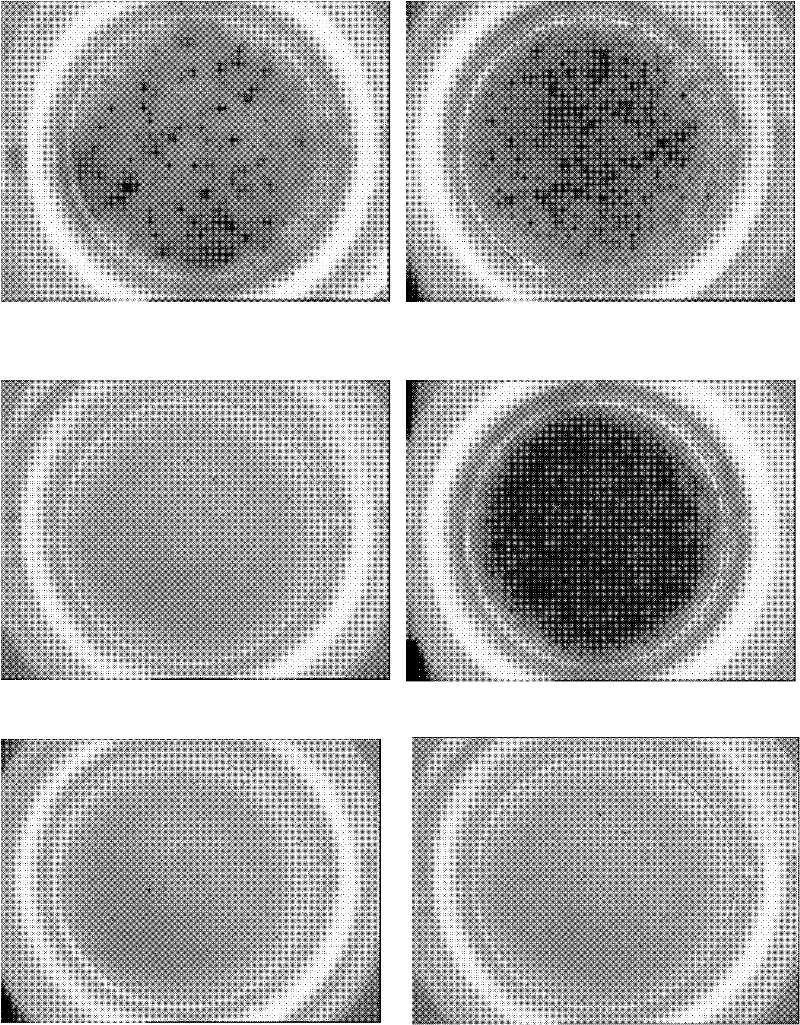
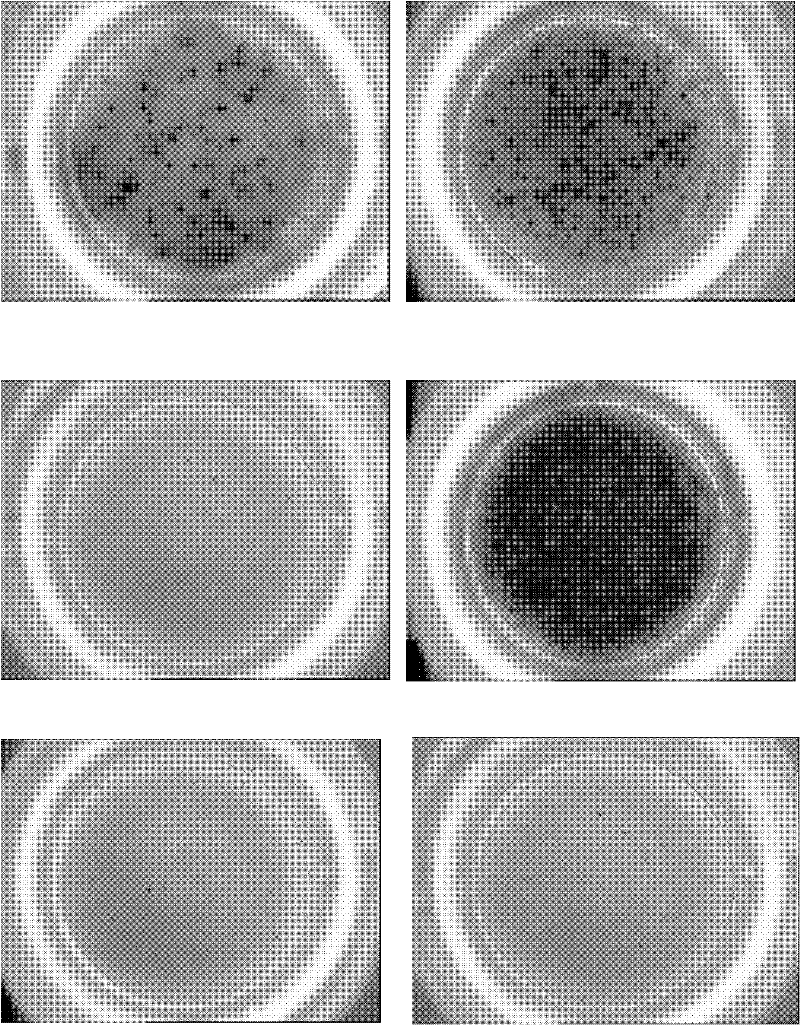
Reagent and method for detecting Mycobacterium tuberculosis infection in vitro
A technology for in vitro detection of Mycobacterium tuberculosis, applied in the field of biomedical testing, can solve the problems of high price, unsatisfactory effect, and difficulty in popularization, and achieve high sensitivity, unsatisfactory overcoming effect, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1M232
[0041] The preparation of embodiment 1M232 polypeptide
[0042] The lymphocytes of PPD-positive tuberculosis patients were detected by ELISPOT, and 21 polypeptides (each polypeptide containing 15 amino acids) of early secretory antigen target-6 (ESAT-6) designed by the Overlap (overlap) method were screened, and 1 A specific T cell reactive Mtb polypeptide, comprising the following steps:
[0043] (1) Design polypeptides by the Overlap method, each polypeptide contains 15 amino acids (aa), and 21 polypeptide sequences are obtained, which are synthesized by a solid-phase formylation synthesizer according to the experimental conditions of the synthesizer manual. Peptides were dissolved in DMSO and prepared as a 10 mg / ml stock solution. Before use, dilute with RPMI1640 culture medium (Note: RPMI is the abbreviation of "Roswell Park Memorial Institute," "Roswell Park Memorial Institute", 1640 is the medium code) to a concentration of 10 μg / ml.
[0044] (2) Collect the blood of P...
Embodiment 2
[0048] Embodiment 2 detects lymphocyte of 310 routine PPD positive tuberculosis patients with ELISPOT
[0049] 1 Materials and methods
[0050] 1.1 Experimental materials The blood of 310 PPD-positive tuberculosis patients was collected from Guangzhou Chest Hospital, about 2-5ml each.
