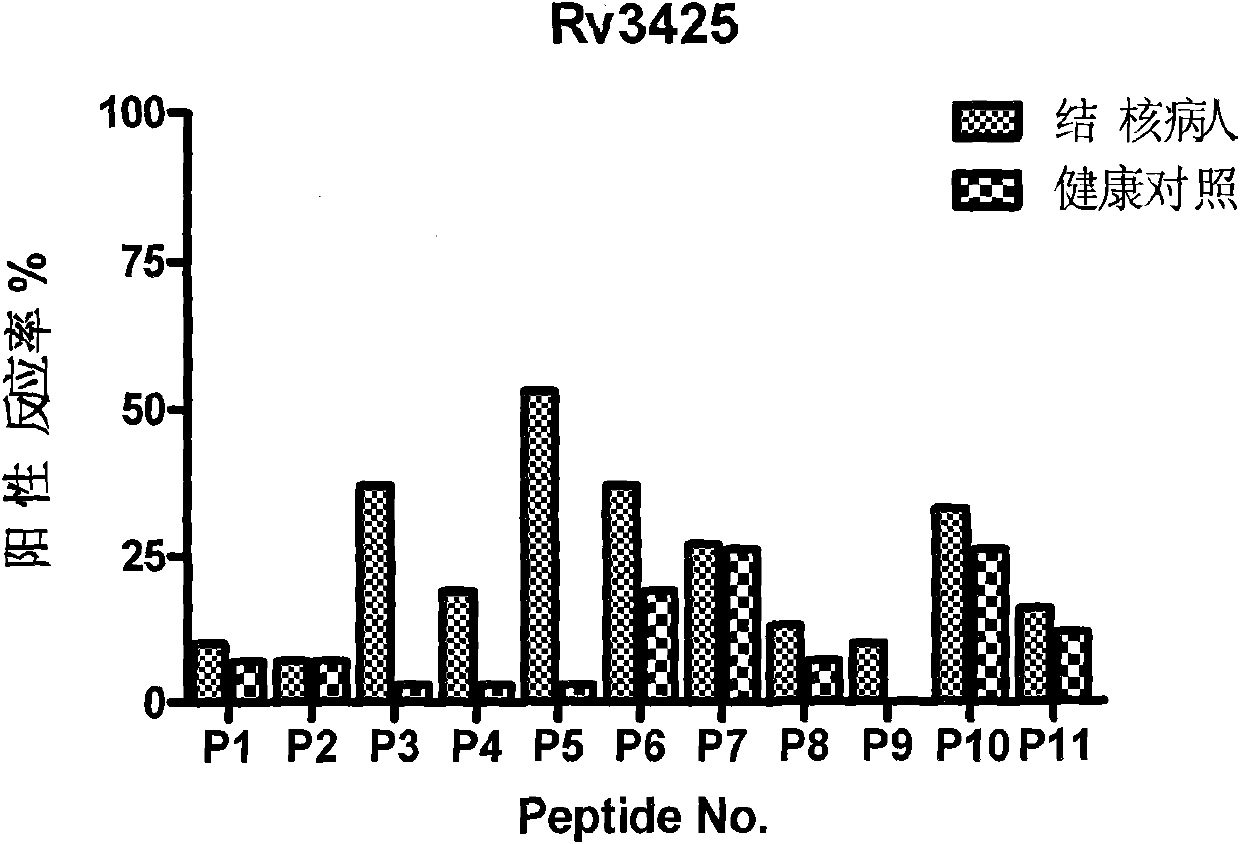
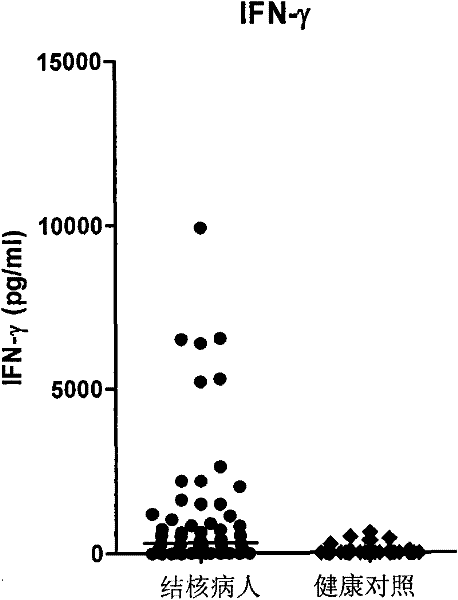
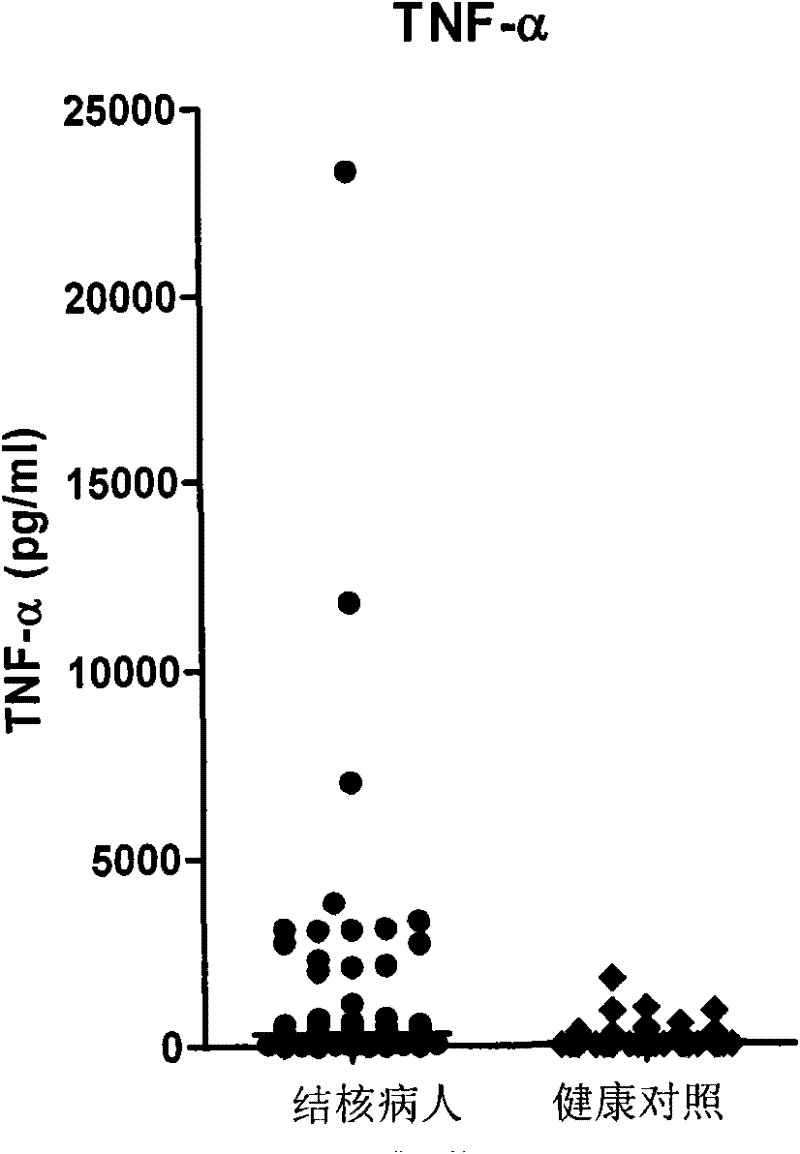
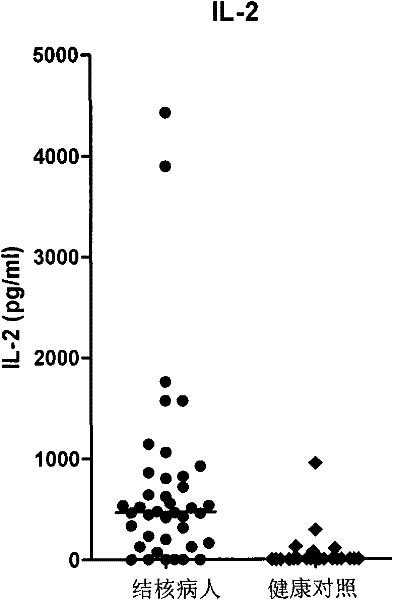
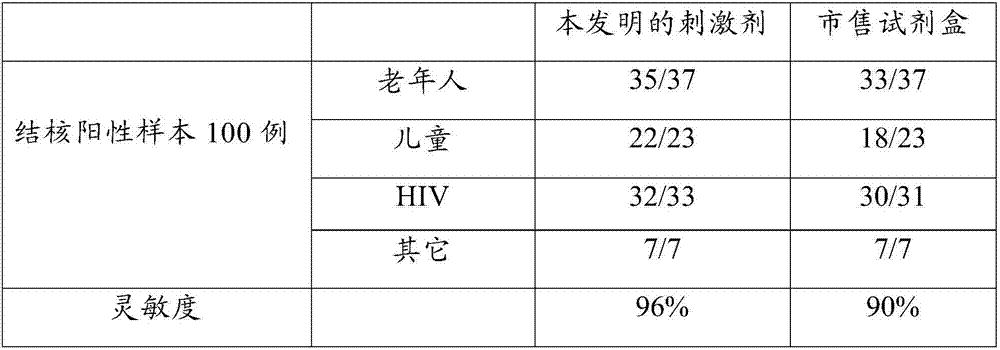
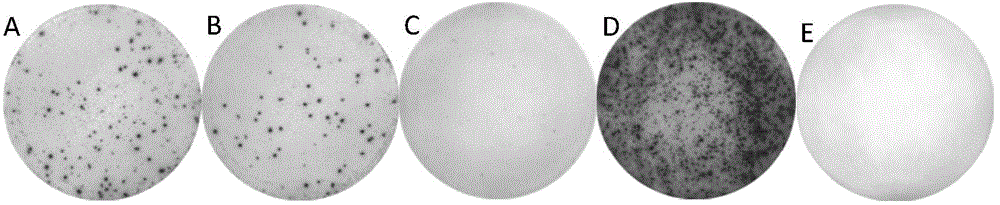
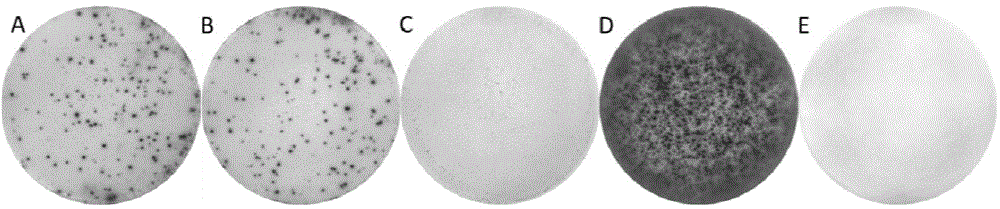
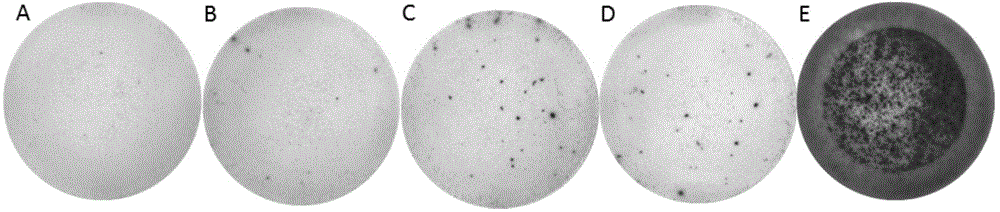
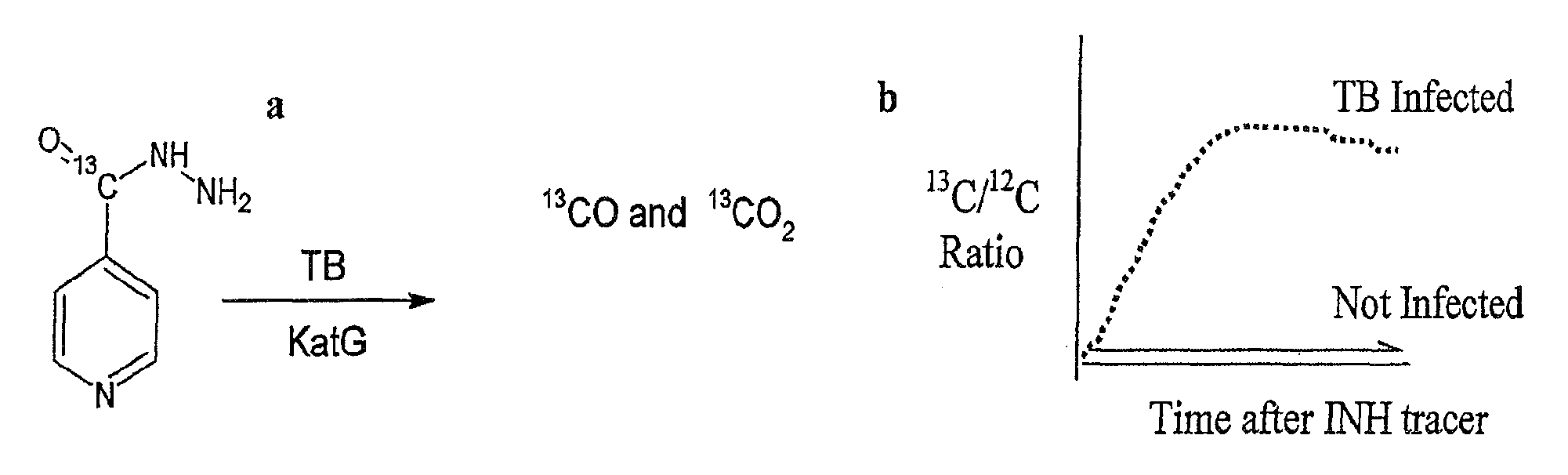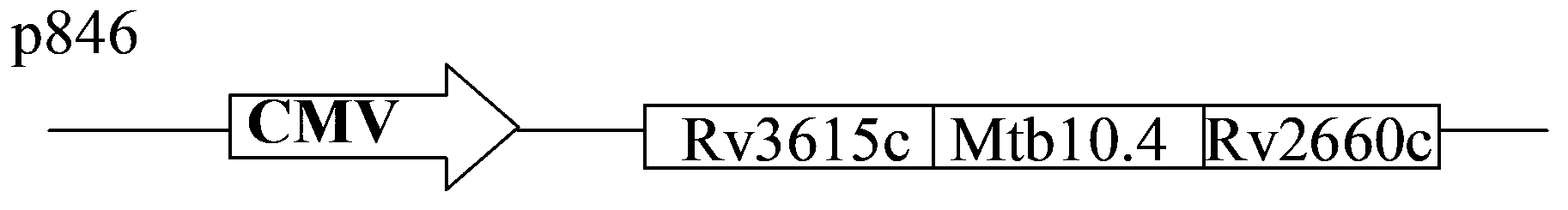Patents
Literature
237 results about "Mycobacterium tuberculosis Infections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tuberculosis (TB) is a disease caused by an infection with the bacteria Mycobacterium tuberculosis complex.
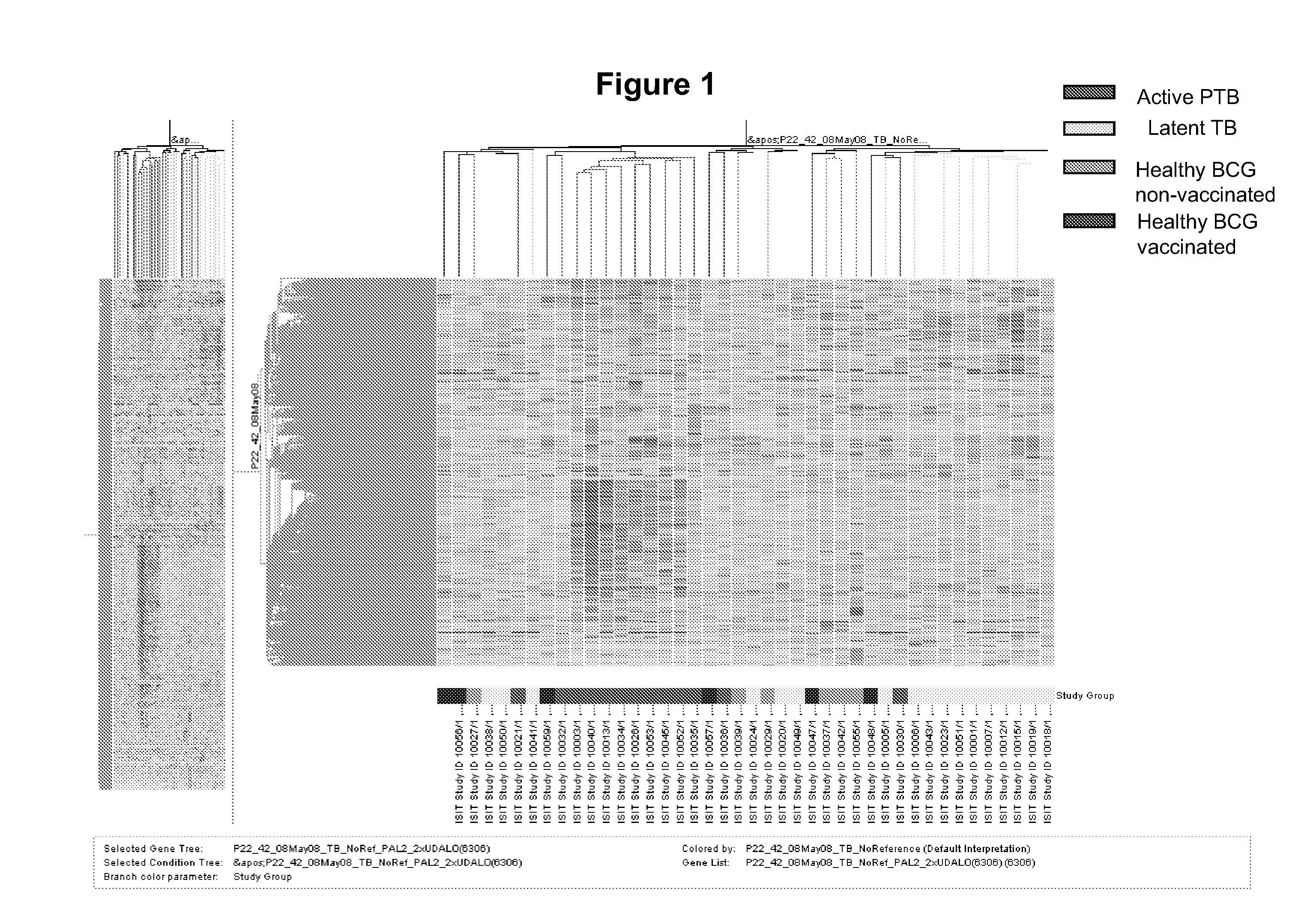
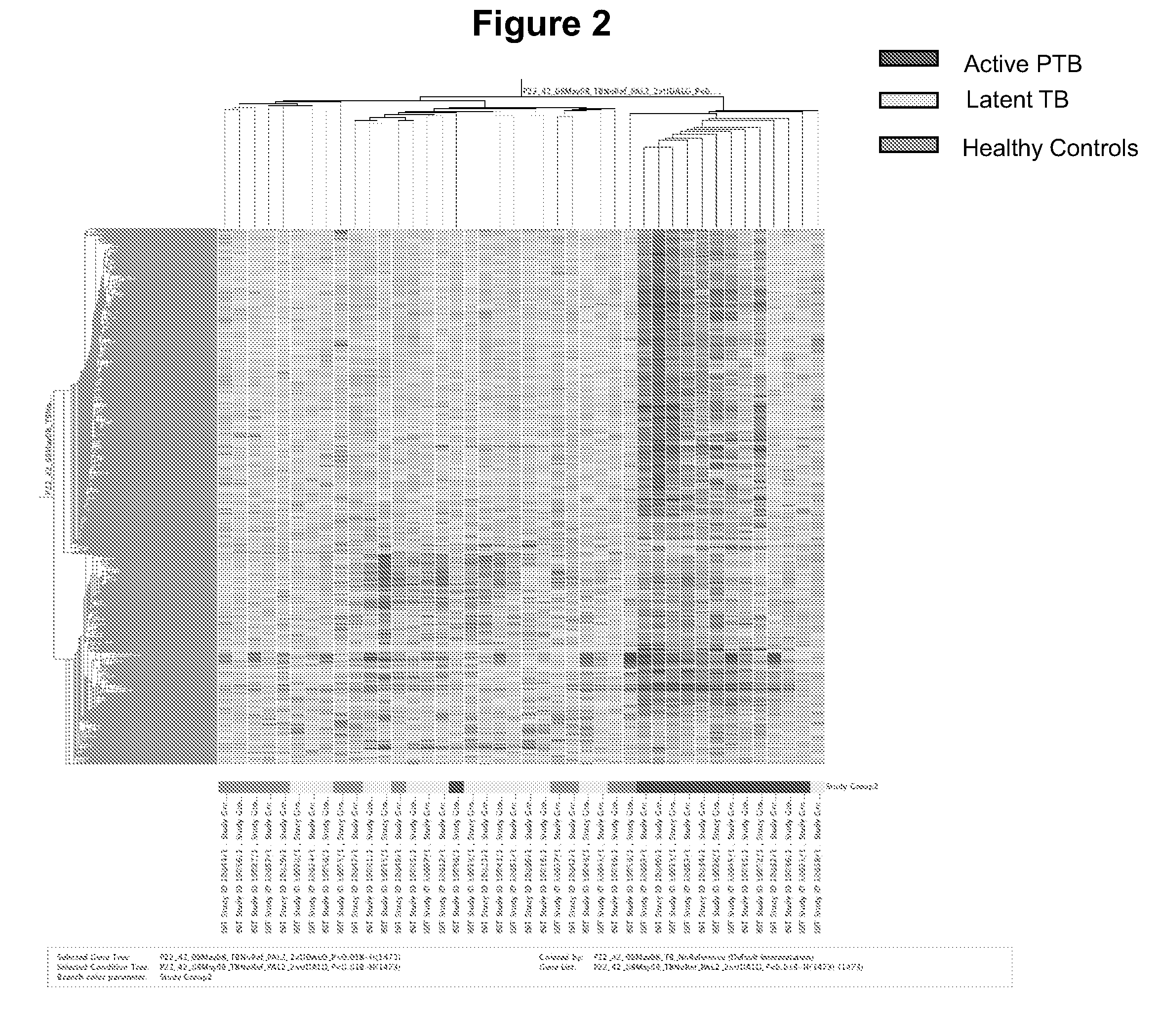
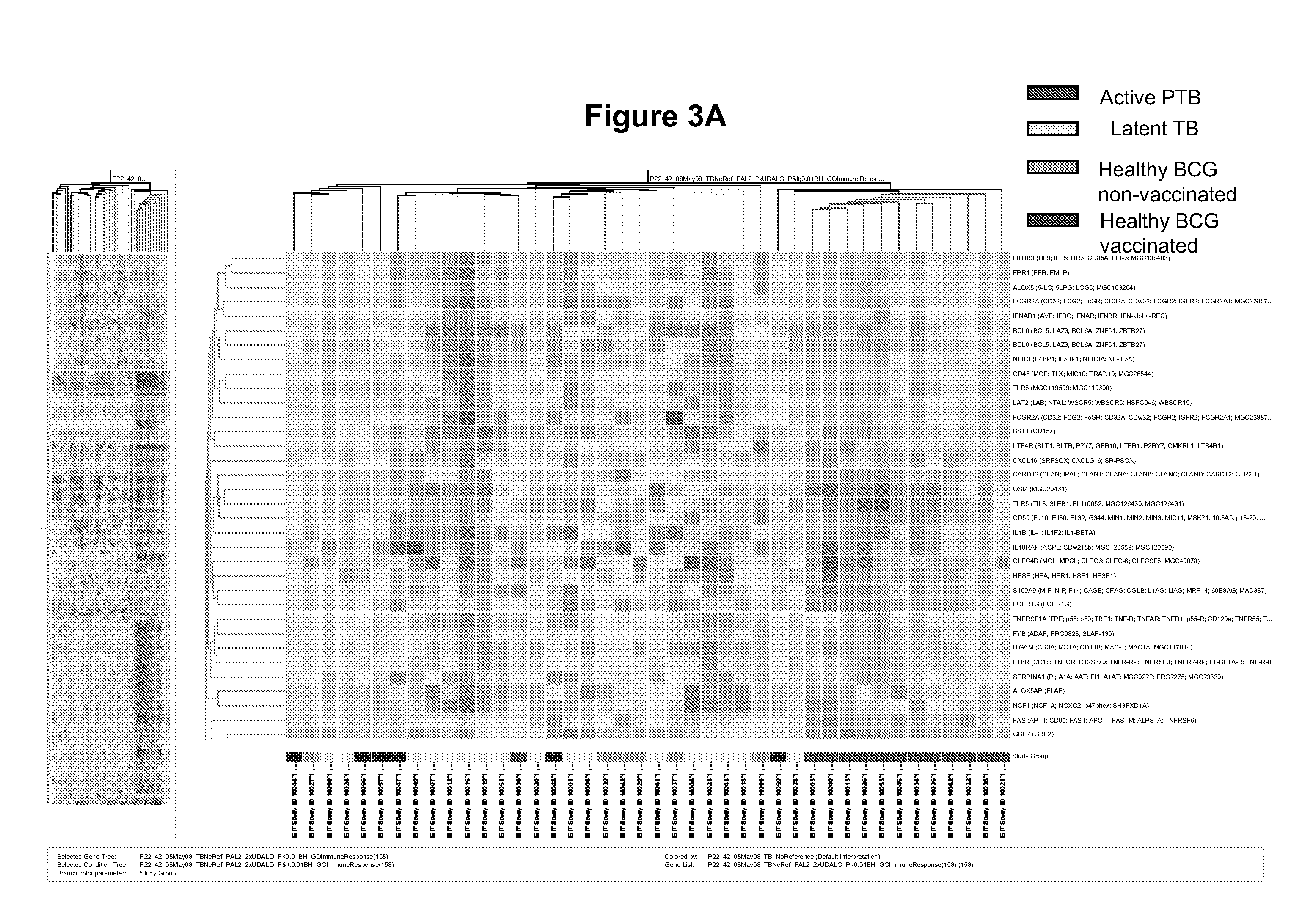
Blood transcriptional signature of mycobacterium tuberculosis infection
InactiveCN102150043AMicrobiological testing/measurementDisease diagnosisInfected patientMycobacterium Infections
The present invention includes methods, systems and kits for distinguishing between active and latent mycobacterium tuberculosis infection in a patient suspected of being infected with mycobacterium tuberculosis, and distinguishing such patients from uninfected individuals, the method including the steps of obtaining a gene expression dataset from a whole blood obtained sample from the patient and determining the differential expression of one or more transcriptional gene expression modules that distinguish between infected and non-infected patients, wherein the dataset demonstrates an aggregate change in the levels of polynucleotides in the one or more transcriptional gene expression modules as compared to matched non- infected patients, thereby distinguishing between active and latent mycobacterium tuberculosis infection.
