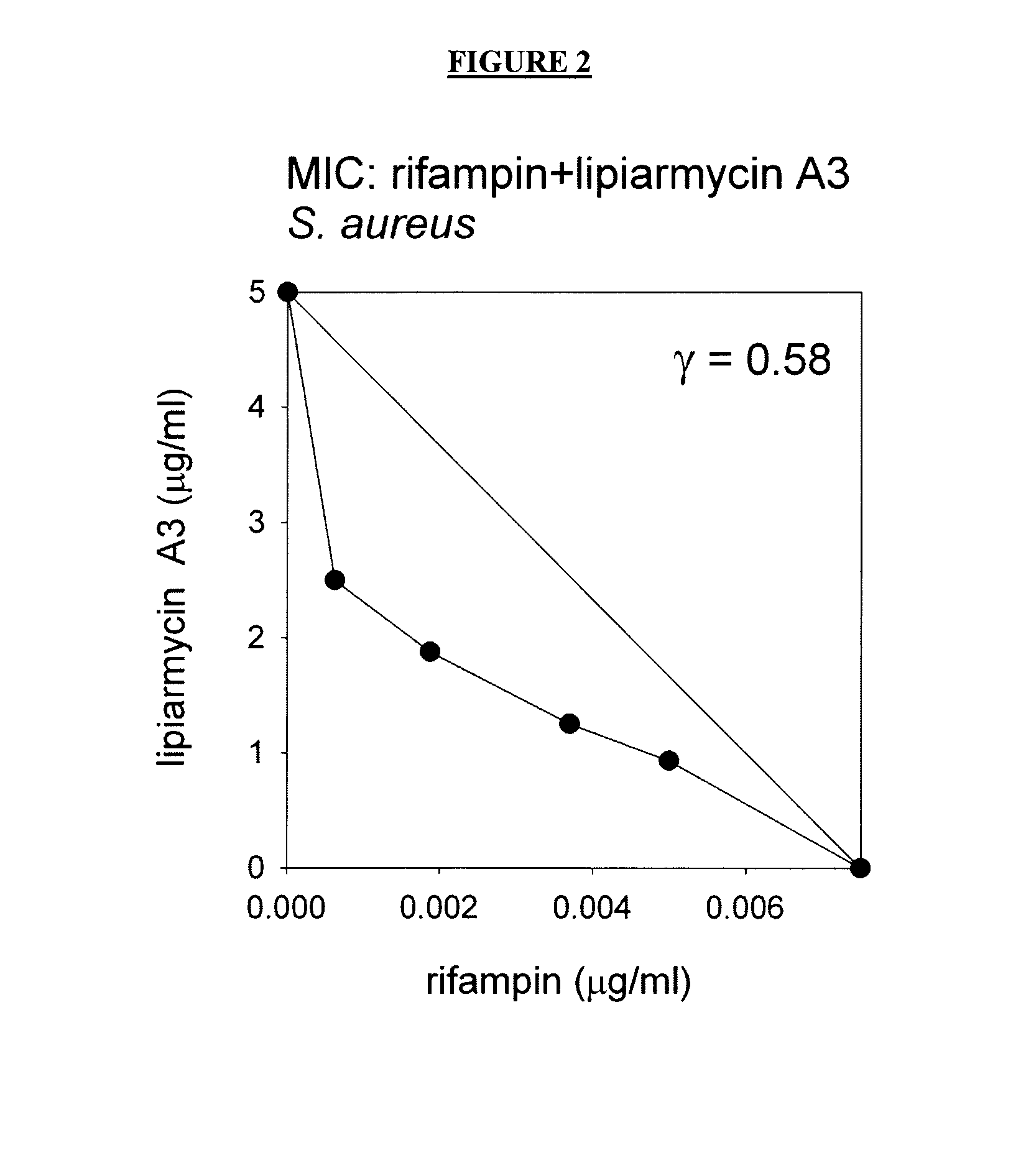
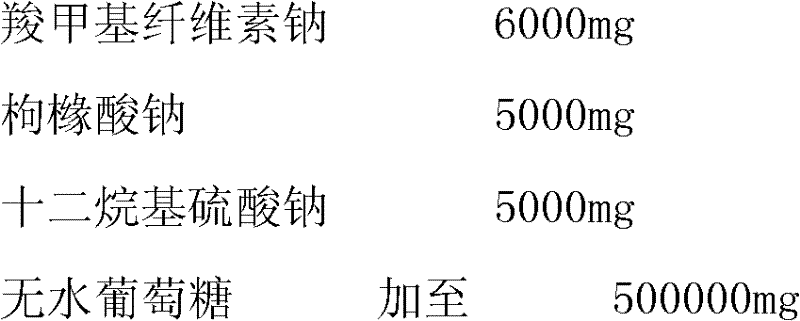
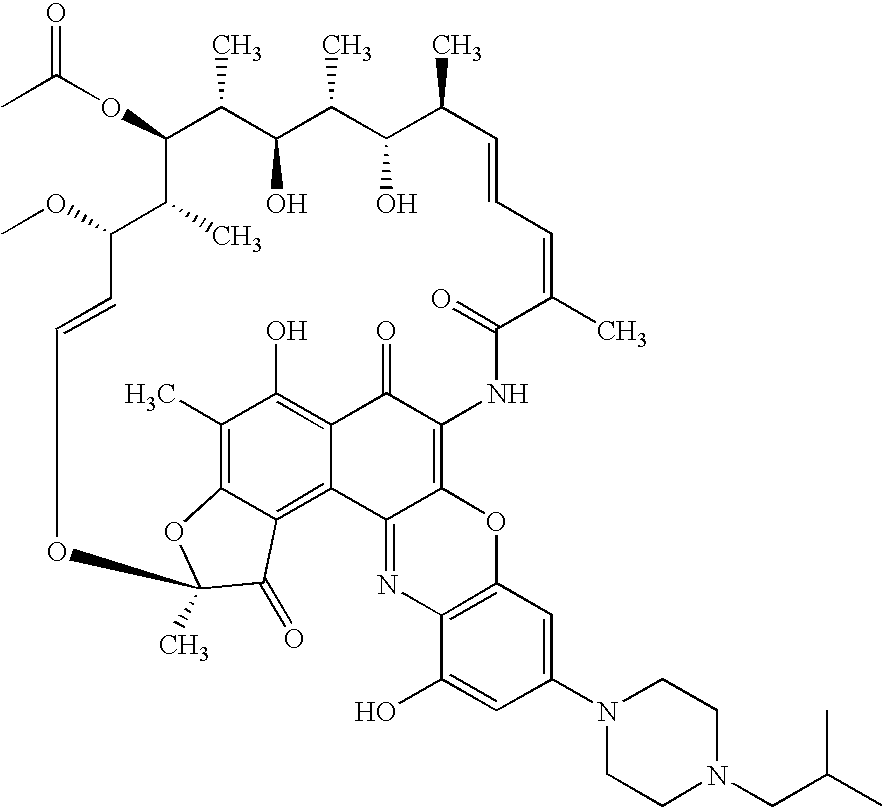
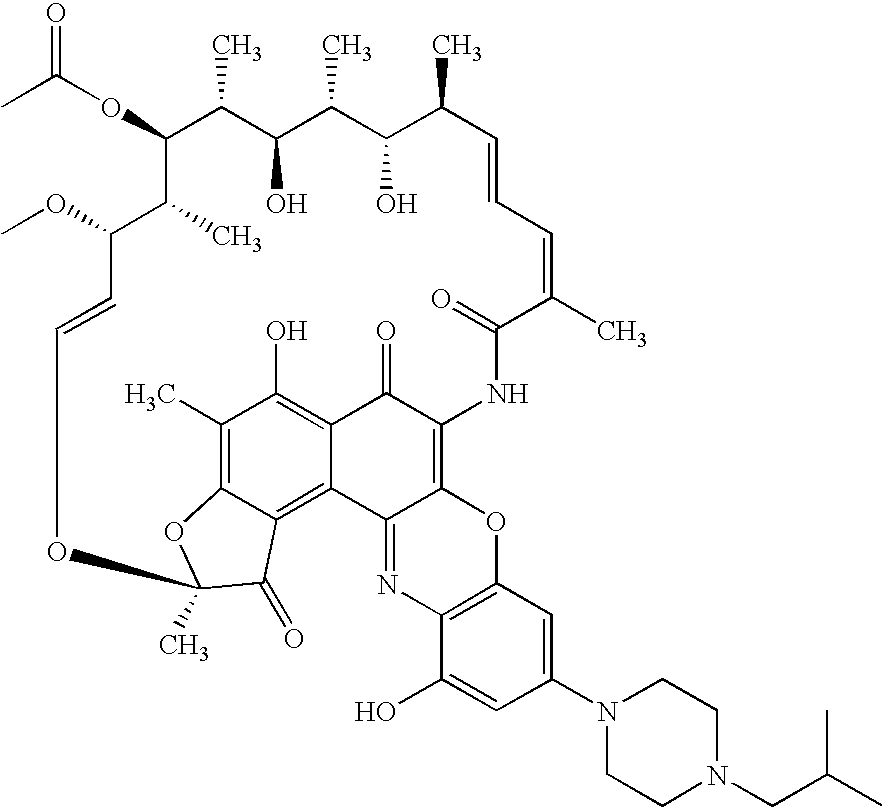
Patents
Literature