[0051] 1.2 Separation of peripheral blood lymphocytes (PBMCs) from tuberculosis patients Ficoll lymphocyte separation medium was used to separate PBMCs, and PBMCs were resuspended in R10 culture medium (10% calf serum in RPMI1640 culture medium) for later use.
[0052] 1.3 ELISPOT analysis Select a 96-well plate with PVDF membrane as the reaction plate, coat it with anti-human γ-interferon (IFNγ) monoclonal antibody (mAb) overnight, add 5 × 10 5 For PBMC and polypeptide M232, 3 wells were set up for each assay and incubated for 18 hours. The following control wells were set up: PMA and Ionomycin were used as positive controls, no polypeptide was used as negative control, no cells were used as...
Embodiment 3
[0055] Example 3 ELISPOT is used to detect the effect of mixed polypeptide M232+M233 (1:1) on lymphocytes of 529 cases of PPD positive tuberculosis patients
[0056] The M233 polypeptide is a polypeptide disclosed in the 200810220523.1 patent application filed by the applicant, and its amino acid sequence is: SEQ ID No.2.
[0057] 1 Materials and methods
[0058] 1.1 Experimental materials The blood of 529 PPD-positive tuberculosis patients was collected from Guangzhou Chest Hospital, about 2-5ml each.
[0059] 1.2 Separation of peripheral blood lymphocytes (PBMCs) from tuberculosis patients Ficoll lymphocyte separation medium was used to separate PBMCs, and PBMCs were resuspended in R10 culture medium (10% calf serum in RPMI1640 culture medium) for later use.
[0060] 1.3 After the synthesis of M232 and M233 polypeptides, they were dissolved in DMSO and prepared as 10 mg / ml stock solutions. Before use, appropriate amounts of M232 and M233 were diluted with RPMI1640 culture s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com