Owner:BAYLOR RES INST +2
Kit and method for detecting mycobacterium tuberculosis infection and application
ActiveCN102004155ALow costQuality assuranceBiological testingMycobacterium InfectionsLatent tuberculosis
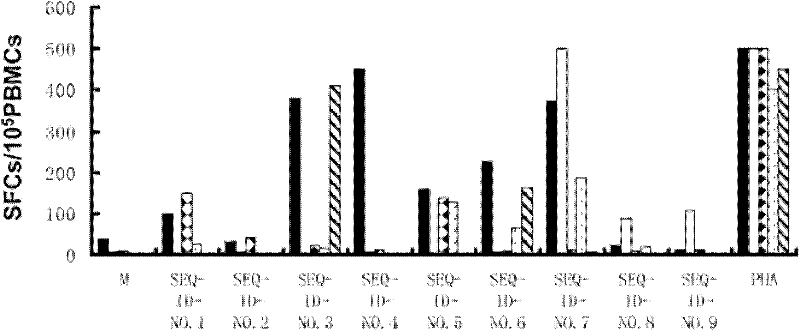
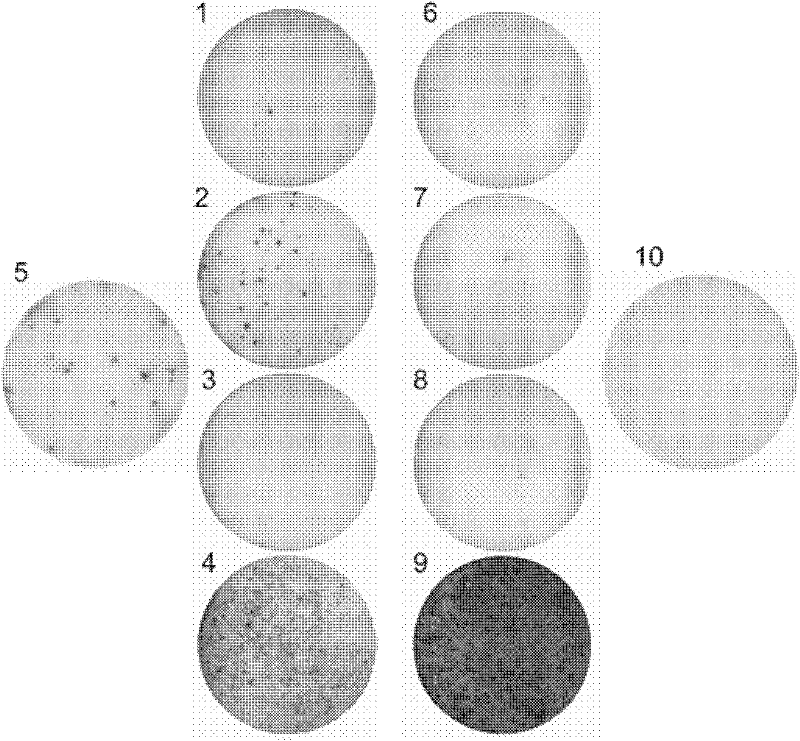
The invention belongs to the field of biomedicine examination, and particularly relates to a kit and a method for detecting mycobacterium tuberculosis infection and application. The invention discloses a novel mycobacterium tuberculosis detection reagent by screening specific T cell epitope of mycobacterium tuberculosis, wherein the reagent contains polypeptide or analog thereof represented by SEQ ID No.1-10. The method detects cell factors released from T cells by using single or more SEQ ID No.1-10 polypeptides to contact the T cells of mycobacterium tuberculosis infected individuals. The method can effectively detect active tuberculosis or latent tuberculosis infection, and is free from disturbance of Bacilli Calmette Guerin (BCG) inoculation vaccines. The invention also discloses a diagnostic kit and other application based on the polypeptide and the method. Compared with the gamma interferon release experiments in the prior art, the method can obviously improve the detection rate without reducing the specificity and has high clinical application value.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Kits for Auxiliary Diagnosis of Tuberculosis
InactiveCN102297968AIncreased sensitivityReduce positive rateImmunoglobulins against cytokines/lymphokines/interferonsDepsipeptidesAIDS diagnosisIfn gamma
The invention discloses a kit for assisted diagnosis of tuberculosis, which comprises a specific antibody composition, wherein the specific antibody composition comprises the following five antibodies: (1) IFN-gamma antibody, (2) TNF-alpha antibody, (3) IL-2 antibody, (4) MIG antibody and (5) IP-10 antibody. The kit disclosed by the invention can distinguish a Mycobacterium tuberculosis infected person from a BCG vaccinee. Compared with the ELISPOT tuberculosis diagnosis method, the tuberculosis diagnosis kit based on multi-molecular marker detection has obviously enhanced sensitivity to active tuberculosis (from 72% to 89%), and the positive rate for normal healthy persons is obviously reduced (from 27% to 16%).
Owner:程小星 +3
Blood transcriptional signature of mycobacterium tuberculosis infection
InactiveUS20110196614A1Microbiological testing/measurementDisease diagnosisInfected patientMycobacterium Infections
The present invention includes methods, systems and kits for distinguishing between active and latent mycobacterium tuberculosis infection in a patient suspected of being infected with mycobacterium tuberculosis, and distinguishing such patients from uninfected individuals, the method including the steps of obtaining a gene expression dataset from a whole blood obtained sample from the patient and determining the differential expression of one or more transcriptional gene expression modules that distinguish between infected and non-infected patients, wherein the dataset demonstrates an aggregate change in the levels of polynucleotides in the one or more transcriptional gene expression modules as compared to matched non-infected patients, thereby distinguishing between active and latent mycobacterium tuberculosis infection.
Owner:BAYLOR RES INST +2
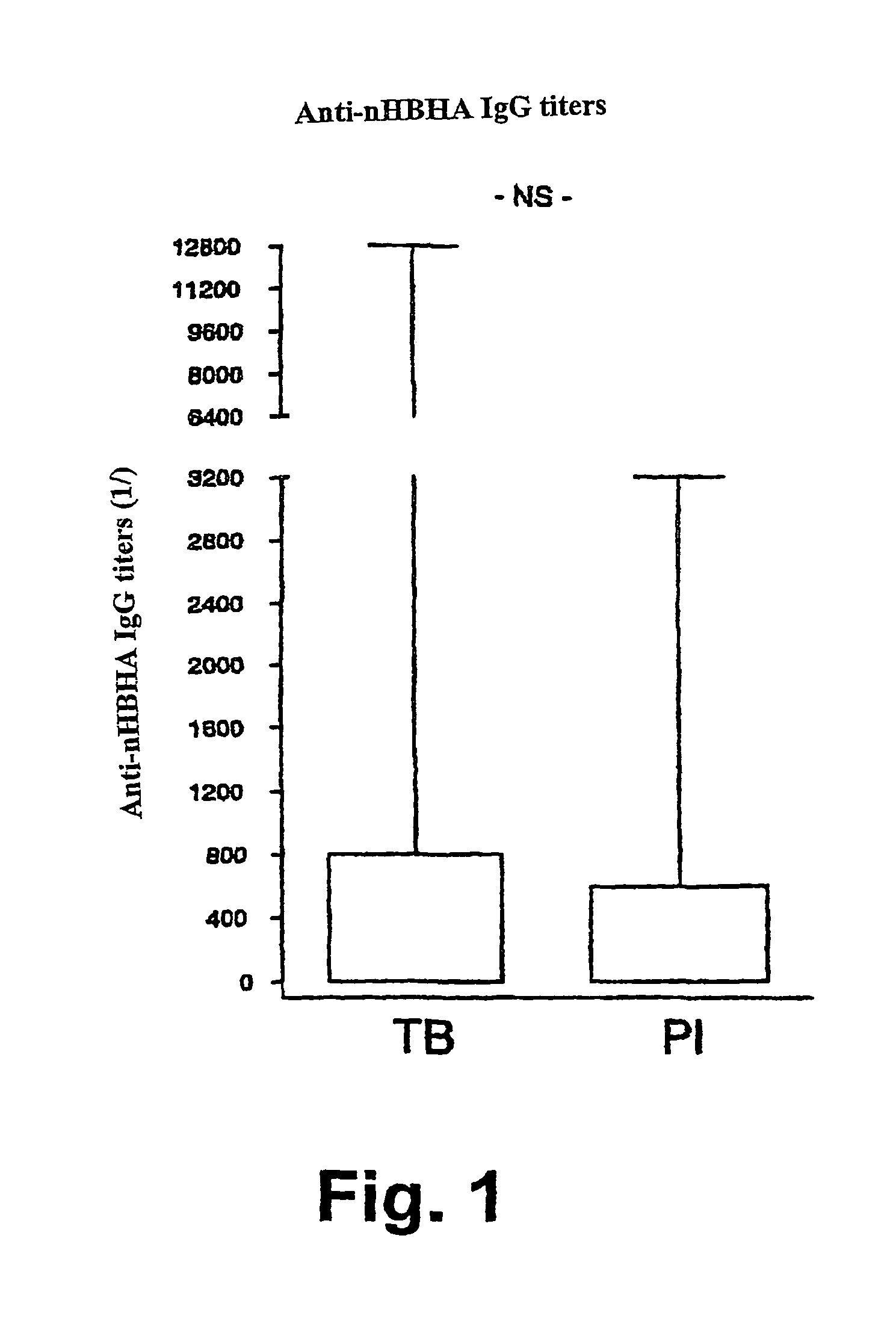
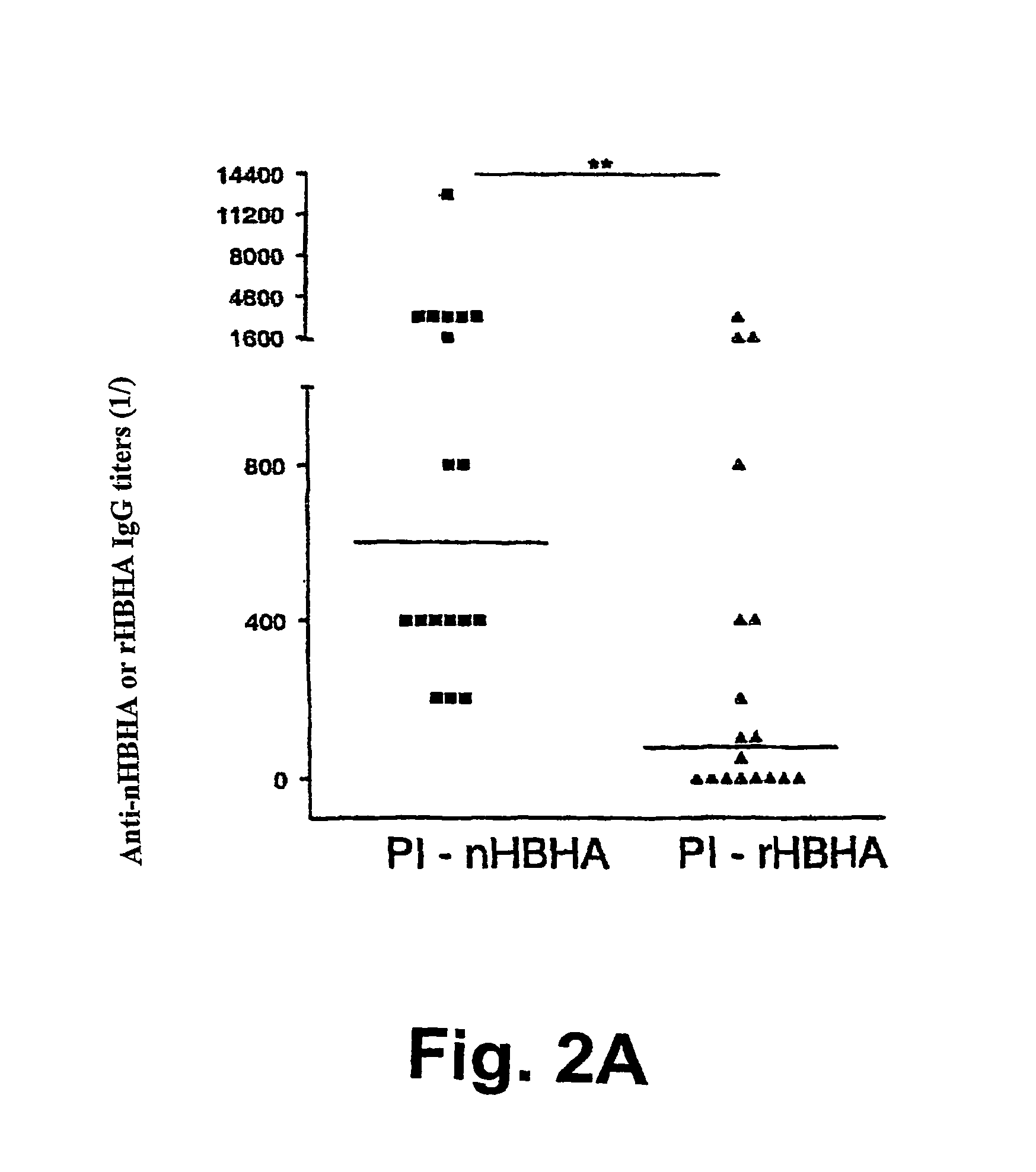
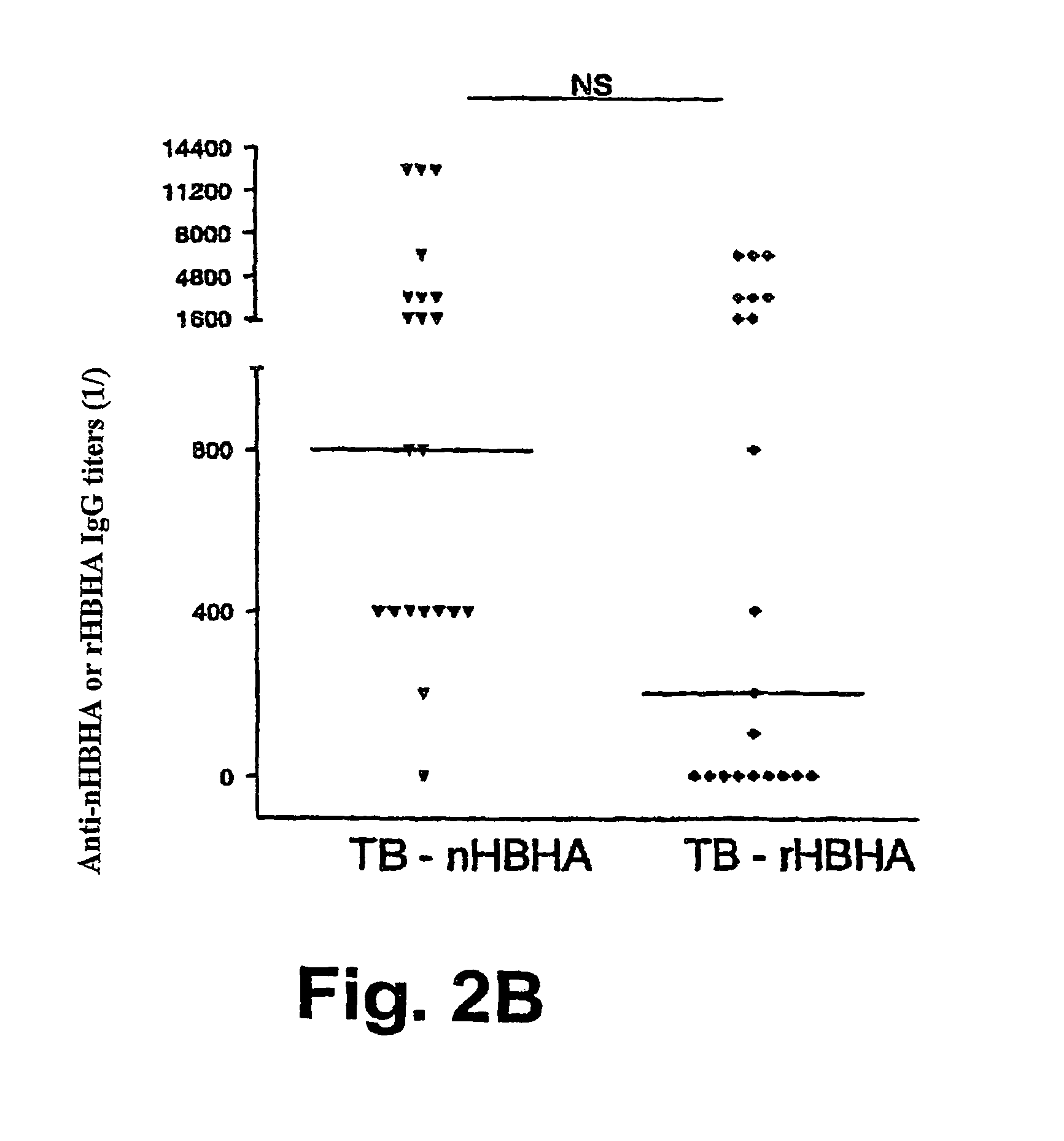
Detection of tuberculosis and infection by Mycobacterium tuberculosis using HBHA
The present invention concerns methods for in vitro detection of an infection by Mycobacterium tuberculosis in mammals, and methods for in vitro distinction between mammals infected with Mycobacterium tuberculosis in which the disease is declared (active form) and mammals which are infected but asymptomatic for tuberculosis (latent form), and a method for in vitro distinction between mammals presenting an active form of tuberculosis and mammals not infected by M. tuberculosis or presenting a latent form of tuberculosis. The present invention also pertains to kits for detection and distinction between infected mammals presenting tuberculosis symptoms and infected mammals with no disease development, and a kit for distinguishing between mammals presenting an active form of tuberculosis and mammals not infected by M. tuberculosis or presenting a latent form of tuberculosis.