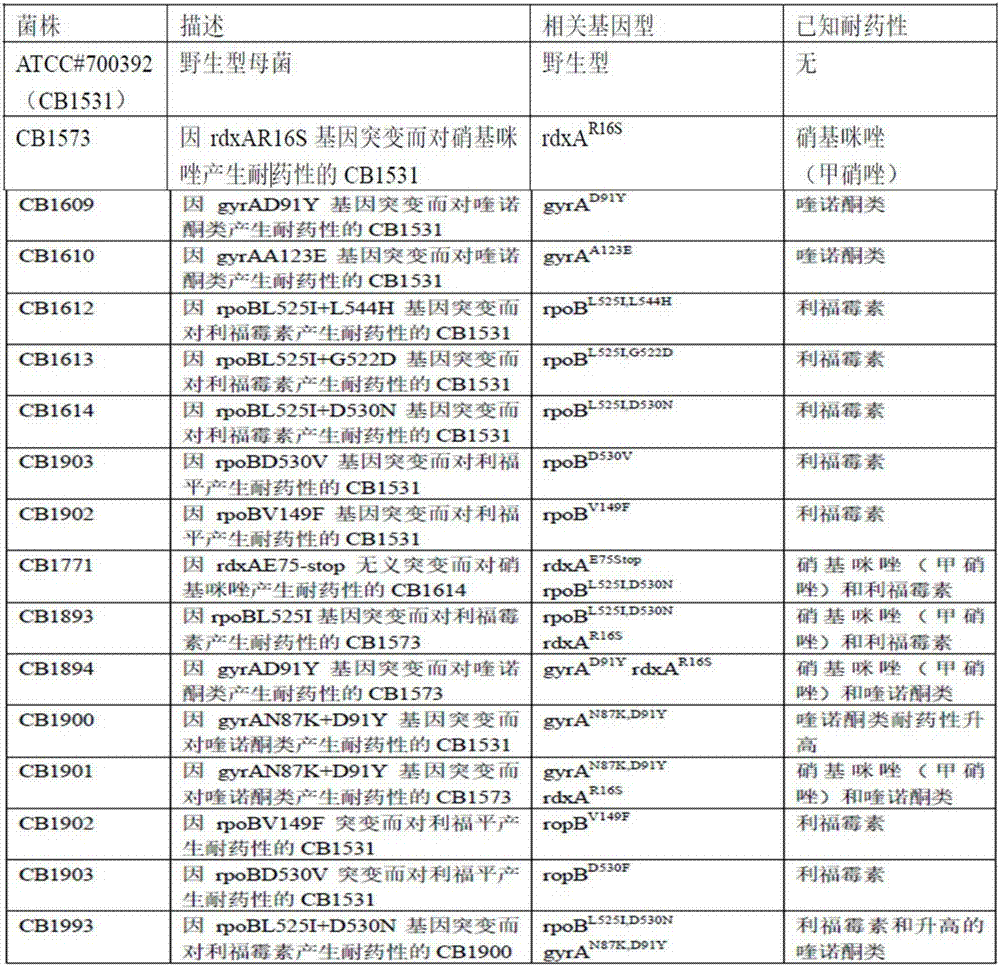
63 results about "Rifamycins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A group of ANTI-BACTERIAL AGENTS characterized by a chromophoric naphthohydroquinone group spanned by an aliphatic bridge not previously found in other known ANTI-BACTERIAL AGENTS. They have been isolated from fermentation broths of Streptomyces mediterranei.
