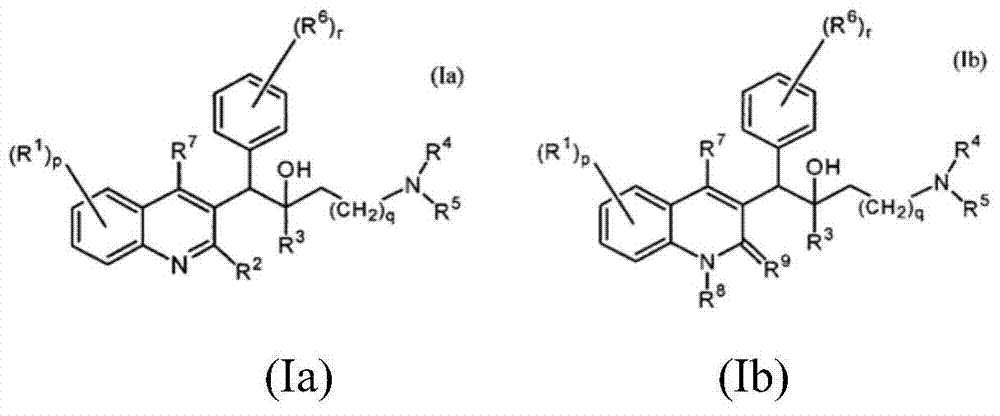
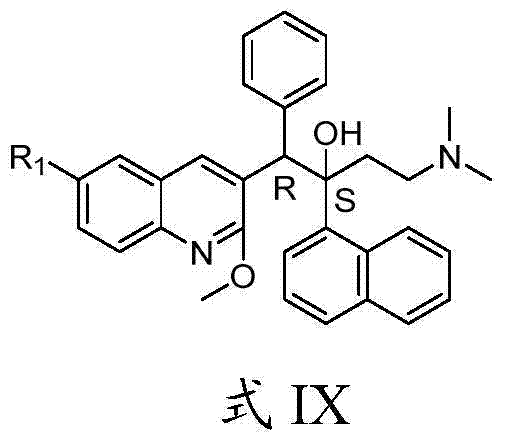
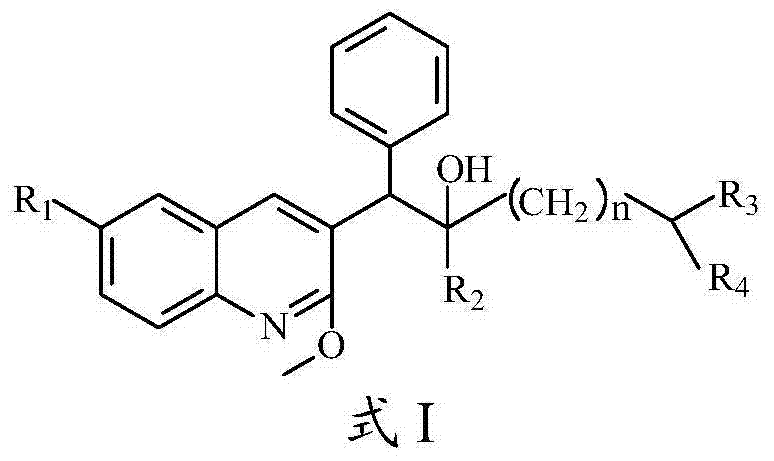
Quinoline, preparation method and application of quinoline
A compound and unsaturated technology, applied in the field of preparation of quinoline derivatives, can solve problems such as interactions, difficulty in patient adherence, and few types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Embodiment 1 Preparation of compound shown in formula II
[0143] Step 1 Preparation of compound shown in formula XII
[0144] Take 220g (1.47mol) of phenylpropionic acid and add it to 400mL of dichloromethane, add 165mL of thionyl chloride under stirring at room temperature, then add 2mL of DMF, stir at room temperature for 1 hour, reflux for 2 hours, concentrate to dryness under reduced pressure, and use 300mL of dichloromethane Methyl chloride was dissolved, and added dropwise at room temperature to a solution of 500 mL of dichloromethane containing 230 g (1.34 mol) of p-bromoaniline and 250 mL of triethylamine. After the dropwise addition was completed, the mixture was stirred overnight at room temperature. Add 400mL of 20% potassium carbonate solution to the reaction solution, stir vigorously for 10 minutes to separate the organic layer, extract the aqueous layer with 400mL of dichloromethane, combine the dichloromethane extracts, wash with 400mL of 20% potassium ...
Embodiment 2
[0152] Embodiment 2 Preparation of compound shown in formula III
[0153] Step 1 Preparation of the compound shown in formula XI (A)
[0154] Take 36.6 g (0.2 mol) of diphenylmethylamine and 26 g (0.2 mol) of epichlorohydrin in 200 mL of isopropanol, and stir at room temperature for 16 hours. The reaction solution was concentrated, 100 mL of acetonitrile and 30 mL of triethylamine were added, heated to 65° C. and stirred for 24 hours. After concentrating, it was dissolved with 400mL of 2N hydrochloric acid, and the obtained solution was washed once with 200mL of ethyl acetate, adjusted to pH=10 with aqueous sodium hydroxide solution, and extracted three times with 500mL of ethyl acetate, and the ethyl acetate extract was washed with saturated brine, anhydrous After drying over sodium sulfate, concentrate, and when the residue is viscous, add 250 mL of petroleum ether and stir vigorously. The eluted solid was collected by filtration and dried to obtain 26 g of the solid compo...
Embodiment 3
[0166] Embodiment 3 Preparation of compound shown in formula III
[0167] Under nitrogen protection, in a 250mL three-necked flask, 6g (24.19mmol) of the compound shown in Formula IV (A) obtained in Step 3 of Example 2 was dissolved in 50mL of anhydrous tetrahydrofuran, and the solution was added dropwise to 3, 5 Difluorobromobenzene 23g (178mmol) and 4.0g magnesium chips were added to the Grignard reagent prepared in 100mL tetrahydrofuran, and after the drop was completed, the temperature was raised to 50°C and stirred for 2.5h. After cooling to room temperature, 100 mL of saturated ammonium chloride aqueous solution was added to terminate the reaction, extracted three times with 50 mL of ethyl acetate each time, and the organic layers were combined. It was washed with water and saturated brine, dried over anhydrous sodium sulfate, concentrated, separated and purified by column chromatography (developing solvent: a mixed solution of petroleum ether and dichloromethane in a vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com