Owner:INSTITUT PASTEUR DE LILLE +3
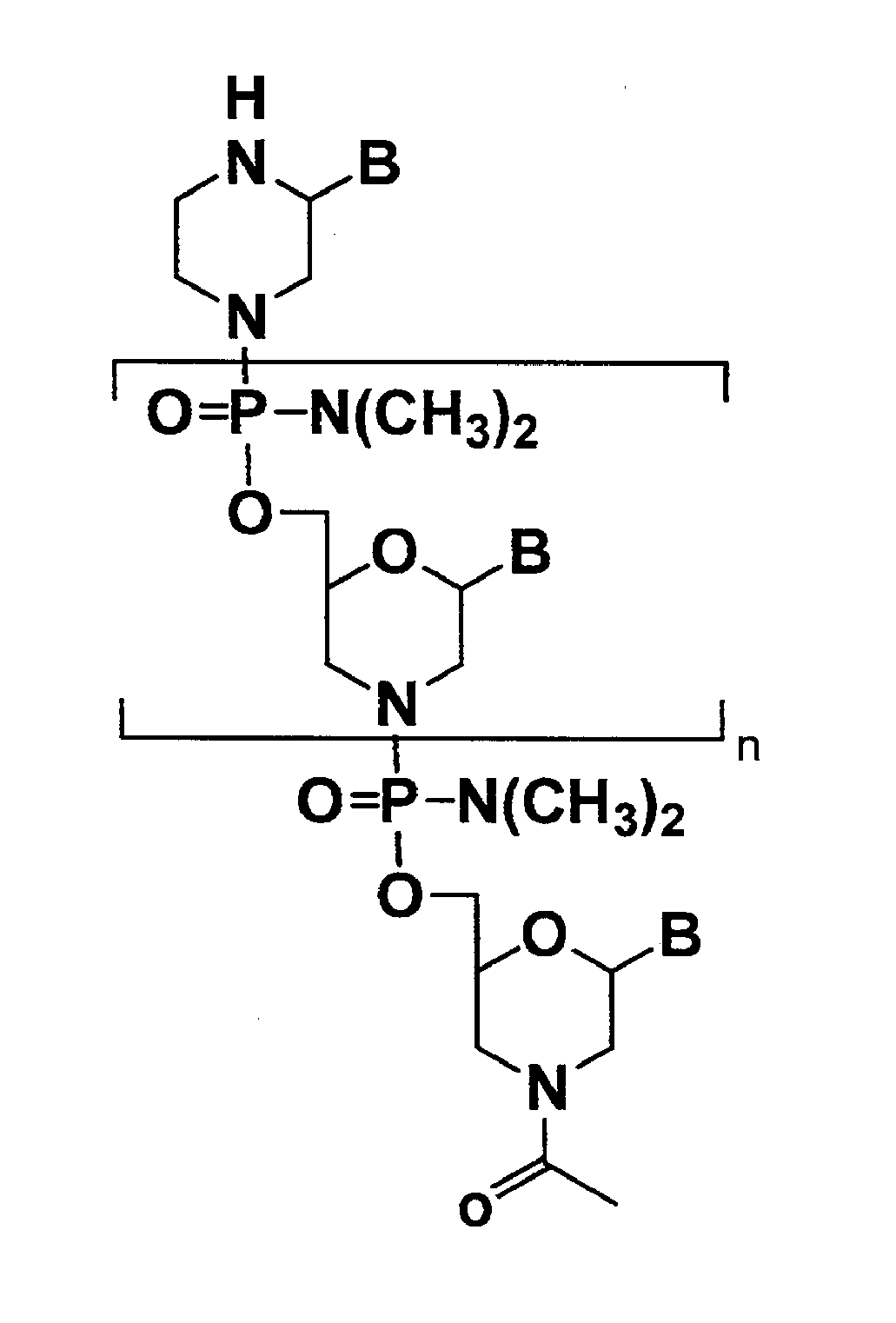
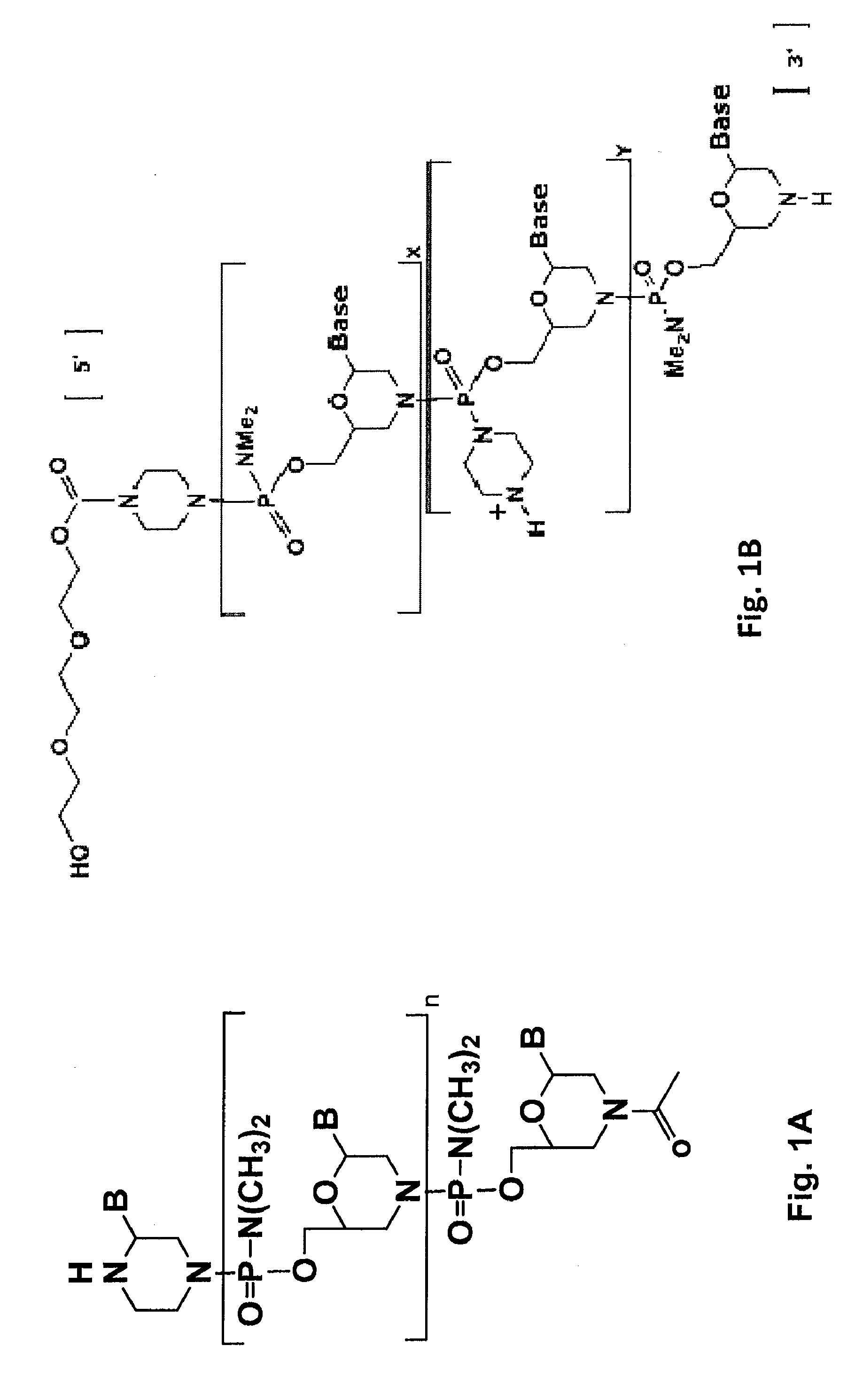
Antisense antibacterial compounds and methods
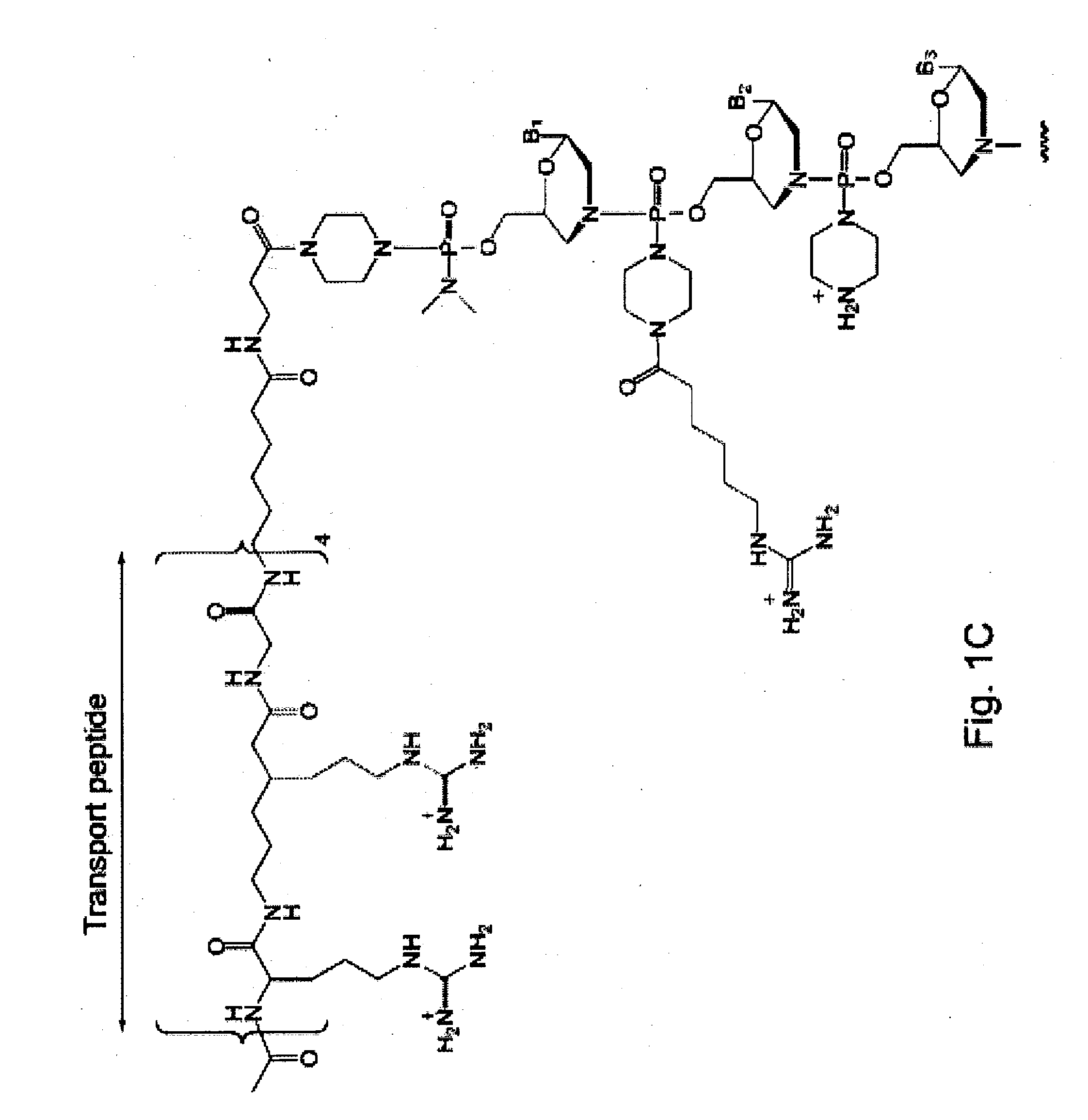
Antibacterial antisense compounds and methods of their use in treating a Mycobacterium tuberculosis infection in a mammalian host are disclosed. The compounds include an antisense oligonucleotide conjugated to a carrier peptide that significantly enhances the antibacterial activity of the oligonucleotide. The antisense oligonucleotides contain 10-20 nucleotide bases and have a targeting nucleic acid sequence complementary to a target sequence containing or within 20 bases, in a downstream direction, of the translational start codon of a bacterial mRNA that encodes a bacterial protein essential for bacterial replication, where the compound binds to a target mRNA with a Tm of between 45° to 60° C. The carrier peptide is an arginine-rich peptide containing between 6 and 14 amino acids. Antisense compounds that target host factor genes that facilitate Mycobacterium tuberculosis infection are also provided, as are methods of using these compounds to treat Mycobacterium tuberculosis infections, alone or in combination with other therapies.
Owner:AVI BIOPHARMA
Application of kelimycin in mycobacterium tuberculosis infection resistance
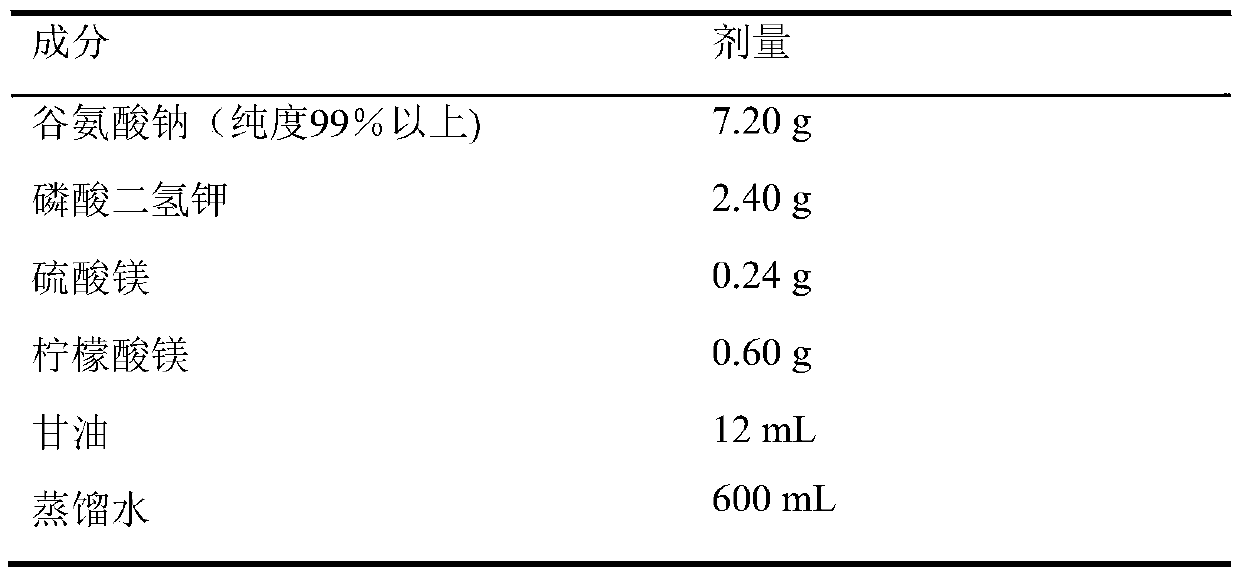
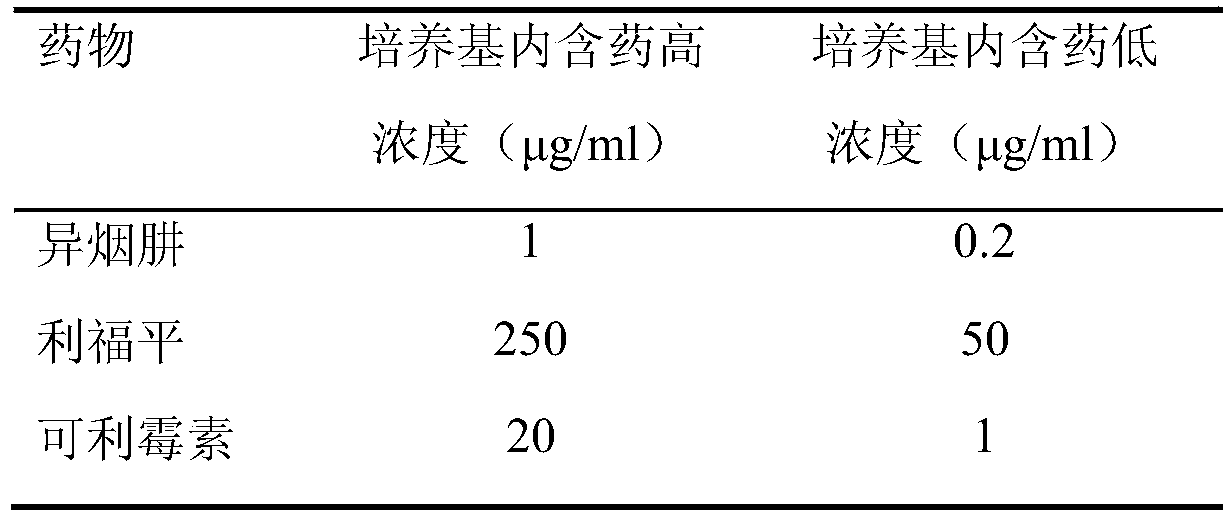
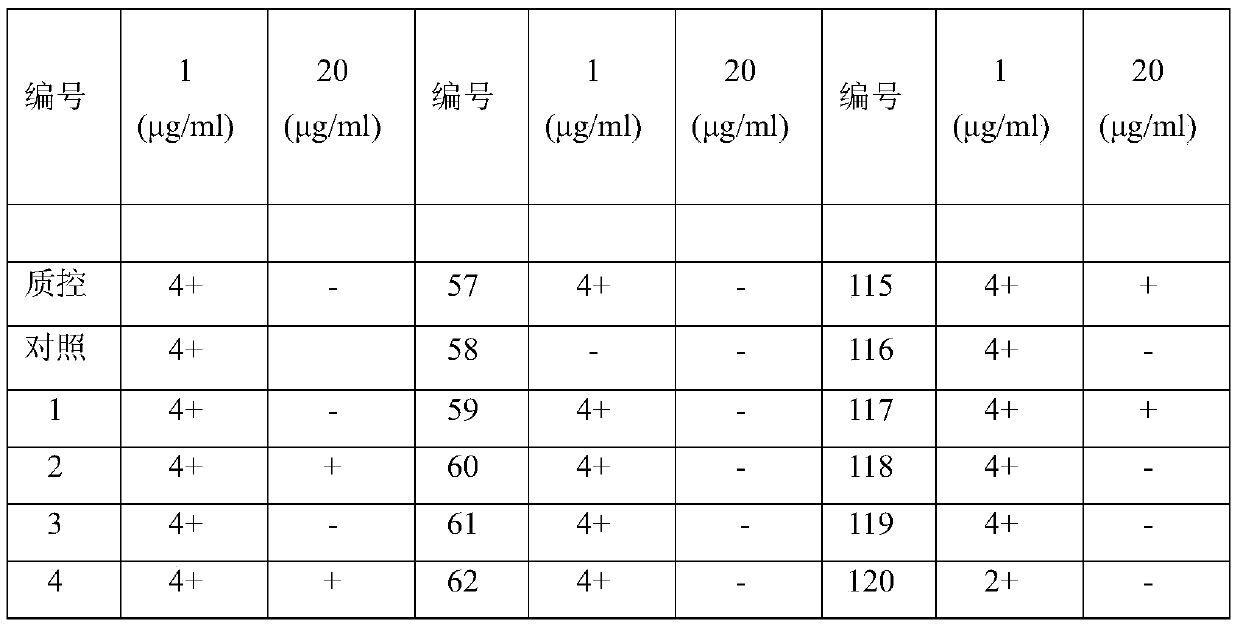
The invention relates to application of kelimycin in mycobacterium tuberculosis infection resistance. The application comprises the following main steps: clinical first-line antitubercular drugs isoniazide and rifamycin are used as contrast, and the antimycobacterial activity of kelimycin is detected by adopting an absolute concentration method. The result indicates that the activity of kelimycin on clinically separated mycobacterium tuberculosis including drug-resistance bacteria is remarkably superior to those of the clinical first-line contrast drugs isoniazide and rifamycin, and novel application of kelimycin is expected to be developed in treatment of tubercle bacillus infected diseases.