Rifamycin analogs and uses thereof
InactiveUS20050137189A1Improve permeabilityGood curative effectAntibacterial agentsBiocideBenzeneAcetylation
The present invention features rifamycin analogs that can be used as therapeutics for treating or preventing a variety of microbial infections. In one form, the analogs are acetylated at the 25-position, as is rifamycin. In another form, the analogs are deacetylated at the 25-position. In yet other forms, benzoxazinorifamycin, benzthiazinorifamycin, and benzdiazinorifamycin analogs are derivatized at various positions of the benzene ring, including 3′-hydroxy analogs, and / or various fused ring systems with the benzene ring at the 4′,5′ or 5′,6′ positions.
Owner:ACTIVBIOTICS PHARMA
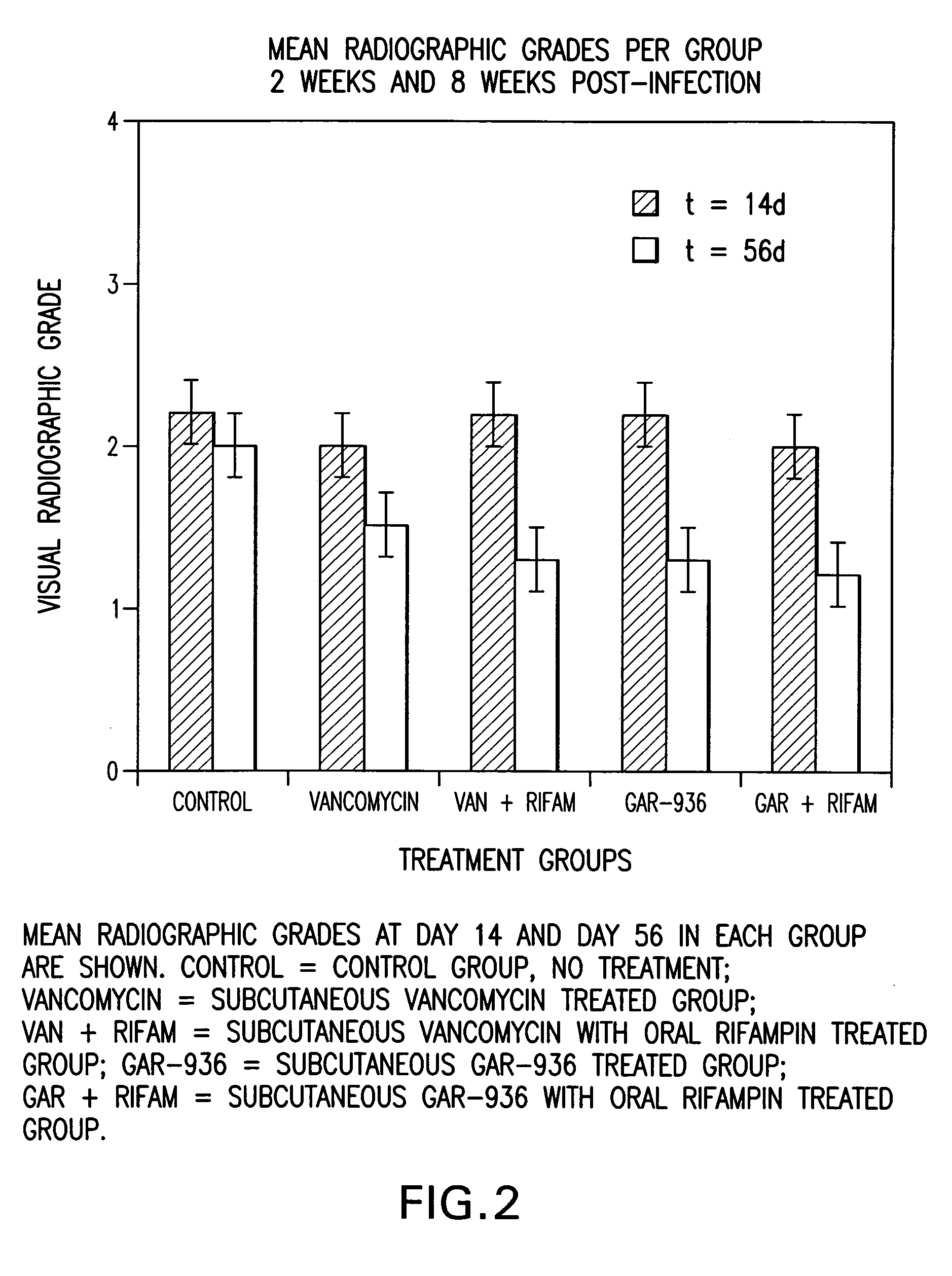
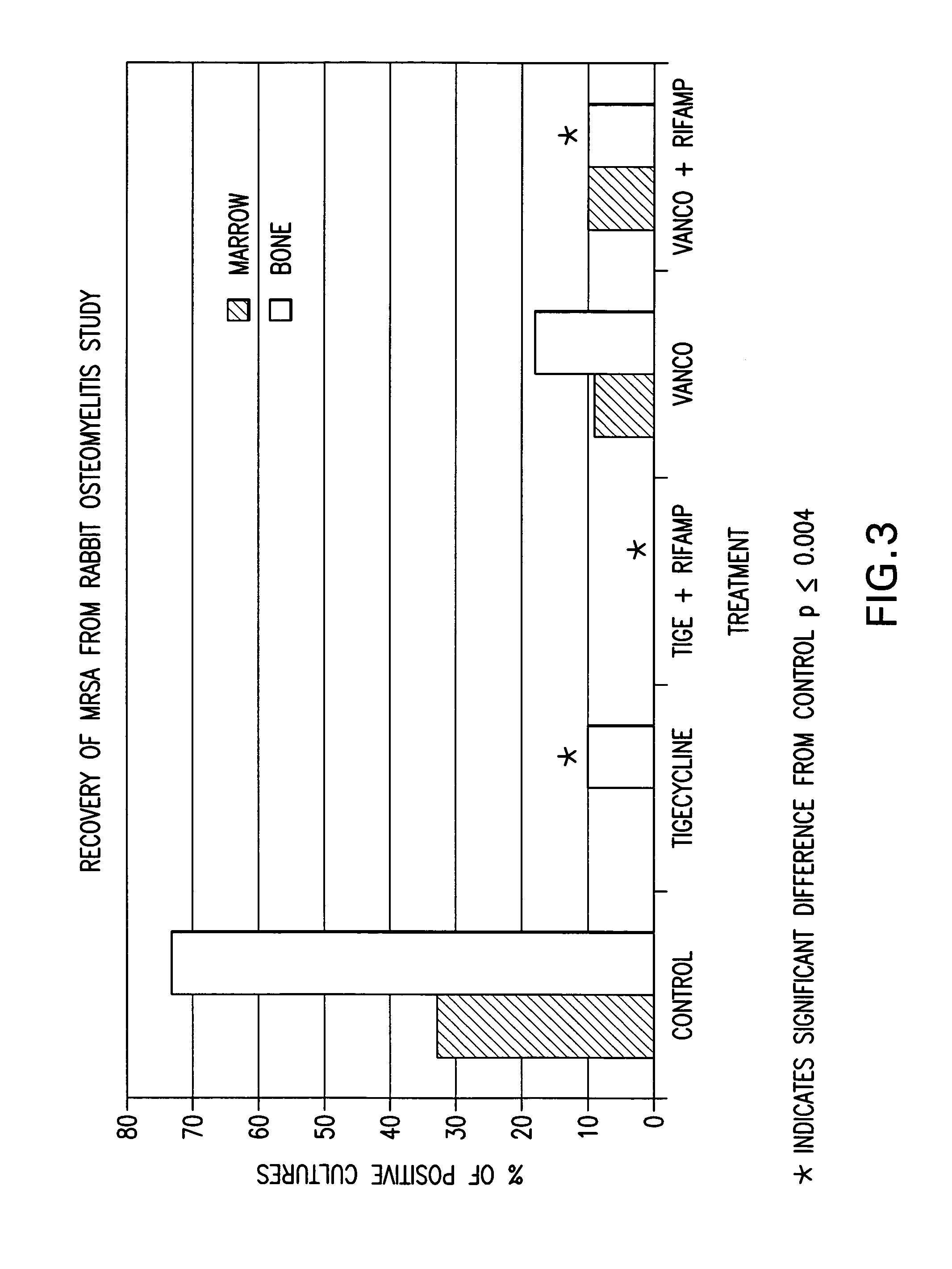
