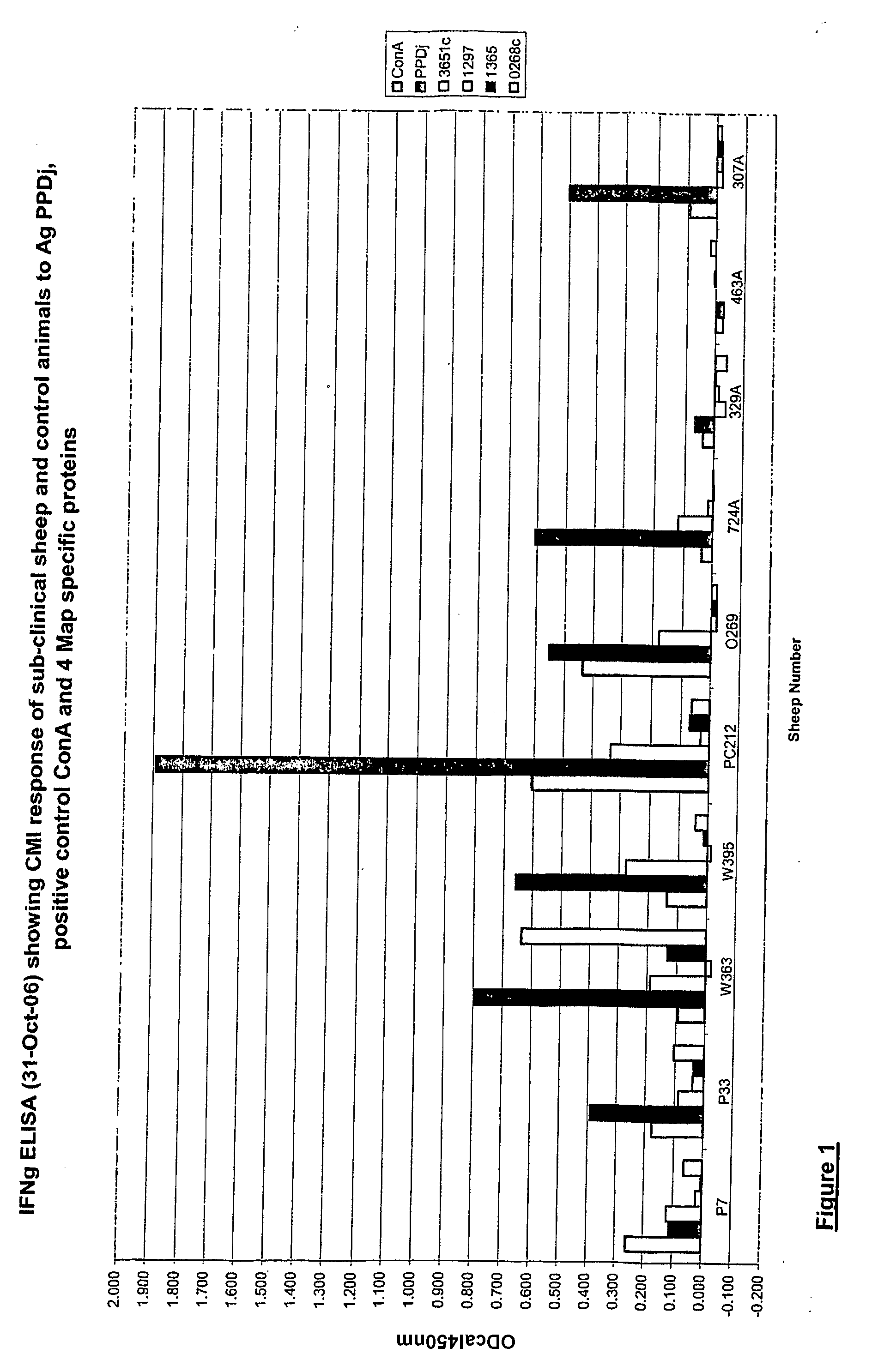
The present invention relates to Mycobacterial infections and provides a method of diagnosing infections of Mycobacterium avium subsp. paratuberculosis (Map), the causative agent of Johne's disease. In addition, the invention also provides as kits for use in the diagnosis of Map infections and vaccines / immunogenic compositions.
Owner:MOREDUN RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com