Owner:SHANGHAI TONGLIAN PHARMA CO LTD
Kit for detecting mycobacterium tuberculosis infection and monitoring clinical treatment effect and application of kit
ActiveCN104020297AIncreased sensitivityImprove featuresDisease diagnosisBiological testingTherapeutic effectSpecific antibody
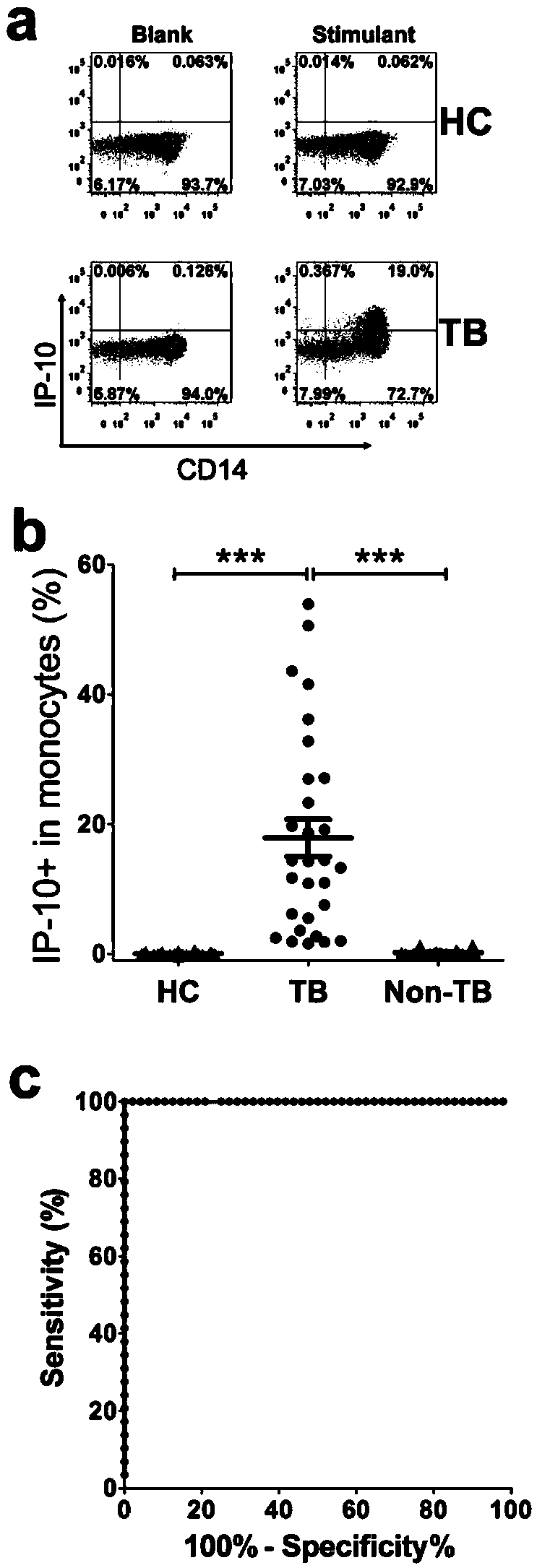
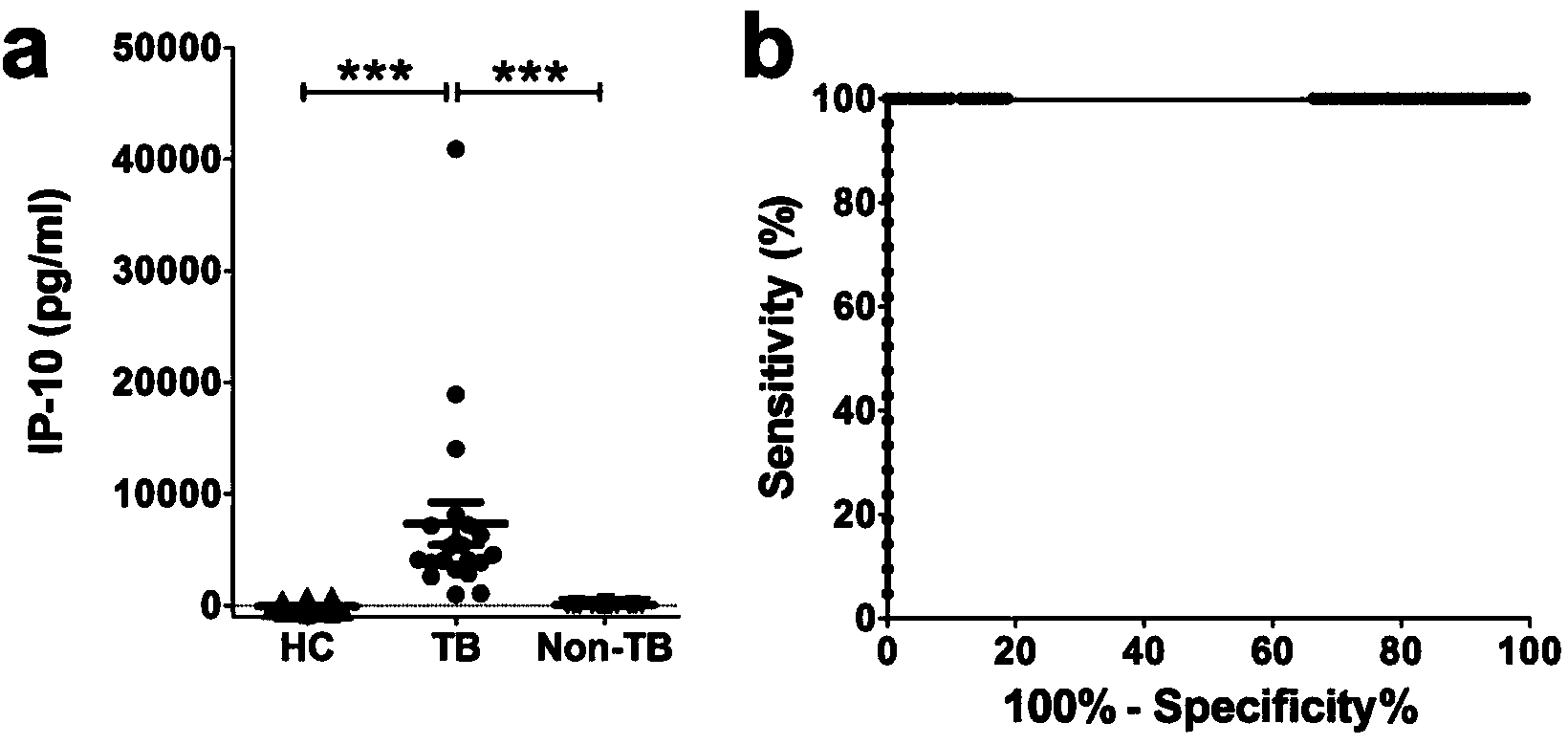
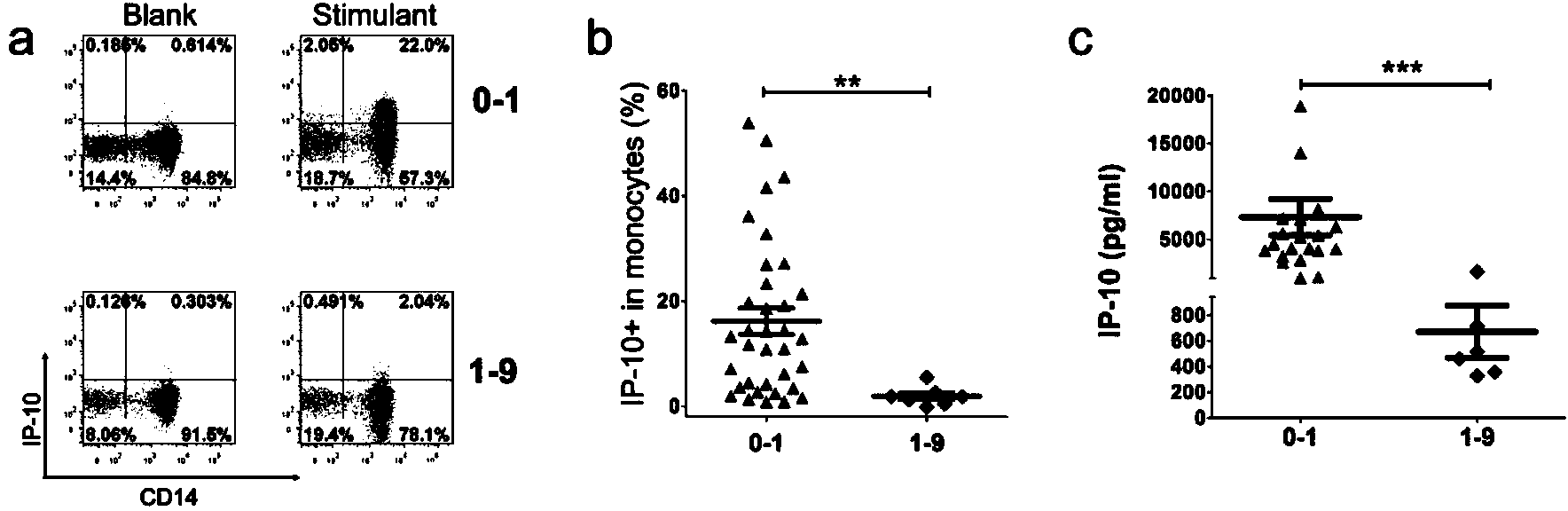
The invention discloses a kit for detecting the mycobacterium tuberculosis infection and monitoring a clinical treatment effect. The kit provided by the invention comprises a specific antibody, namely an IP-10 antibody and / or CD14 antibody, an antigen irritant and a positive contrast irritant. The kit disclosed by the invention can be used for diagnosing an active tuberculosis patient or a tuberculosis latent infection patient and is not affected by BCG (bacillus calmette-guerin) inoculation. The sensitivity and the specificity of the kit for diagnosing the active tuberculosis patient are higher than those of a commercial T-SPOT.TB kit and can be up to 100 percent. After the antituberculosis therapy is performed for 1 month, the detection rates of over 80 percent of clinical tuberculosis patients are converted to be negative, so that the kit can be used for detecting the clinical antituberculosis treatment effect.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Epitope polypeptide applicable to mycobacterium tuberculosis infection detection and application thereof
ActiveCN102516356AHigh T cell reactivityDiagnosis of infectionAntibacterial agentsPeptide/protein ingredientsEpitopeMycobacterium Infections
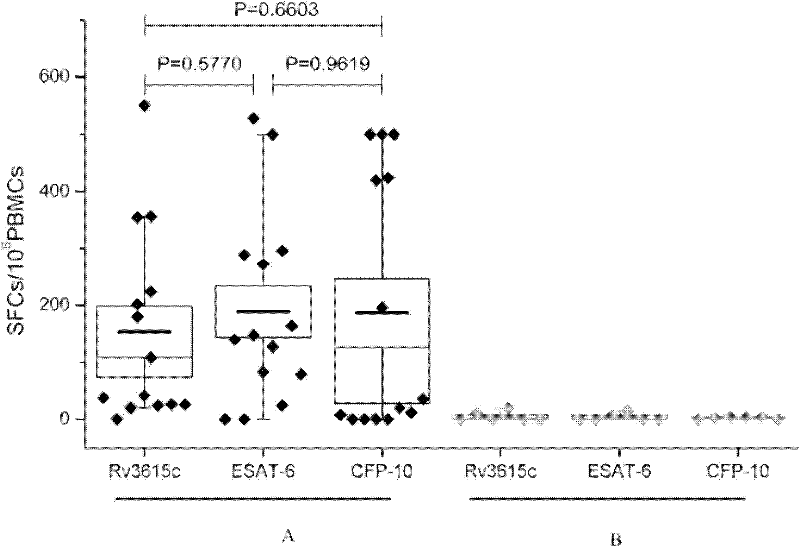
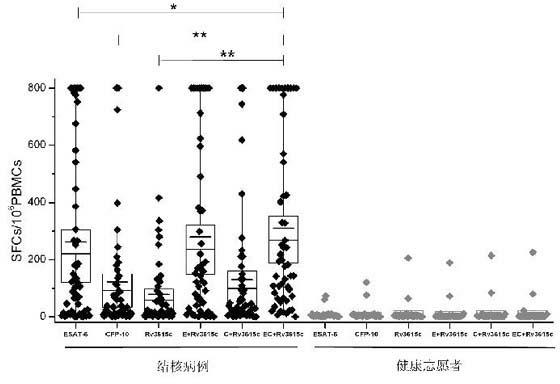
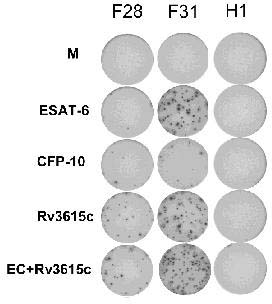
The invention discloses certain mycobacterium tuberculosis specific antigen epitope polypeptides, and particularly discloses an antigen Pv3615c antigen epitope polypeptide consisting of 8-11 continuous polypeptide fragments. The clinical detection practicability of the antigen polypeptide is evaluated according to the reaction sensitivity and specificity of the antigen polypeptide in a tuberculosis case. As proved by a result, an antigen polypeptide has high T cell reactivity in a tuberculosis patient, and the positive rate is up to 64 percent (9 / 14). Compared with the result of a healthy volunteer, the antigen epitope polypeptide has the specificity of up to 100 percent (6 / 6). Mycobacterium tuberculosis infection can be diagnosed effectively by applying an antigen peptide library specific T cell gamma interferon.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Compounds for diagnosis of tuberculosis and methods of their use
InactiveUS20010012888A1Detect presenceBacteriaAntibody mimetics/scaffoldsAntigenMycobacterium Infections
Compounds and methods for diagnosing tuberculosis are disclosed. The compounds provided include polypeptides that contain at least one antigenic portion of one or more M. tuberculosis proteins, and DNA sequences encoding such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of M. tuberculosis infection in patients and biological samples. Antibodies directed against such polypeptides are also provided.
Owner:CORIXA CORP
Methods of Diagnosis and Treatment of M.Tuberculosis Infection and Reagents Therefor
InactiveUS20090011426A1Reduce in quantityImprove solubilityAntibacterial agentsPeptide/protein ingredientsImmunogenic peptideImmunogenicity
The present invention provides diagnostic, prognostic and therapeutic reagents for infection of an animal subject such as a human by M. tuberculosis, and conditions associated with such infections, such as, for example, tuberculosis. More particularly, the present invention provides a recombinant protein of M. tuberculosis designated “BSX” (SEQ ID NO: 1) and immunogenic epitopes thereof such as, for example, comprising SEQ ID NOS: 34, 25 and 45, that are useful in antibody-based diagnostic applications. The present invention also provides antibodies against BSX and its immunogenic peptides that are useful for antigen-based diagnostic and prognostic tests, and for therapy and vaccine formulations.
Owner:PROTEOME SYST LTD
Use of an il12 receptor-beta 1 splice variant to diagnose active tuberculosis
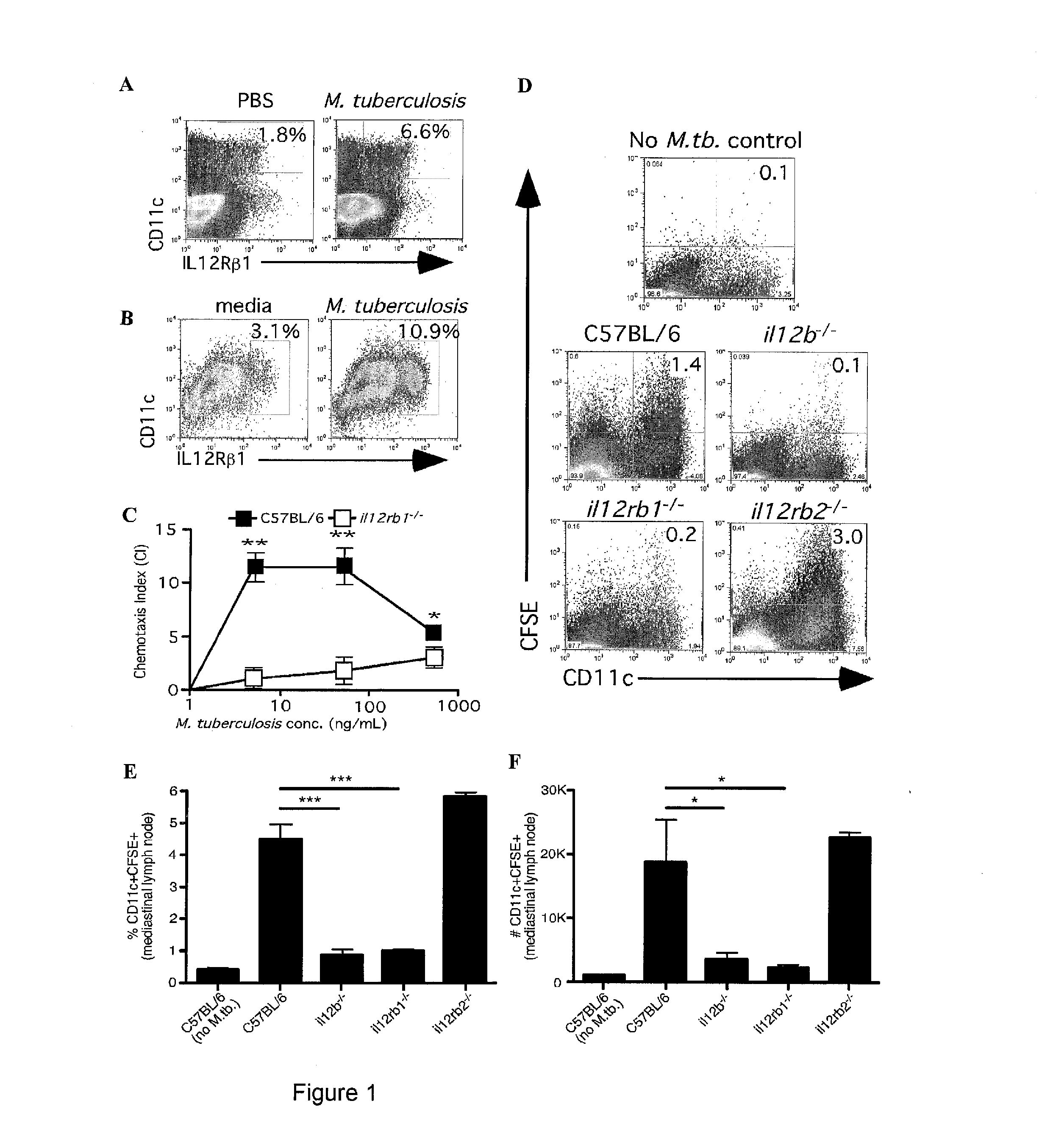
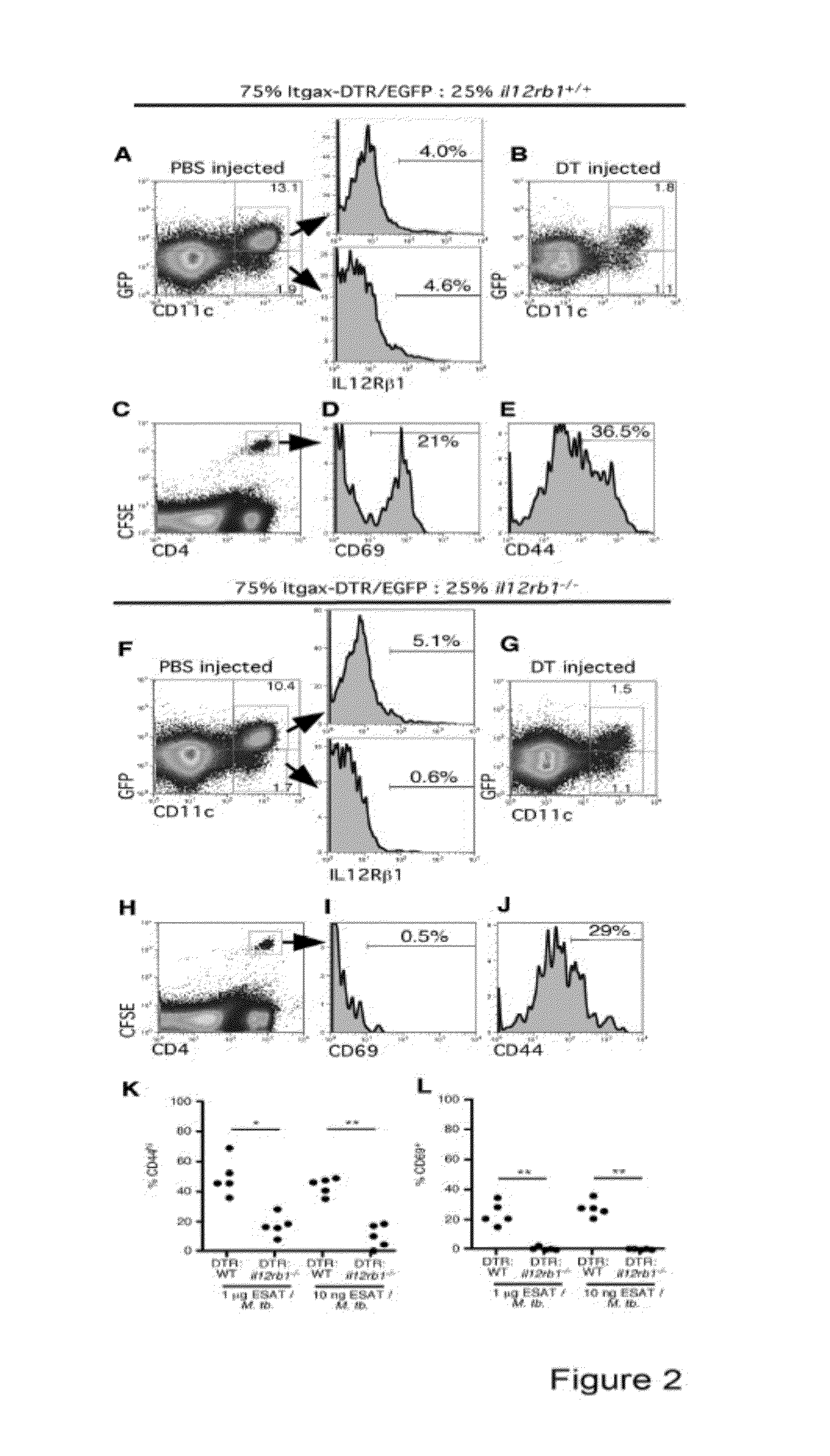
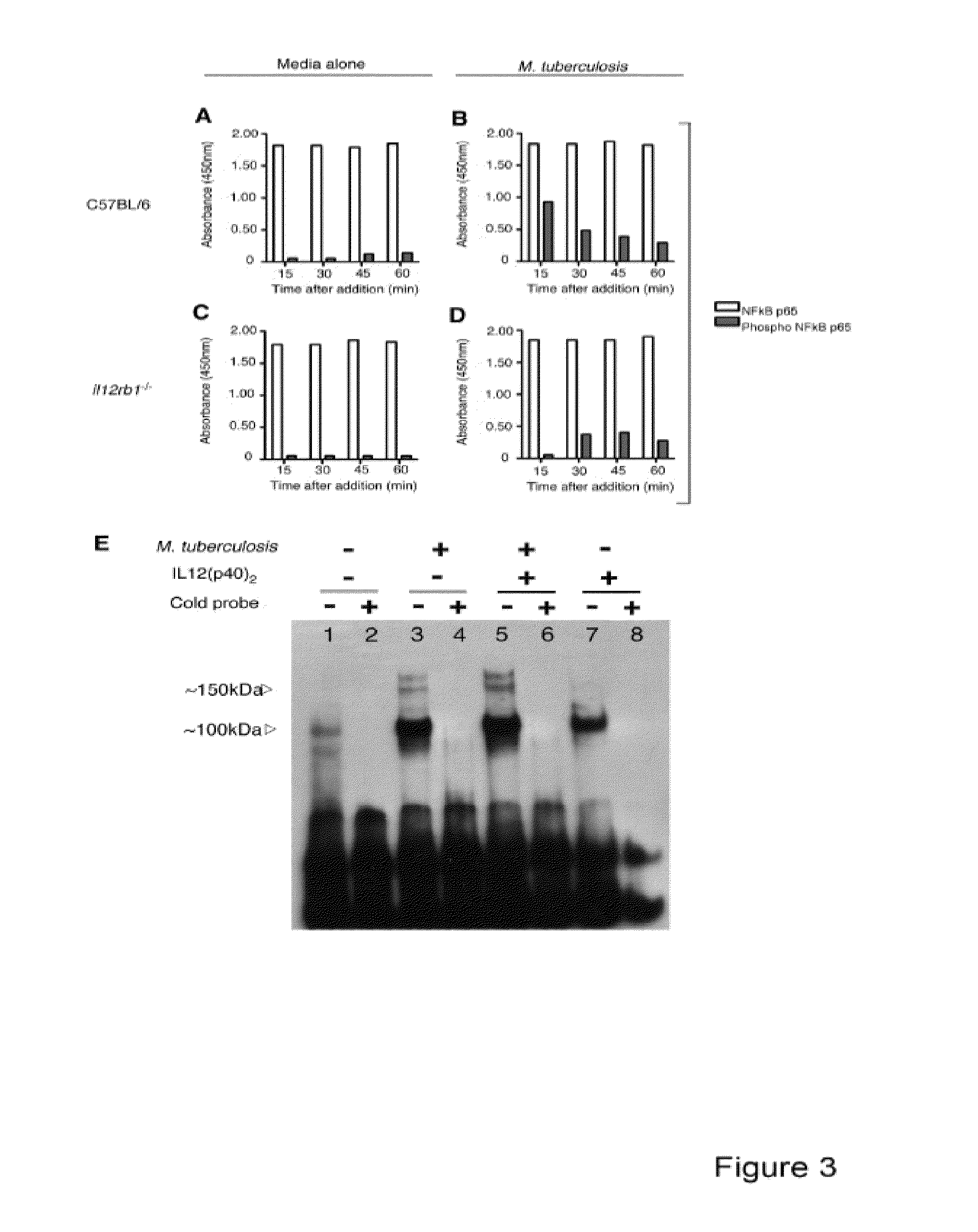
InactiveUS20140227324A1Improve responseAltered functionBacterial antigen ingredientsMicrobiological testing/measurementMycobacterium InfectionsLatent tuberculosis
The present invention describes compositions for both diagnostic and therapeutic applications. In one embodiment, the present invention contemplates a method of identifying an active M. tuberculosis infection. In another embodiment, the present invention contemplates a method of monitoring a M. tuberculosis infection. In yet another embodiment, the present invention contemplates a method of monitoring a patient's response to treatment for an active M. tuberculosis infection. In a further embodiment, the present invention contemplates a method of monitoring a patient's response to treatment for an active M. tuberculosis infection.