Use of tigecycline, alone, or in combination with rifampin to treat osteomyelitis and/or septic arthritis
The present invention is directed to a method for treating bone or bone marrow infections, joint infection or infection of the tissues surrounding the joint by administration of the antibiotic tigecycline alone or in combination with a rifamycin antibiotic. In a preferred embodiment the bone or bone marrow infection causes osteomyelitis. In another embodiment the joint infection or infection of the tissues surrounding the joint causes septic arthritis. The invention is also directed to manufacture of a medicament for treatment of bone and / or bone marrow infections, or joint infections and / or infections in tissues surrounding the joint with tigecycline alone or in combination with rifampin.
Owner:WYETH +1
Aminomethylene amide analogs of pyrazinamide with intracellular antimycobacterial activity against pyrazinamide-resistant mycobacteria combined with a rifamycin
InactiveUS6399607B1Delay disintegration and absorptionSuitable for manufactureBiocideCarbohydrate active ingredientsDiseaseAryl
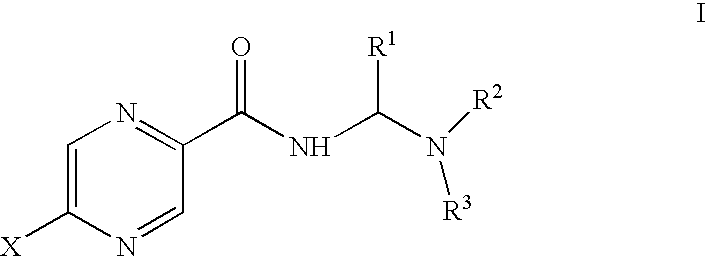
Methods for treating diseases involving pyrazinamide-resistant mycobacteria comprise administering to a mammal in need of treatment a therapeutically effective amount of a combination of rifamycin and a compound of formula I:whereinR1 is hydrogen haloalkyl, or lower alkyl;R2 and R3 are independently chosen from alkyl, substituted alkyl, cycloalkyl, aryl, substituted aryl, alkylaryl and substituted alkylaryl, or R2 and R3 taken together form a five- or six-membered heterocyclic or substituted heterocyclic ring; andX is hydrogen, halogen, or lower alkyl;or a pharmaceutically acceptable salt thereof.