Owner:TRUDEAU INST INC
Tuberculosis antigen specific whole blood IFN-gamma diagnosis kit, method for producing the same and method for using same
InactiveCN101493454ADiagnostic advantageShorten the timeBiological testingEscherichia coliEnzyme linked immunoassay
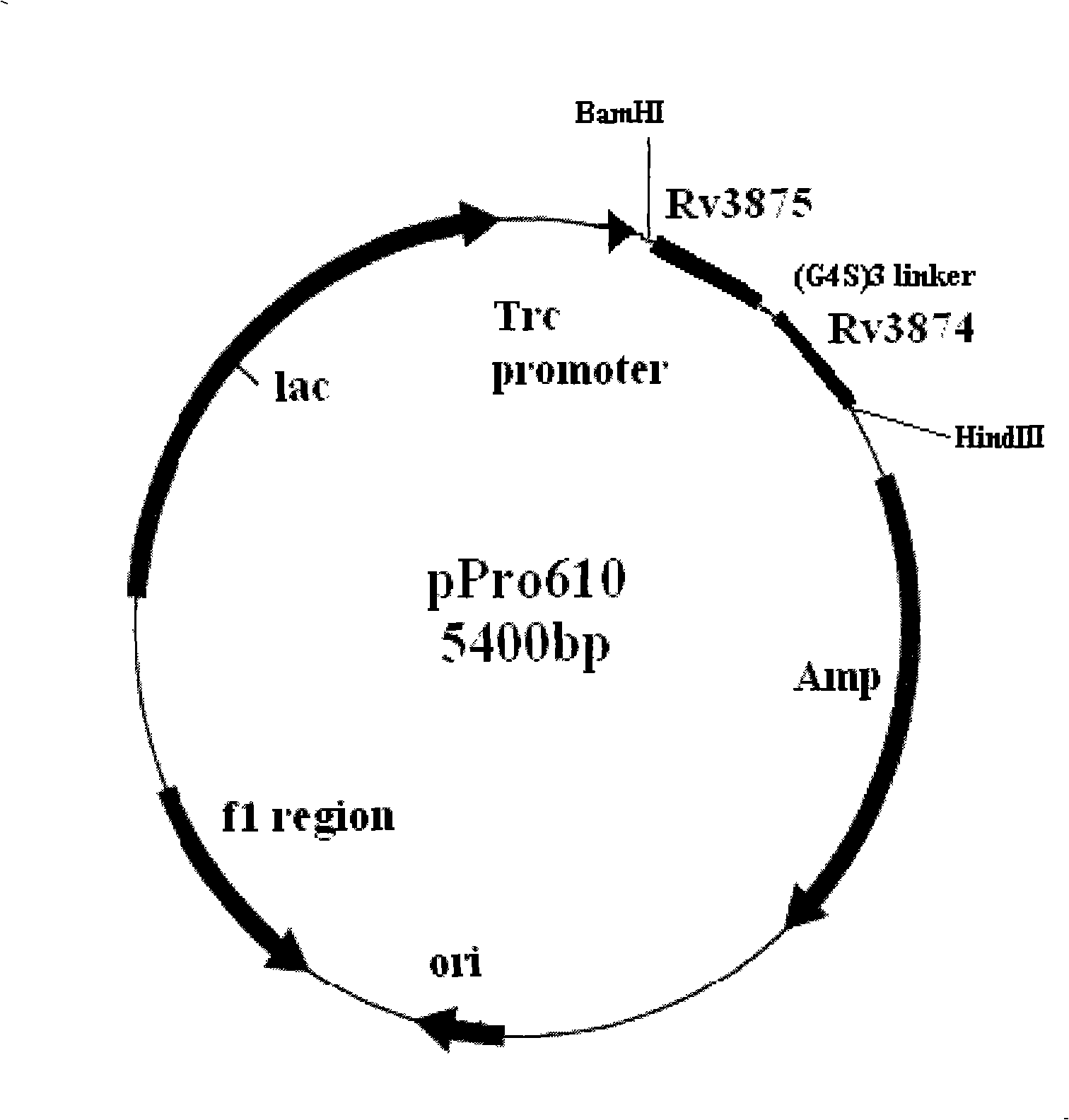
The invention relates to a diagnostic kit for tuberculosis and mycobacterium tuberculosis infectors, a preparation method and an application method thereof. By using the linker for encoding 15 amino acid (G4S1) 3, the encoding genes (SEQ.ID.NO.6) of a mycobacterium tuberculosis specific antigen Rv3875 and Rv3874 are connected in series, and then inserted into an E. coli expression vector, and the high-efficiency expression and purification for the fusion protein of Rv3875 and Rv3874 in the E. coli are achieved. The recombinant protein at least comprises 8 T cell epipositions which can be used for cell immunity diagnosis, and a diagnostic kit and diagnostic method for a whole blood IFN-Gamma release analysis method are established by taking the protein as the basis and combining the human IFN-Gamma enzyme-linked immunoassay technology, and can be used for the early, specific diagnosis and screening of tuberculosis and mycobacterium tuberculosis infectors.
Owner:范雄林
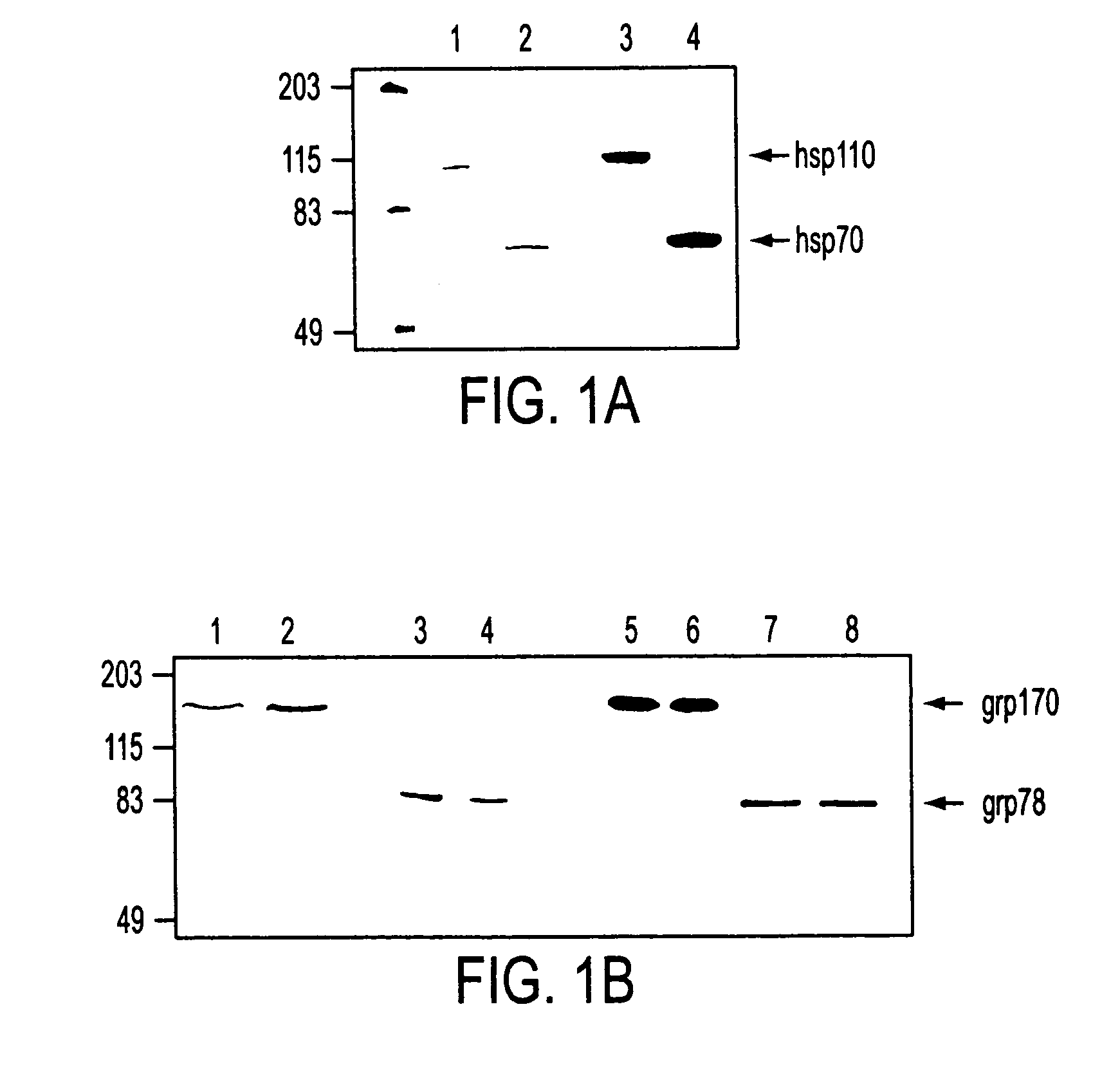
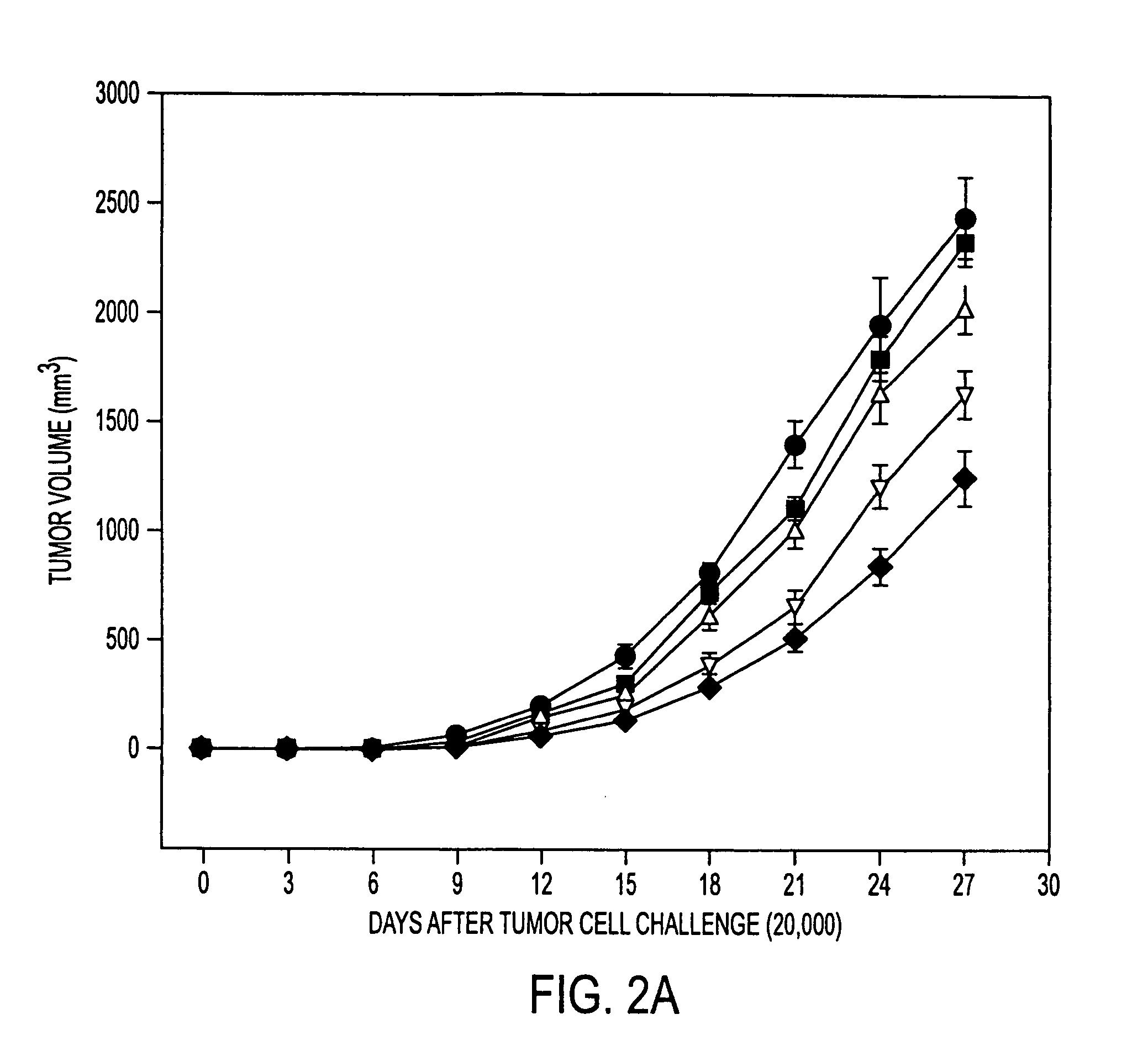
Stress protein compositions and methods for prevention and treatment of cancer and infectious disease
InactiveUS20050202035A1Good curative effectIncrease pressureAntibacterial agentsPeptide/protein ingredientsInfected cellAbnormal tissue growth
Pharmaceutical compositions comprising a stress protein complex and related molecules encoding or cells presenting such a complex are provided. The stress protein complex comprises an hsp110 or grp170 polypeptide complexed with an immunogenic polypeptide. The immunogenic polypeptide of the stress protein complex can be associated with a cancer or an infectious disease. The pharmaceutical compositions of the invention can be administered to a subject, thereby providing methods for inhibiting M. tuberculosis-infection, for inhibiting tumor growth, for inhibiting the development of a cancer, and for the treatment or prevention of infectious disease. The invention further provides a method for producing T cells directed against a tumor cell or a M. tuberculosis-infected cell, wherein a T cell is contacted with an APC that is modified to present an hsp110 or grp170 polypeptide and an immunogenic polypeptide associated with a tumor or with the M. tuberculosis-infected cell. Included in the invention are T cells produced by this method and a pharmaceutical composition comprising such T cells. The T cells can be contacted with a M. tuberculosis-infected cell in a method for killing a M. tuberculosis-infected cell, or with a tumor cell in a method for killing a tumor cell.
Owner:HEALTH RES INC
Protein for diagnosis and prevention of tuberculosis
InactiveCN104211787AImproving immunogenicityIncreased sensitivityAntibacterial agentsBacterial antigen ingredientsMycobacterium obuenseImmunogenicity
The invention relates to a protein for diagnosis and prevention of tuberculosis, a kit containing the protein and an application of the protein in preparation of the kit or preparation of a medicine for preventing and curing tuberculosis. Specifically, 21 proteins with good immunogenicity are screened from whole genome of mycobacterium tuberculosis. The proteins can be used for detecting mycobacterium tuberculosis infection or diagnosing tuberculosis and have good sensitivity and specificity. Or the proteins are used for preparing a vaccine so as to prevent or curing tuberculosis.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
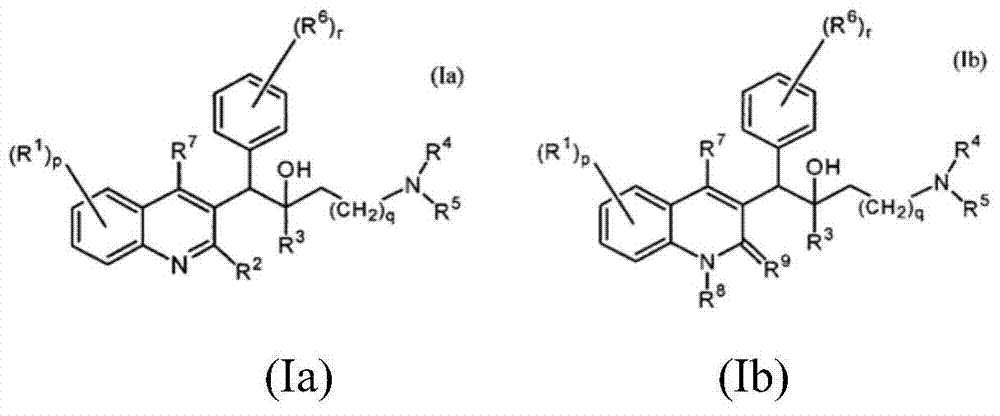
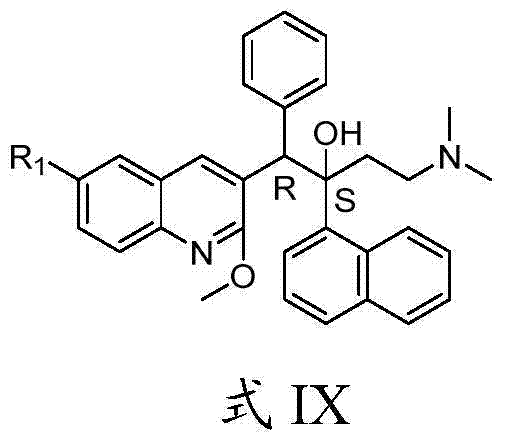
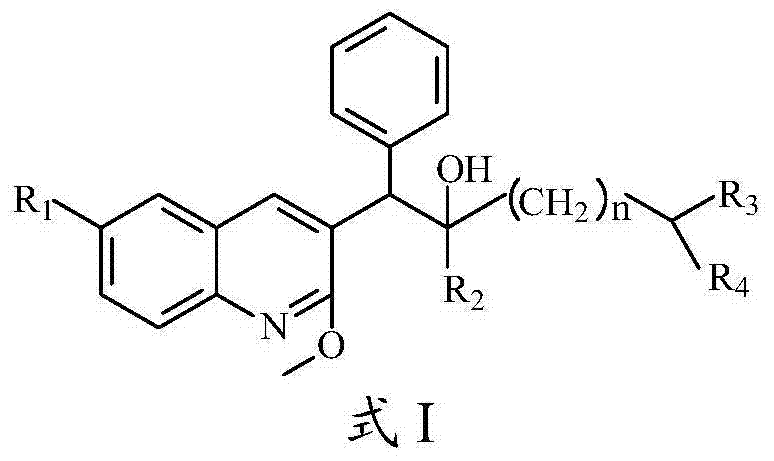
Quinoline, preparation method and application of quinoline
The invention belongs to the technical field of pharmaceutical chemistry and discloses a quinoline, and a preparation method and an application of the quinoline. The quinoline is a chemical compound shown as Formula I as shown in the specification, or an optical isomer, racemate, a diastereoisomer, pharmaceutically acceptable salt or solvate of the chemical compound. The quinoline has a significant killing effect on mycobacterium tuberculosis, is applicable to preparing drugs for treating or preventing diseases or symptoms caused by mycobacterium tuberculosis infection.
Owner:CHONGQING PHARMA RES INST
Vaccines comprising tb 10.4
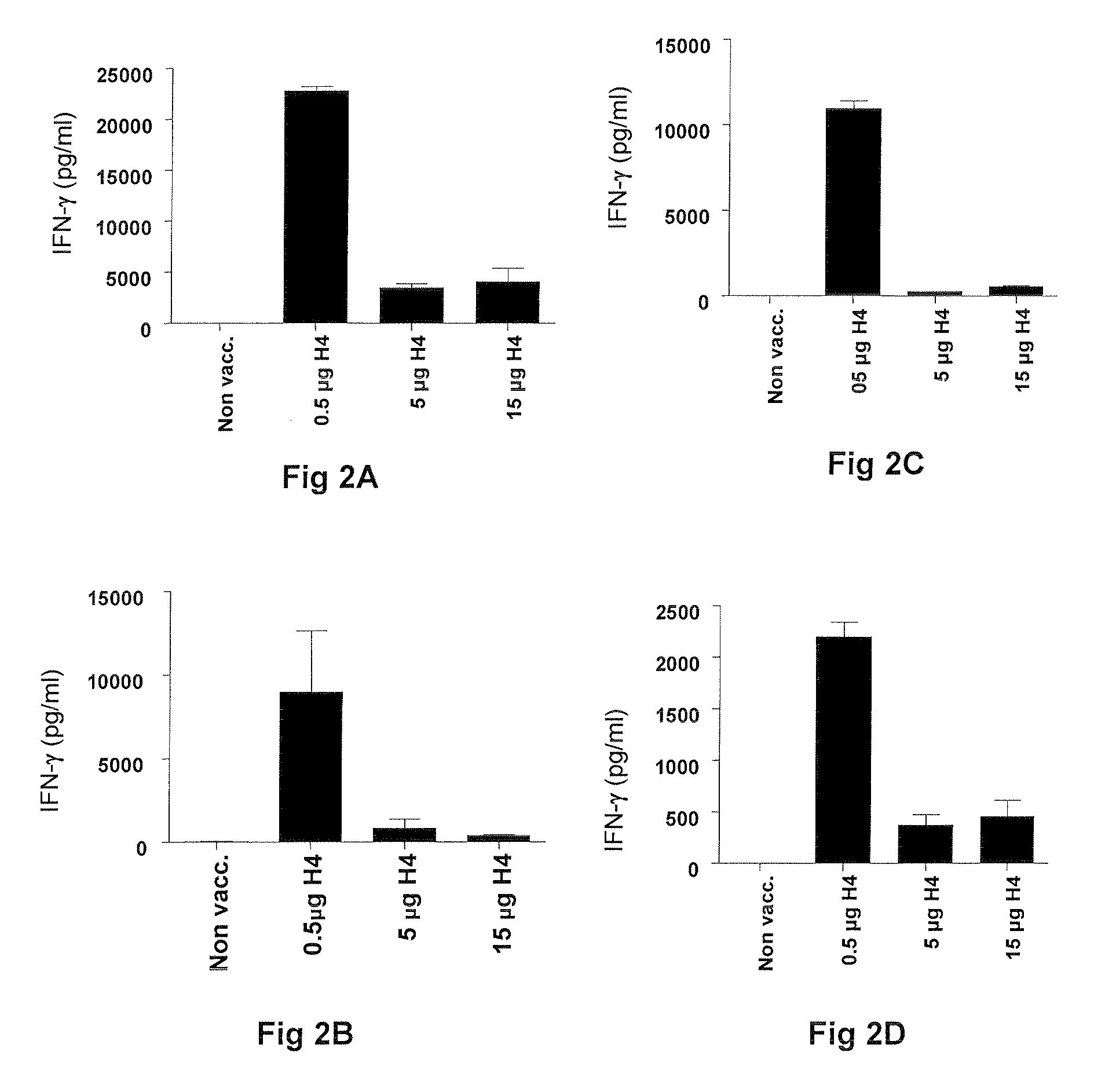
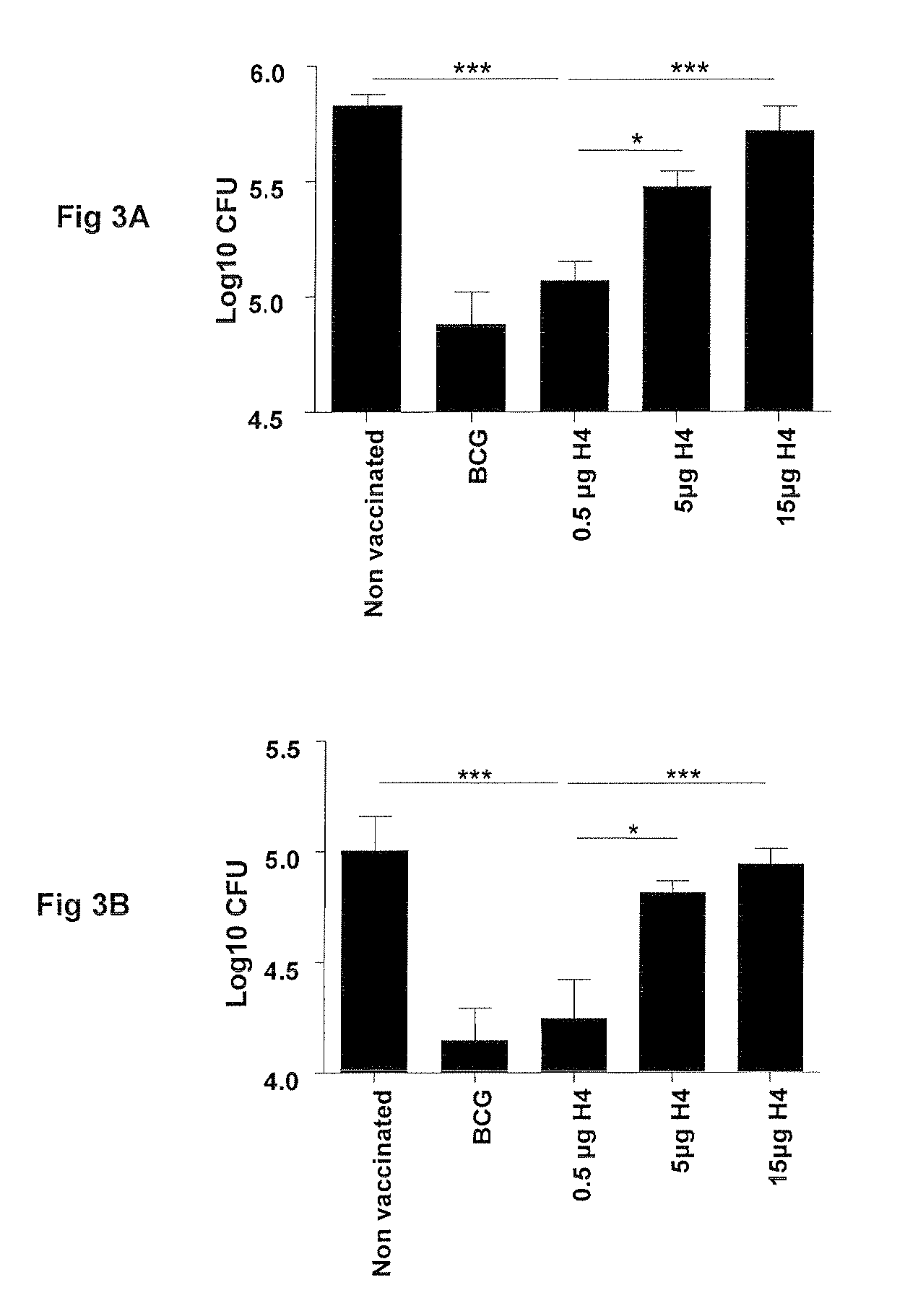
Vaccination with the combination of Ag85B-TB10.4 and IC31® adjuvant generated a high amount of polyfunctional CD4+T cells expressing high levels of IFN-γ, TNF-α, and IL-2. This in turn led to significant protection against infection with M. tuberculosis in the mouse aerosol challenge model of tuberculosis. Both the immunogenicity of the vaccine and its ability to protect against TB infection was highly dependent on the antigen dose. Thus, whereas the standard antigen dose of 5 μg, as well as 15 μg, did not induce significant protection against M. tuberculosis, reducing the dose to 0.5 μg increased both the immunogenicity of the vaccine as well as its protective efficacy to a level comparable to that observed in BCG vaccinated mice. Thus, the IC31® adjuvant, with the specified antigen dose, can induce a strong protective Th1 response against M. tuberculosis.
Owner:STATENS SERUM INST
Non-invasive rapid diagnostic test for M. tuberculosis infection
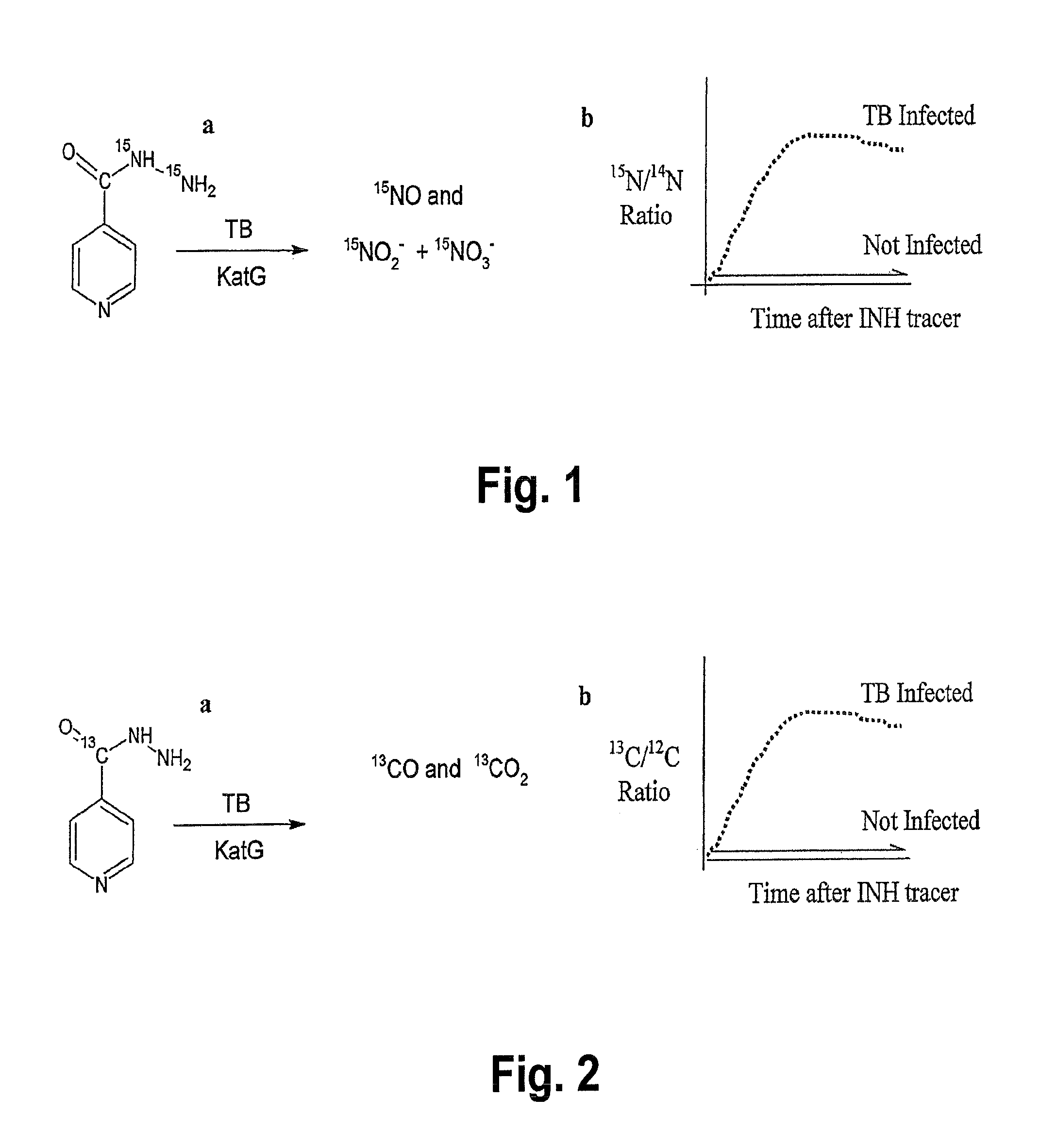
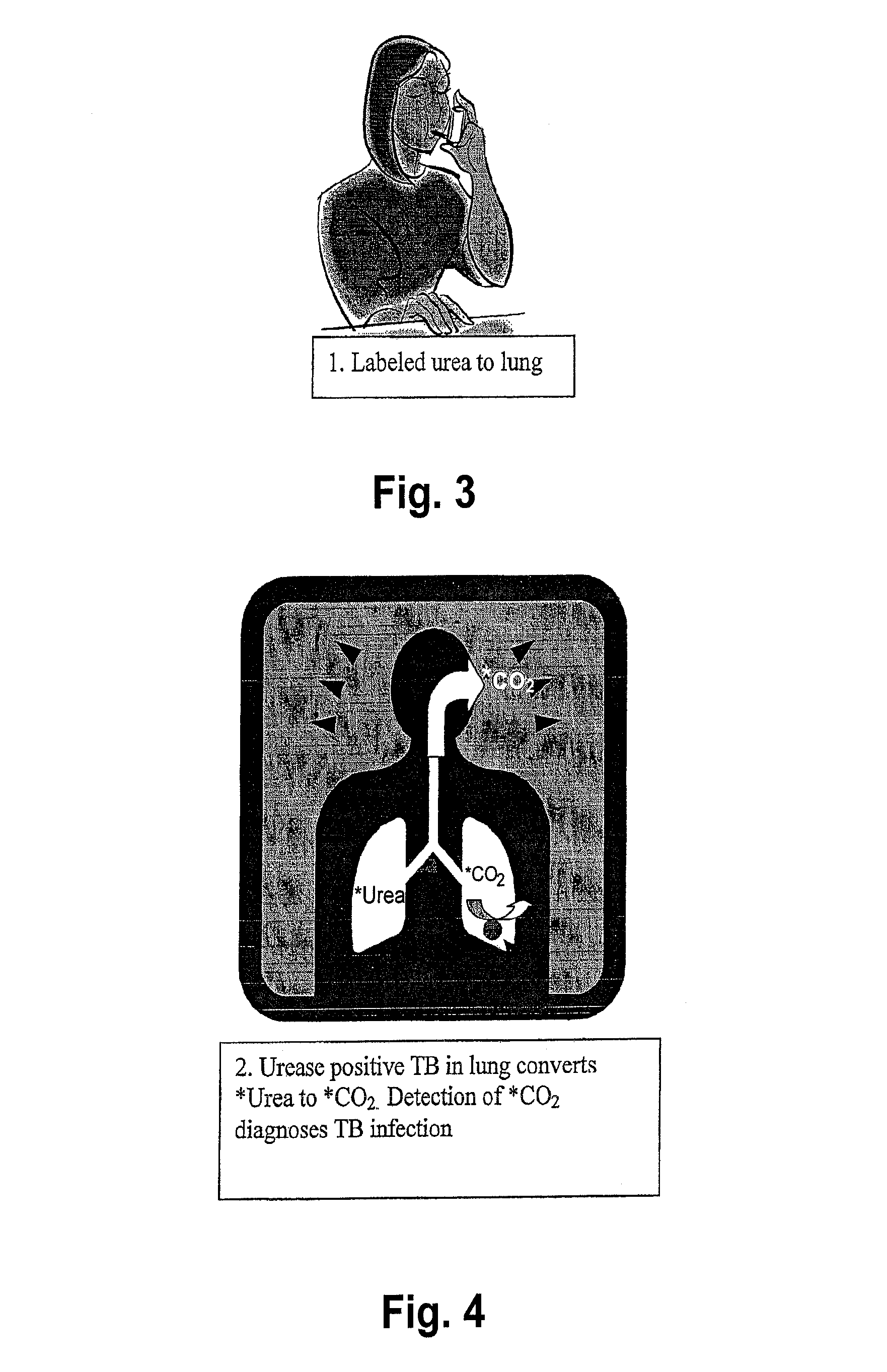
This invention relates to a test for detecting a Mycobacterium tuberculosis (tuberculosis or TB) infection in a patient or subject, specifically a diagnostic test, including a breath test, whereby patients are provided a small dose of an isotopically labeled TB drug, Isoniazid (INH) orally or directly to the lungs of the patient or subject. If TB is present, a TB enzyme mycobacterial peroxidase KatG oxidizes the INH; and KatG specific metabolites, in particular, isotopically labeled nitric oxide (NO), nitrites, nitrates, carbon monoxide (CO) or carbon dioxide converted from carbon monoxide of INH cleavage are measured. Other embodiments relate to a diagnostic breath test for detecting TB utilizing isotopically labeled urea (preferably, carbon-13 labeled urea), alone or in combination with isotopically labeled isoniazid (preferably, nitrogen-15 labeled isoniazid), wherein M. tuberculosis organism, if present in the patient or subject's lungs (or other tissues), will metabolize the isotopically labeled urea to isotopically labeled carbon dioxide (CO2) such that a determination of the residence of M. tuberculosis, including residence of an isoniazid resistant strain of M. tuberculosis, may be made.
Owner:STC UNM
Diagnostic mycobacterium tuberculosis test
ActiveUS20120128708A1Low specificityHigh sensitivityAntibacterial agentsBacterial antigen ingredientsMycobacterium InfectionsGene
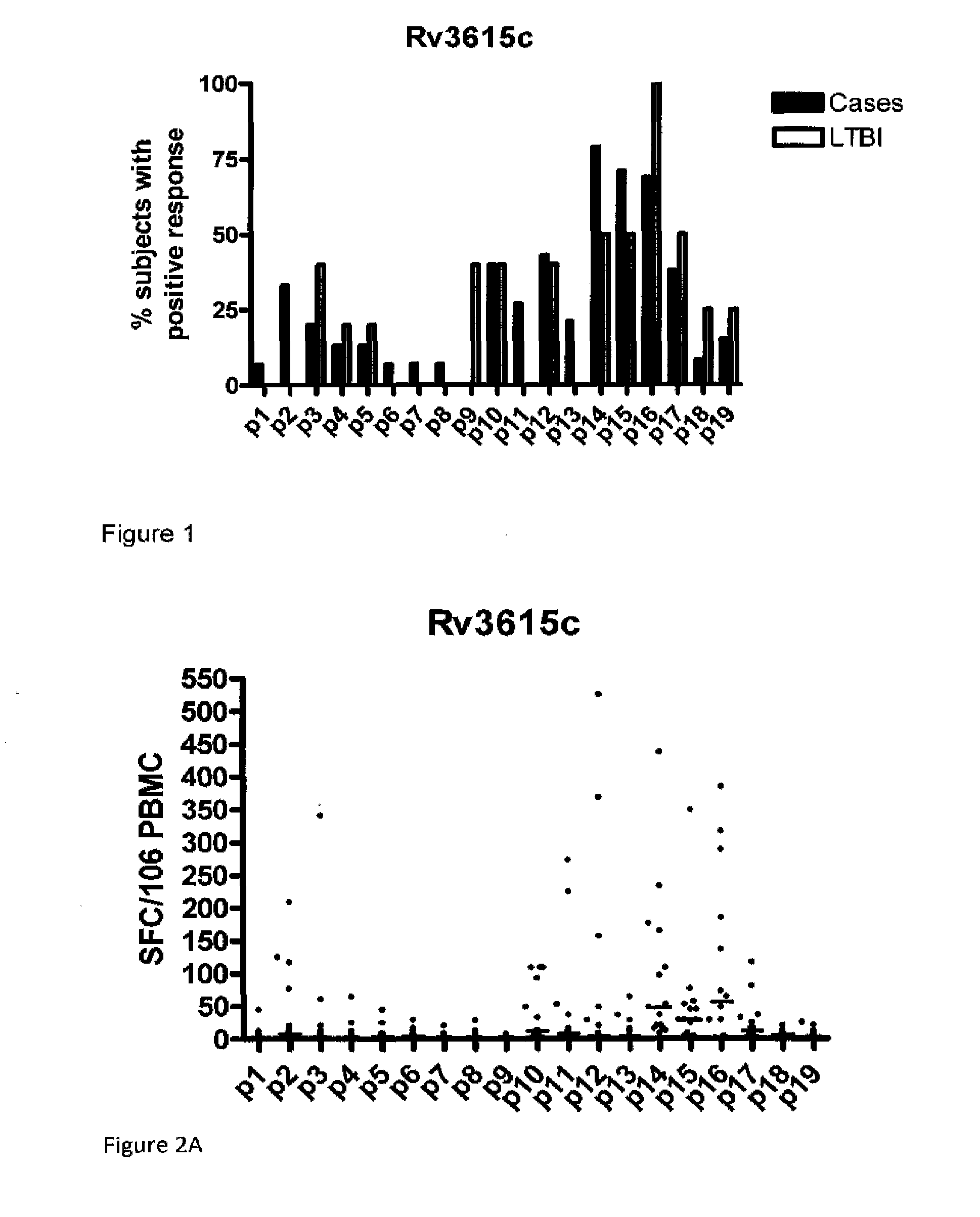
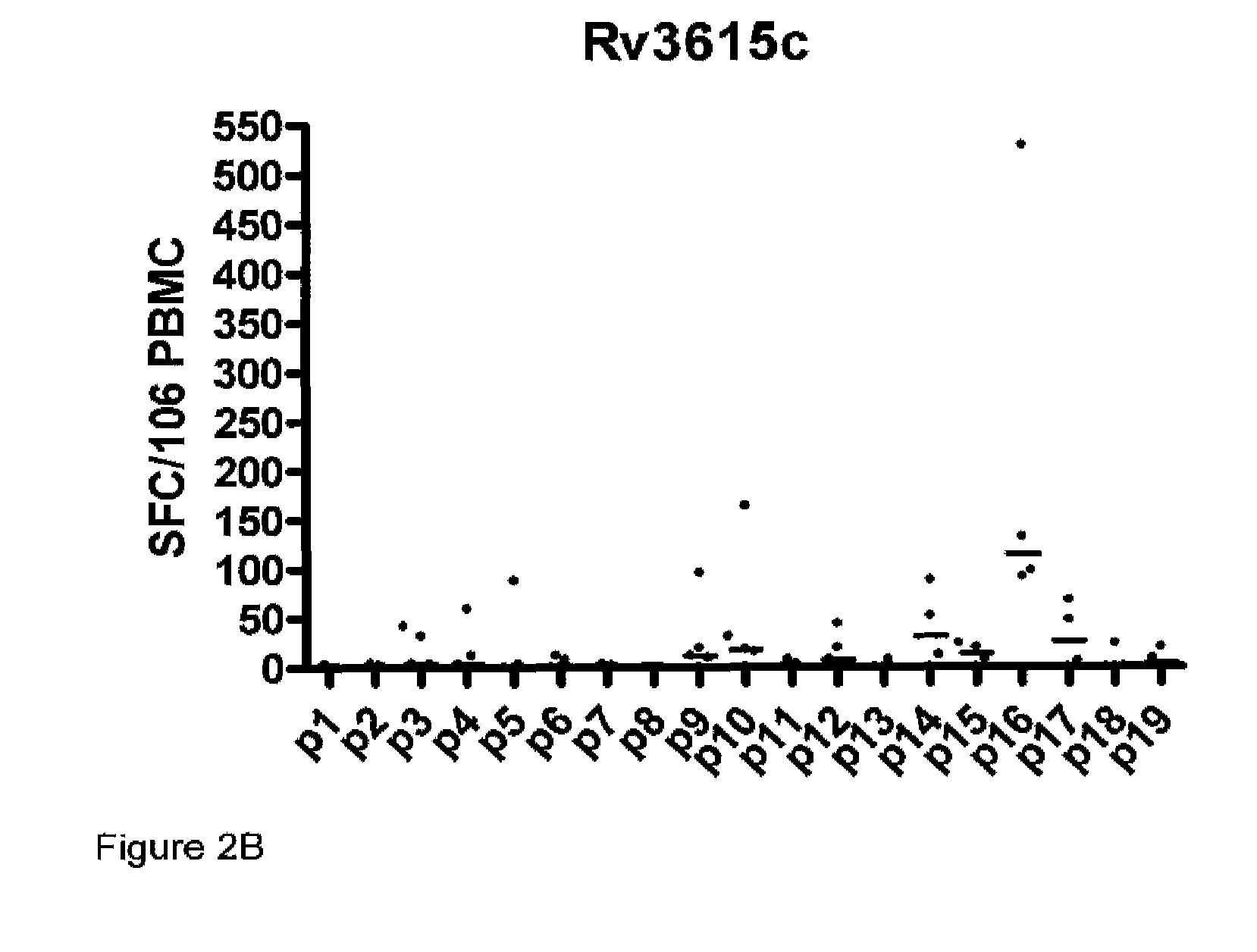
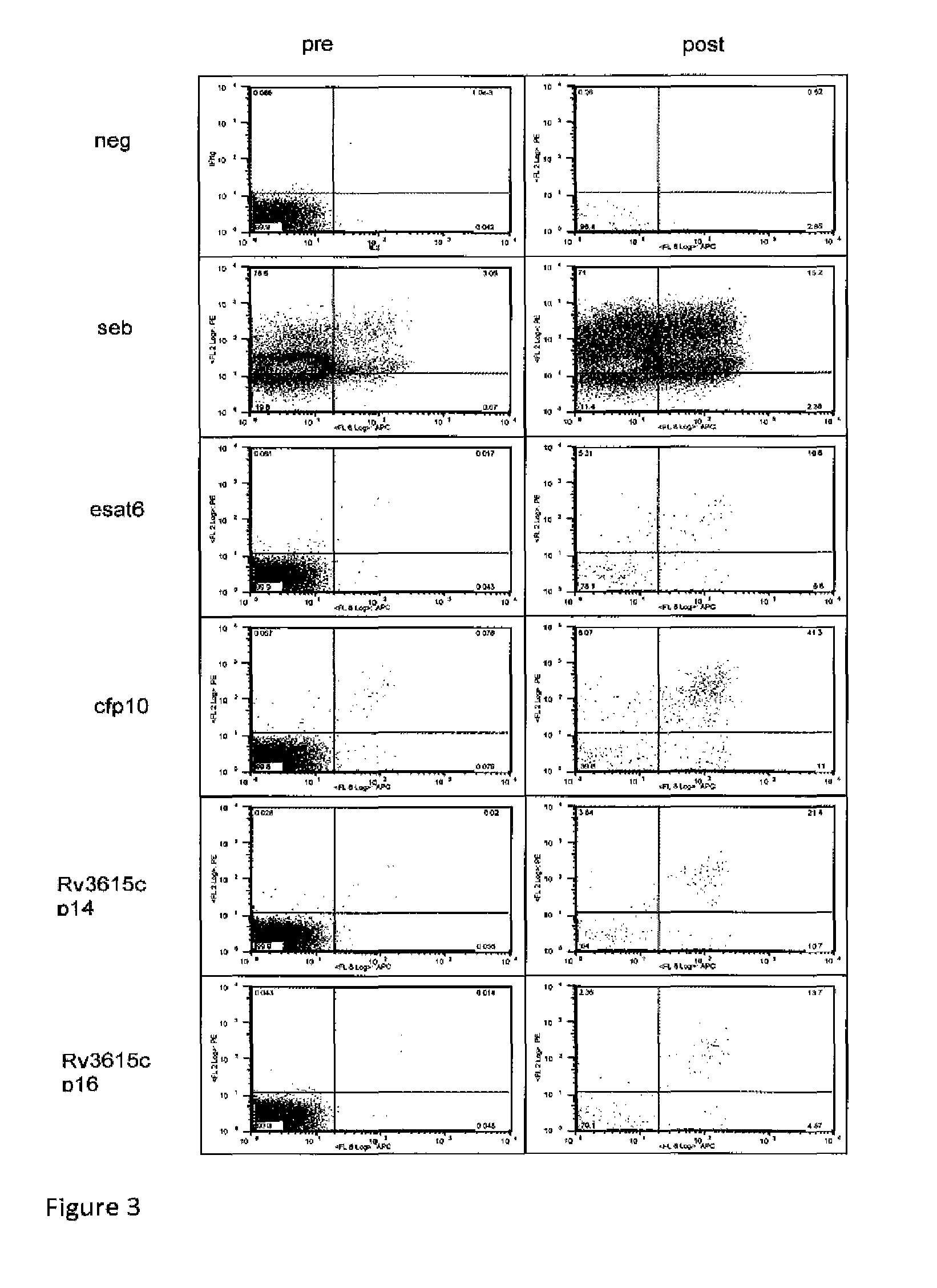
A method of diagnosing Mycobacterium tuberculosis infection in a human, or of determining whether a human has been exposed to Mycobacterium tuberculosis, comprising (i) contacting T-cells from said human with one or more of (a) a peptide having the sequence shown in SEQ ID NO 20, (b) a peptide having or comprising the sequence of at least 8 consecutive amino acids of the sequence shown in SEQ. ID NO 20; or (c) a peptide having or comprising a sequence which is capable of binding to a T-cell receptor which recognises a peptide as defined in (a) or (b); and (ii) determining whether any of the said T-cells recognise said peptide, wherein steps (i) and (ii) are optionally carried out in vitro. The peptide is the product of the RV3615c gene.
Owner:LALVANI AJIT
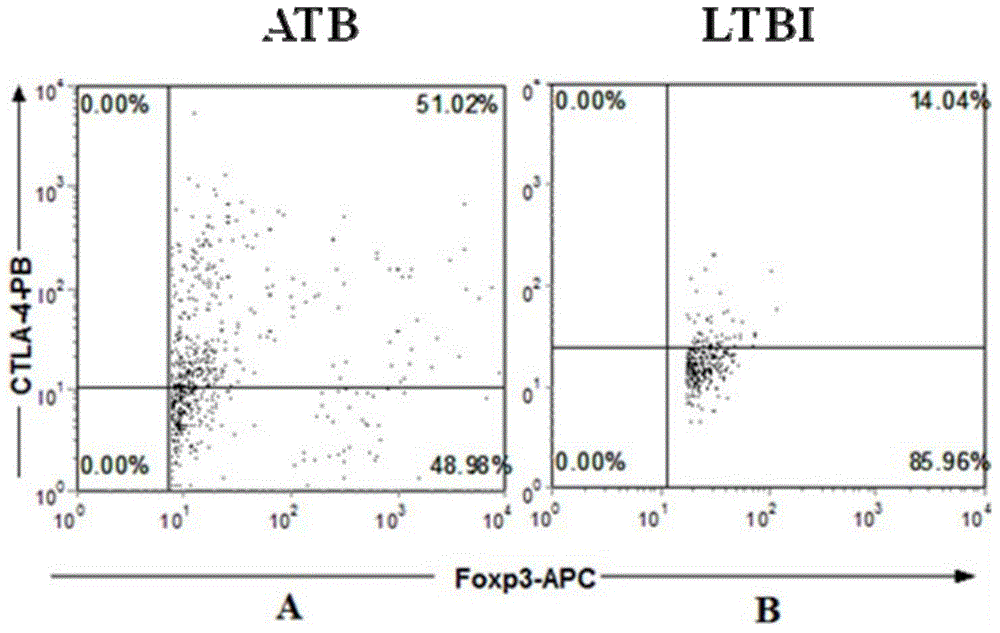
Diagnostic kit for distinguishing active and latent mycobacterium tuberculosis infection
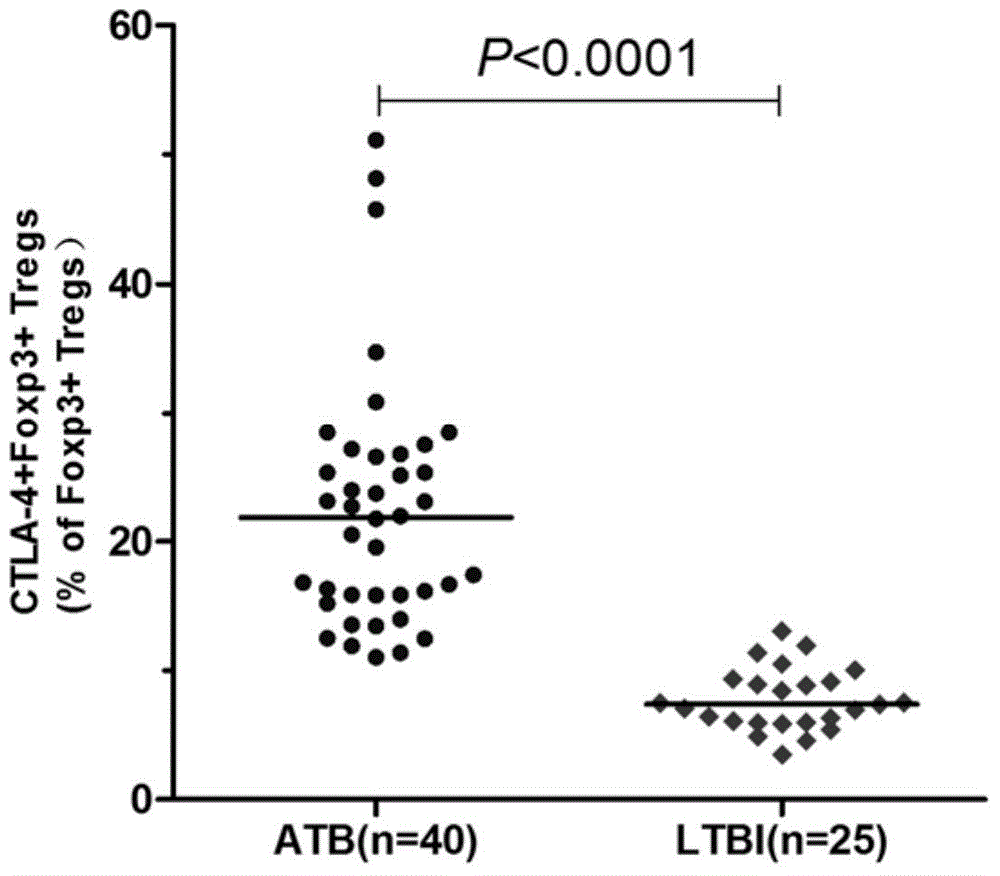
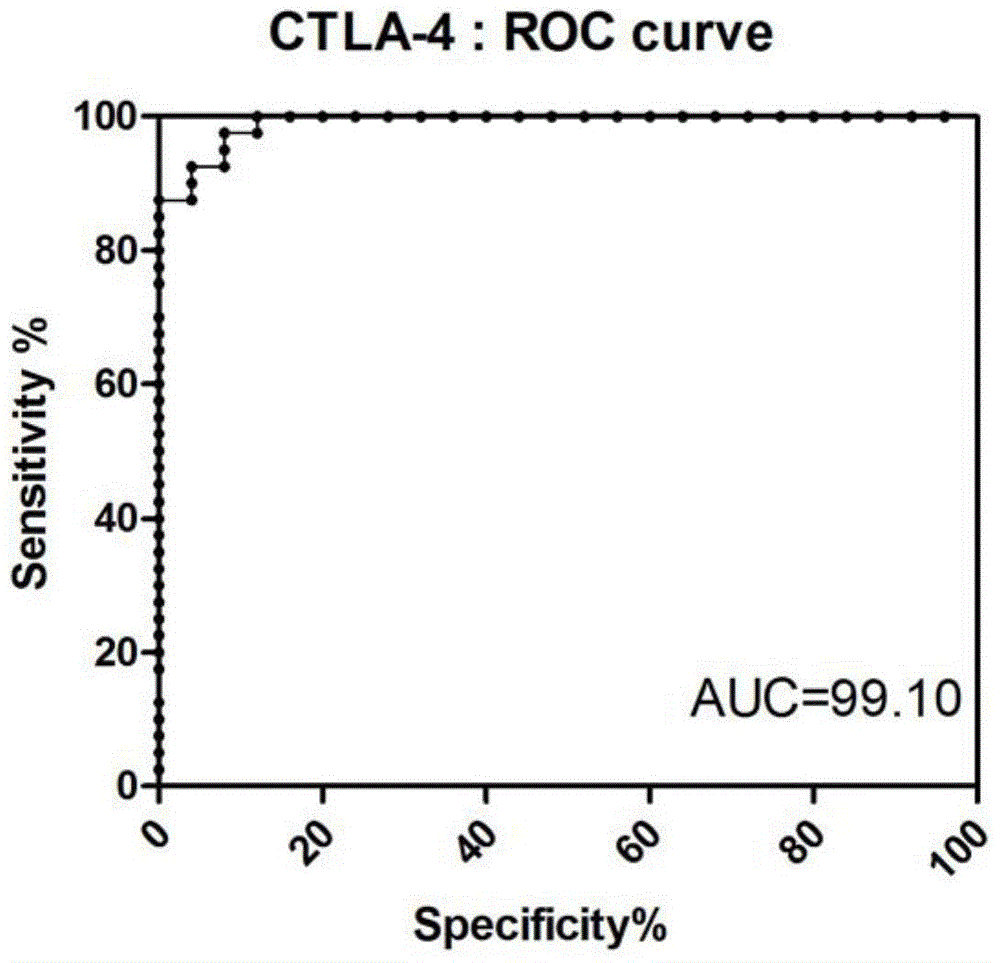
The invention provides a diagnostic kit for distinguishing active and latent mycobacterium tuberculosis infection with cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) serving as a diagnosis marker and a method for distinguishing active and latent mycobacterium tuberculosis infection by means of the diagnostic kit. The diagnostic kit comprises a CTLA-4, CD3, CD4, CD25, FoxP3 fluorescent antibody, erythrocyte lysate, membrane breaking liquid, washing buffer, phosphate buffer, fetal calf serum, stationary liquid and a streaming pipe. The diagnostic kit can be used for detecting the CTLA-4 expression quantity of peripheral venous blood of a patient and distinguishing active and latent mycobacterium tuberculosis infection, sensitivity and accuracy are high, and a strong basis is provided for clinic differential diagnosis.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Use of acellular short corynebacteria preparation in preparation of medicine for treating tuberculosis
InactiveCN1465354AImprove biological activityReduce pollutionAntibacterial agentsBacteria material medical ingredientsTherapeutic effectCorynebacterium amycolatum
The present invention discloses a non-cell corynebacterium parvum preparation (NCP) and its application in preparation of medicine for curing tuberculosis, in which the described NCP is a non-cell preparation prepared by breaking corynebacterium parvum, its grain size is less than 100 nm, and its heat source is less than 160 Eu / ml, protein content is 10-40% (g / g), nucleic acid content is 3-25% (g / g), the rest is polysaccharide. The tests show that it can obtain good therapeutic effect.
Owner:上海昌润生物科技有限公司
MTB (Mycobacterium Tuberculosis) infection diagnosis kit
ActiveCN105954521AInfection diagnosisStrong specificityBiological material analysisBiological testingBiotin-streptavidin complexInsulin-like growth factor
The invention belongs to the field of biomedicine examination, and relates to an MTB (Mycobacterium Tuberculosis) infection diagnosis kit. The MTB infection diagnosis kit provided by the invention is prepared from the following components: an antigen stimulant, a pre-coated ELISPOT (Enzyme-Linked Immunospot Assay) plate of a capture antibody, an insulin-like growth factor, a detection antibody, HRP (Horse Radish Peroxidase)-labeled streptavidin, a 3-amino-9-ethyl carbazole developing solution, antibody diluent and a positive control stimulant; the antigen stimulant is selected from one or multiple polypeptides in sequences as shown in SEQ ID NO.1 to 12. The MTB infection diagnosis kit provided by the invention is high in sensitivity and specificity and stable in property; meanwhile, when the MTB infection diagnosis kit provided by the invention is applied to in-vitro detection on MTB, the detection time can be saved, the detection steps can be simplified, and the detection efficiency can be increased.
Owner:WUHAN DANGKANG XING ZHONG BIOTECHNOLOGY CO LTD
Antigenic polypeptide pool capable of detecting mycobacterium tuberculosis infection, and application thereof
ActiveCN107011418AQuick checkSpecific detectionBiological material analysisDepsipeptidesPeripheral blood mononuclear cellMycobacterium Infections
The invention discloses an antigenic polypeptide pool capable of detecting mycobacterium tuberculosis infection. The antigenic polypeptide pool specifically stimulates mycobacterium tuberculosis infected fresh whole blood to specifically secrete IFN-gama and increase the sensitivity. The invention provides a new detection reagent for detecting mycobacterium tuberculosis infection, experiments prove that peripheral blood can be directly utilized to conduct antigenic simulation without separation of peripheral blood mononuclear cell, the experimental data shows that the antigenic polypeptide pool has high sensitivity and specificity for detecting mycobacterium tuberculosis infection, and is simple and convenient to operate and low in cost, thus having high clinical application values.
Owner:武汉海吉力生物科技有限公司
Antigen stimulant and kit for detecting mycobacterium tuberculosis infection, and application of antigen stimulant
ActiveCN104597239AIncreased sensitivityImprove featuresMicrobiological testing/measurementBiological testingMycobacterium InfectionsStimulant
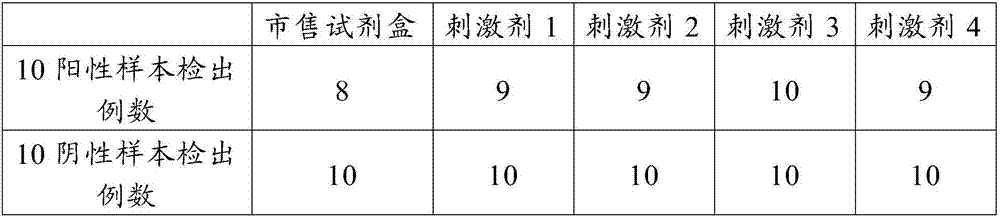
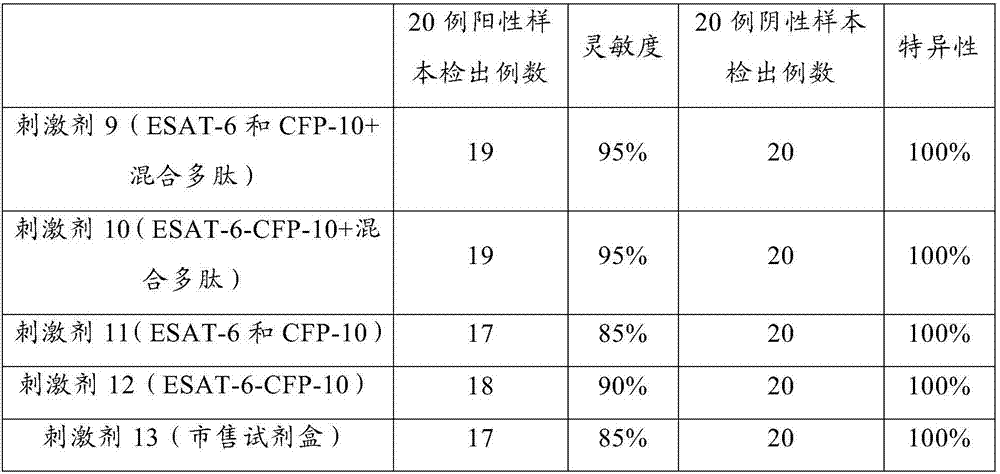
The invention provides an antigen stimulant for detecting mycobacterium tuberculosis infection, and a kit comprising the antigen stimulant. The invention also provides an application of the antigen stimulant in reagents for detecting mycobacterium tuberculosis infection. The antigen stimulant comprises at least one polypeptide or analogues thereof in polypeptides shown as the sequences 1-11 in a sequence table, wherein the polypeptides respectively come from tuberculosis specific antigen polypeptides ESAT-6 and tuberculosis specific antigen polypeptides CFP-10. According to the antigen stimulant provided by the invention, peripheral blood T lymphocytes of tuberculosis infection patients can be effectively stimulated to generate IFN-gamma, so that the tuberculosis infection can be diagnosed at high sensitivity and high specificity, and the influence on BCC inoculation or other underlying diseases can be avoided.
Owner:SUN YAT SEN UNIV +1
Compositions and methods for treating mycobacterial infections
InactiveUS20100172845A1Reduced stateAvoid stickingAntibacterial agentsOrganic active ingredientsRegimenMycobacterium Infections
Disclosed are compositions and improved methods for effective treatment of Mycobacterial infections in susceptible animals. Also disclosed are regimens for preventing, reducing, or ameliorating the emergence of symptoms of Mycobacterium tuberculosis infection in susceptible individuals, as well as methods for reducing the spread of tubercular infections in at-risk populations.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
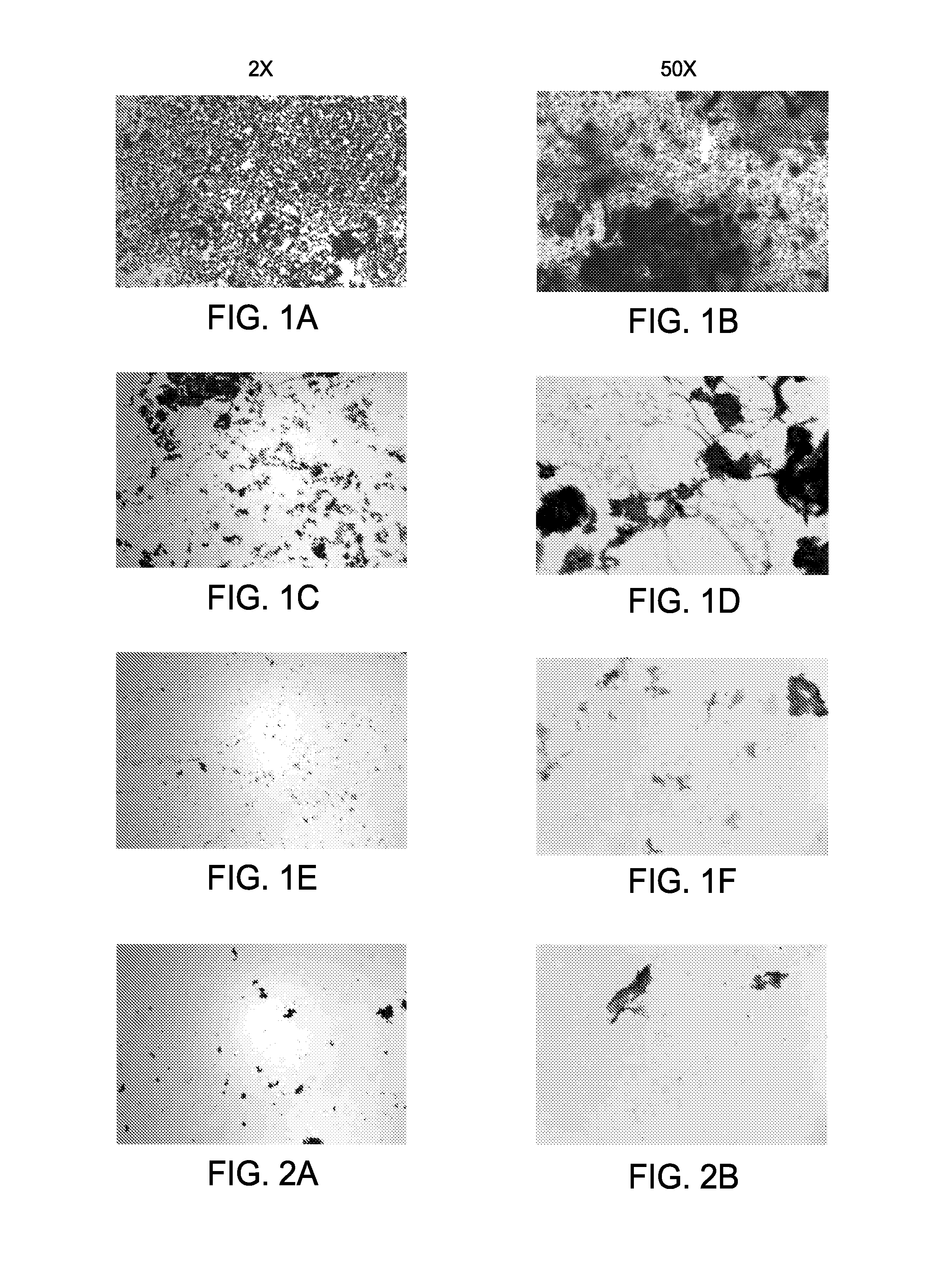
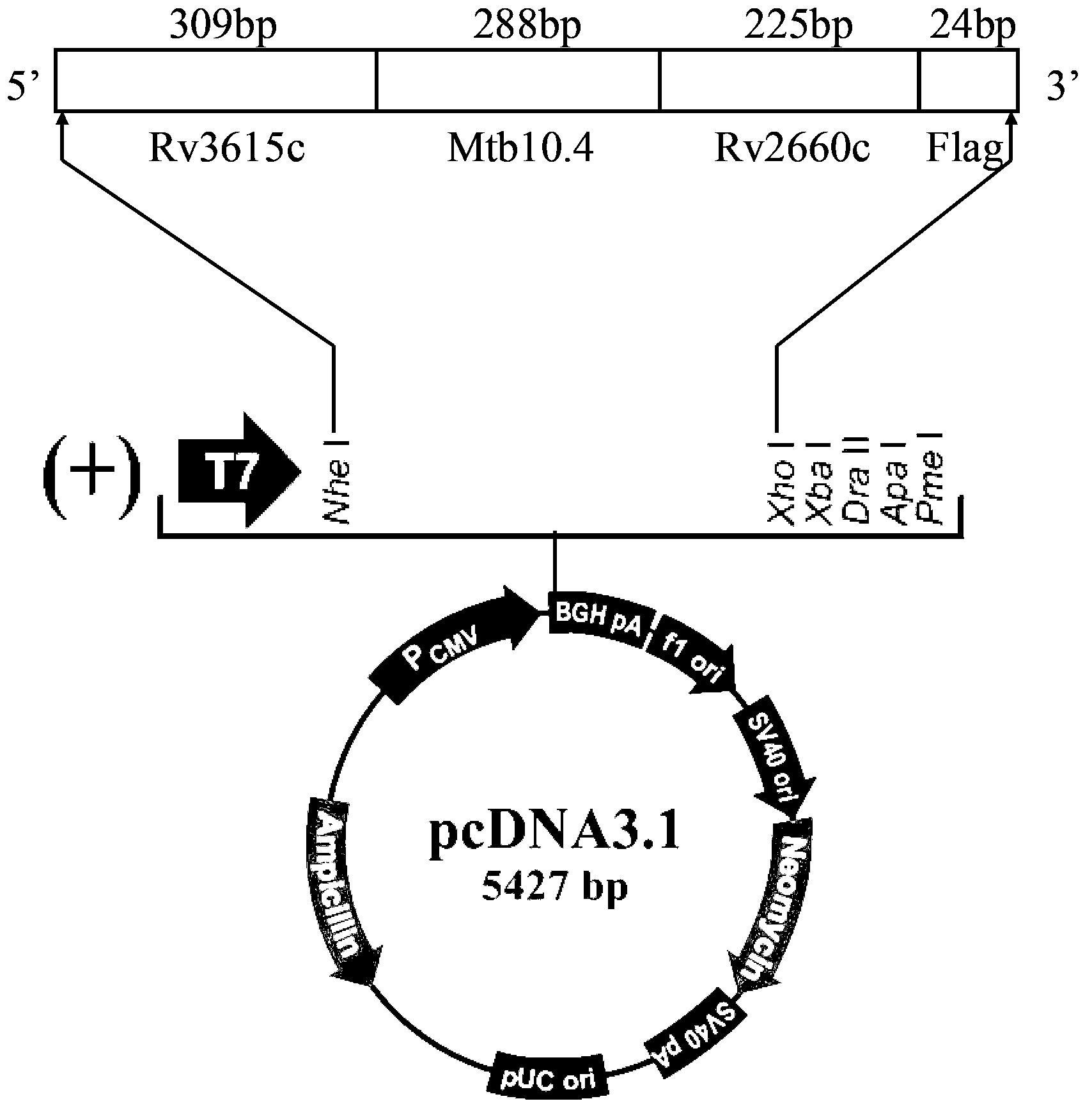
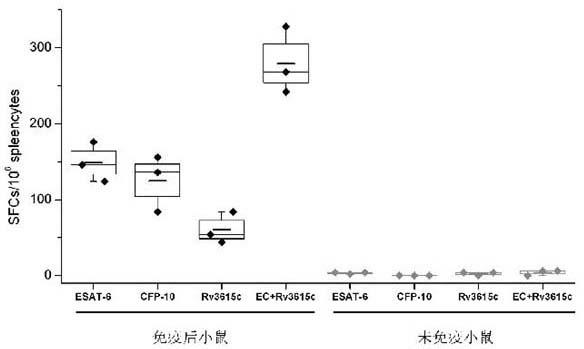
Three-antigen fusion gene vaccine of mycobacterium tuberculosis as well as preparation method and application of three-antigen fusion gene vaccine
The invention discloses a fusion protein gene which is formed by sequentially connecting an antigen gene Rv3615c, an antigen gene Mtb10.4 and an antigen gene Rv2660c in series from a 5' end to a 3' end. The invention also provides a three-antigen fusion gene vaccine of mycobacterium tuberculosis as well as a preparation method and application of the three-antigen fusion gene vaccine of the mycobacterium tuberculosis, wherein the three-antigen fusion gene vaccine is composed of a carrier, and a segment of the fusion protein gene is inserted to the carrier. Rat immunological experiments show that the three-antigen fusion gene vaccine of the mycobacterium tuberculosis can be used for inducing a better cellular immunological response. The three-antigen fusion gene vaccine of the mycobacterium tuberculosis, disclosed by the invention, can be used for inducing a specific killing response and a stronger CD4<+> and CD8<+>T cellular immune protection reaction having an important protection effect on mycobacterium tuberculosis infection, can be used for effectively resisting the mycobacterium tuberculosis infection, and plays a better protection effect on tuberculosis infected rats.
Owner:SUZHOU UNIV
Tuberculosis diagnostic composition and application thereof
ActiveCN102608333AReduce false negativesReduce irritation holesAntibacterial agentsBacterial antigen ingredientsAntigenMycobacterium Infections
The invention relates to a tuberculosis diagnostic composition and application of the tuberculosis diagnostic composition, in particular provides the tuberculosis diagnostic composition which consists of optional two or three of antigen ESAT-6 derived polypeptide, antigen CFP-10 derived polypeptide and antigen Rv3615c derived polypeptide. The invention also provides application of the tuberculosis diagnostic composition in a specific T cellular immune reaction caused by the detection of mycobacterium tuberculosis infection in vitro and application of the preparation of a tuberculosis diagnostic agent. Compared with the existing diagnostic technology, the diagnostic cost is reduced, the detection design and the operation process are simplified, and the detection sensitivity is obviously improved.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +2
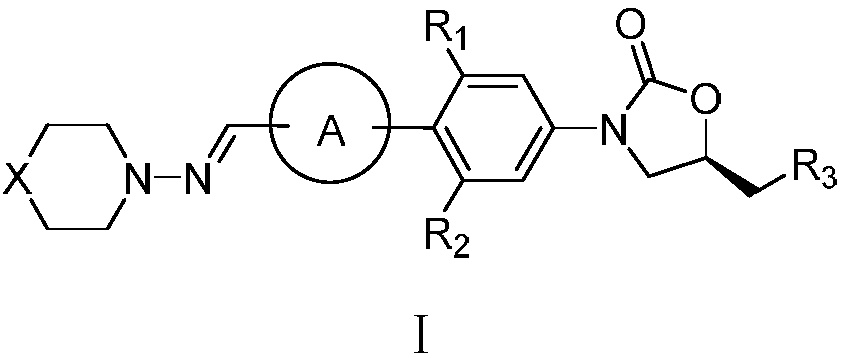
Oxazolidinone compound containing combined aromatic hydrazine and its preparation method
The present invention relates to oxazolidinone compounds containing biarylhydrazone structures represented by general formula I, or optical isomers, pharmaceutically acceptable salts and / or solvates thereof, their preparation methods and compounds containing said compounds pharmaceutical composition. Wherein the substituents R1, R2, R3, X and ring A have the meanings given in the specification. The present invention also relates to the use of the compounds and their pharmaceutically acceptable salts, solvates or prodrugs as antibacterial drugs in treatment, especially the use in the treatment of Gram-positive bacteria infection and Mycobacterium tuberculosis infection.
Owner:SHENYANG PHARMA UNIVERSITY
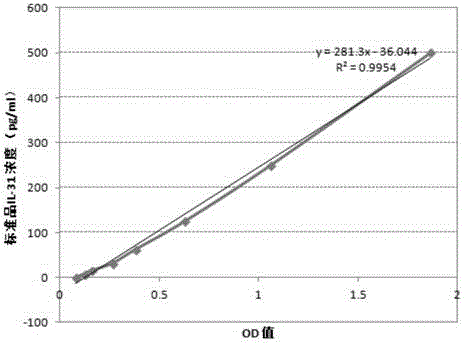
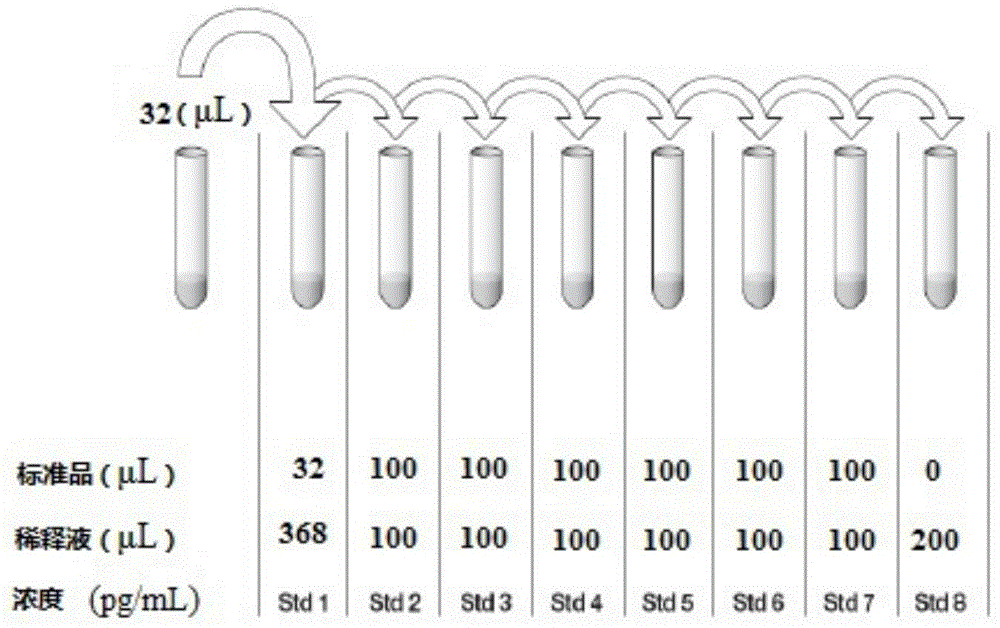
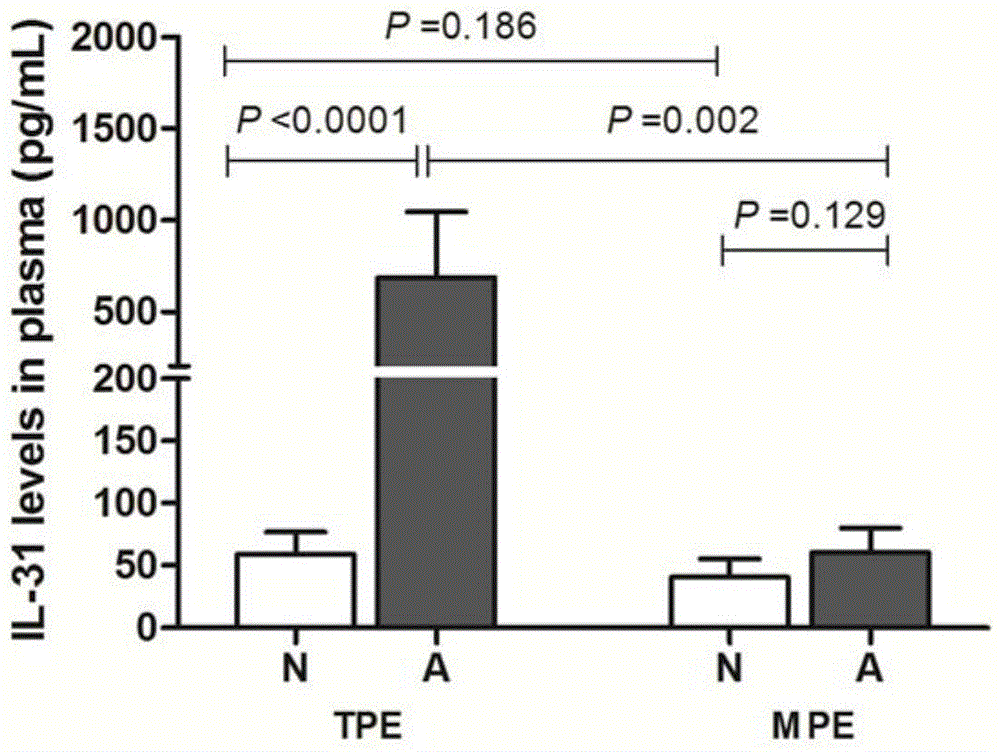
Kit for diagnosing mycobacterium tuberculosis infection based on tuberculosis specificity IL-31 detection
InactiveCN104897893AIncreased sensitivityImprove accuracyBiological material analysisAntigenMycobacterium Infections
The invention provides a kit for diagnosing mycobacterium tuberculosis infection based on tuberculosis specificity IL-31 detection. The kit comprises an IL-31 capture antibody, an IL-31 detection antibody, tuberculosis specific antigen polypeptide, a diluent, a standard substance, a developing solution, a stop solution, a buffer solution, a solid carrier and a micro-plate sealer. The kit is used for detecting the IL-31 expression quantity in plasma, can be used for tuberculosis diagnosis, and is relatively high in sensitivity and accuracy, and fast to operate, so that the diagnosing efficiency of tuberculosis can be improved.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Biomarker for diagnosing mycobacterium tuberculosis infection and related kit
InactiveCN106501530AImprove diagnostic efficiencyEasy to operateBiological material analysisBiological testingAntigenInfection diagnosis
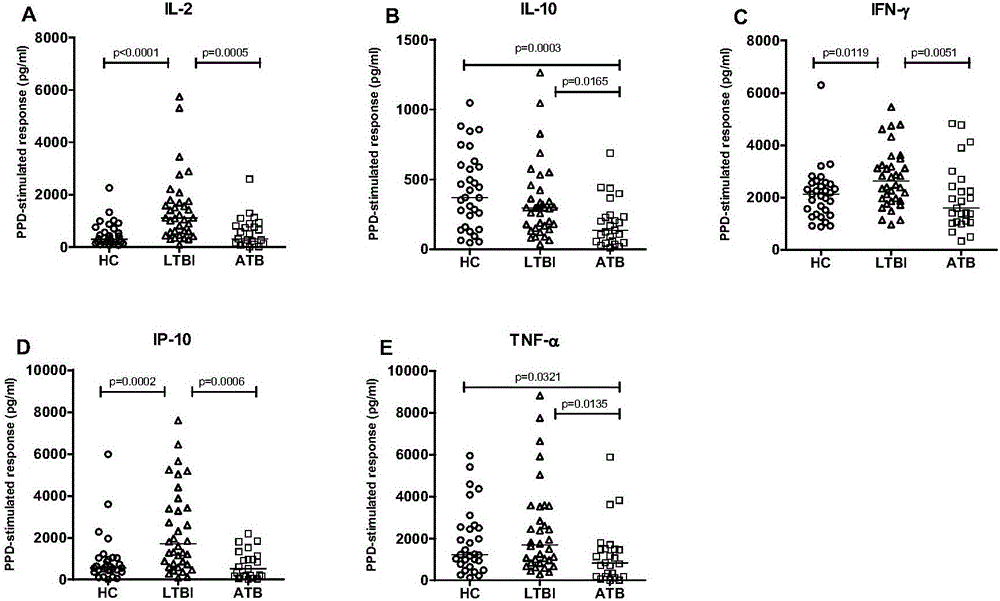
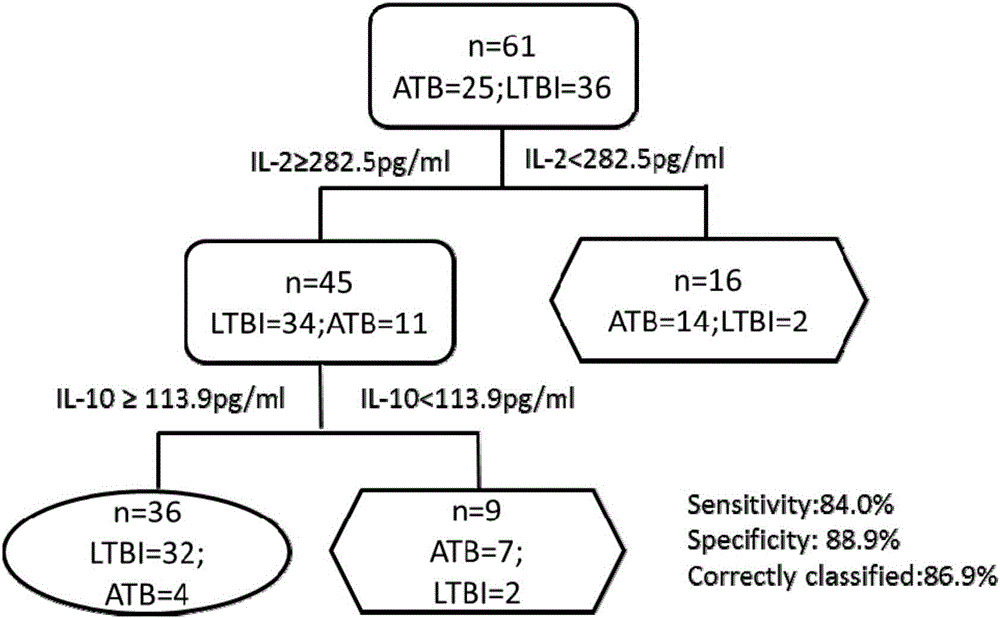
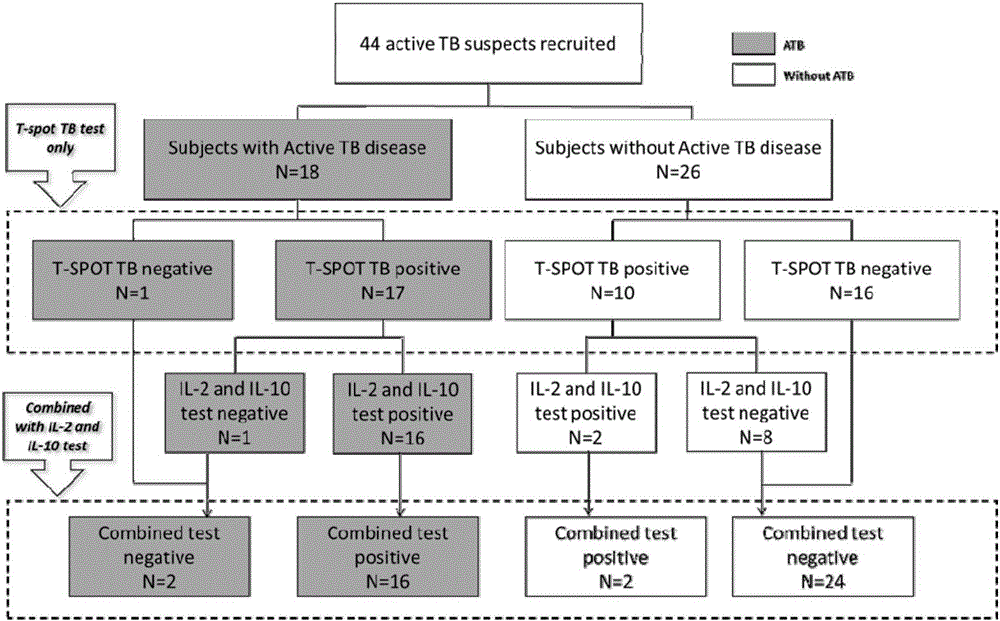
The invention discloses a biomarker for diagnosing mycobacterium tuberculosis infection. The biomarker comprises IL-2 and IL-10. The invention proves that cell factors IL-2 and IL-10 can serve as markers for tuberculosis infection diagnosis for the first time, the tuberculosis infection can be diagnosed by measuring the concentration of the IL-2 and the IL-10 after peripheral blood monouclear cells are stimulated by tuberculosis antigen, and active tuberculosis and latent tuberculosis infection are further distinguished. A tuberculosis infection blood detection reagent and a kit which are prepared on the basis of the two cell factors provide new technological basis for quick diagnosis of the active tuberculosis, have the advantages of high sensitivity, high specificity and the like, are quick in operation, reasonable and practical, and can obviously improve the diagnosis efficiency of the active tuberculosis.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com