Owner:RES FOUND STATE UNIV OF NEW YORK THE
Application of kelimycin in mycobacterium tuberculosis infection resistance
The invention relates to application of kelimycin in mycobacterium tuberculosis infection resistance. The application comprises the following main steps: clinical first-line antitubercular drugs isoniazide and rifamycin are used as contrast, and the antimycobacterial activity of kelimycin is detected by adopting an absolute concentration method. The result indicates that the activity of kelimycin on clinically separated mycobacterium tuberculosis including drug-resistance bacteria is remarkably superior to those of the clinical first-line contrast drugs isoniazide and rifamycin, and novel application of kelimycin is expected to be developed in treatment of tubercle bacillus infected diseases.
Owner:SHANGHAI TONGLIAN PHARMA CO LTD
Targeted therapeutics and uses thereof
InactiveUS7122525B2Advantageous biodistributionHigh activityAntibacterial agentsBiocideDiseaseMedicine
Owner:ACTIVBIOTICS PHARMA
Antibacterial agents: combination of a rifamycin and a switch region inhibitor
ActiveUS20150031640A1Reduces minimum effective doseReduces spontaneous resistance frequencyBiocideCarbohydrate active ingredientsTreatment failureCo administration
It has been determined that co-administration of a rifamycin and a switch-region inhibitor 1) results in synergistic antibacterial effects, enabling efficacy at low, subtoxic doses, and / or 2) results in a low spontaneous resistance frequency, enabling treatment of high-titer infections without treatment failure due to spontaneous resistance. Accordingly, certain embodiments provide composition comprising a rifamycin and a switch region inhibitor, as well as methods of use thereof.
Owner:RUTGERS THE STATE UNIV
Compound rifaximin dry suspension for preventing and treating endometritis of livestock and preparation method for same
ActiveCN102512417ALess resistant bacteriaAntibacterial and anti-inflammatoryAntibacterial agentsPowder deliverySodium new houttuyfonateVeterinary Drugs
The invention belongs to the field of veterinary medicine, and particularly relates to a compound rifaximin dry suspension for preventing and treating the endometritis of livestock and a preparation method for the same. The rifaximin and sodium new houttuyfonate dry suspension is obtained by using rifaximin and sodium new houttuyfonate as active ingredients, and preparing with pharmaceutically acceptable auxiliary materials. The preparation method comprises the following steps of: performing superfine crushing treatment on raw materials at first; uniformly mixing the treated rifaximin and sodium new houttuyfonate with right amount of filler, suspending aid, surfactant, lubricant, adsorbent and pH buffer according to an equivalent incremental mixing method; subpackaging and sterilizing for1 hour by flowing steam at 100 DEG C. Rifaximin is a novel rifamycin board-spectrum semisynthetic antibiotic medicine, with the advantages of board-spectrum antibacterium, antitoxin and the like, andcapable of preventing and treating the endometritis of dairy cow well; and sodium new houttuyfonate is antibacterial, anti-inflammatory and capable of enhancing immunity as well as resisting bacteriaand diminishing inflammation by cooperating with rifaximin, and has great effect of preventing and treating the endometritis of dairy cow.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Treatment of athersclerotic disease
InactiveUS20080009487A1Reduce frequencyReduce the numberOrganic active ingredientsCardiovascular disorderVascular diseaseIntimal media thickness
The invention features a method of inhibiting the progression of intima-media thickening, or reducing the intima-media thickness (IMT) in arteries in a patient in need thereof by administering to the patient a rifamycin in an amount effective to inhibit the progression of intima-media thickening, or reduce the IMT. The invention also features a method for treating or preventing cerebral vascular disease in a patient in need thereof by administering to the patient a rifamycin in an amount effective to treat the cerebral vascular disease in the patient.
Owner:ACTIVBIOTICS PHARMA
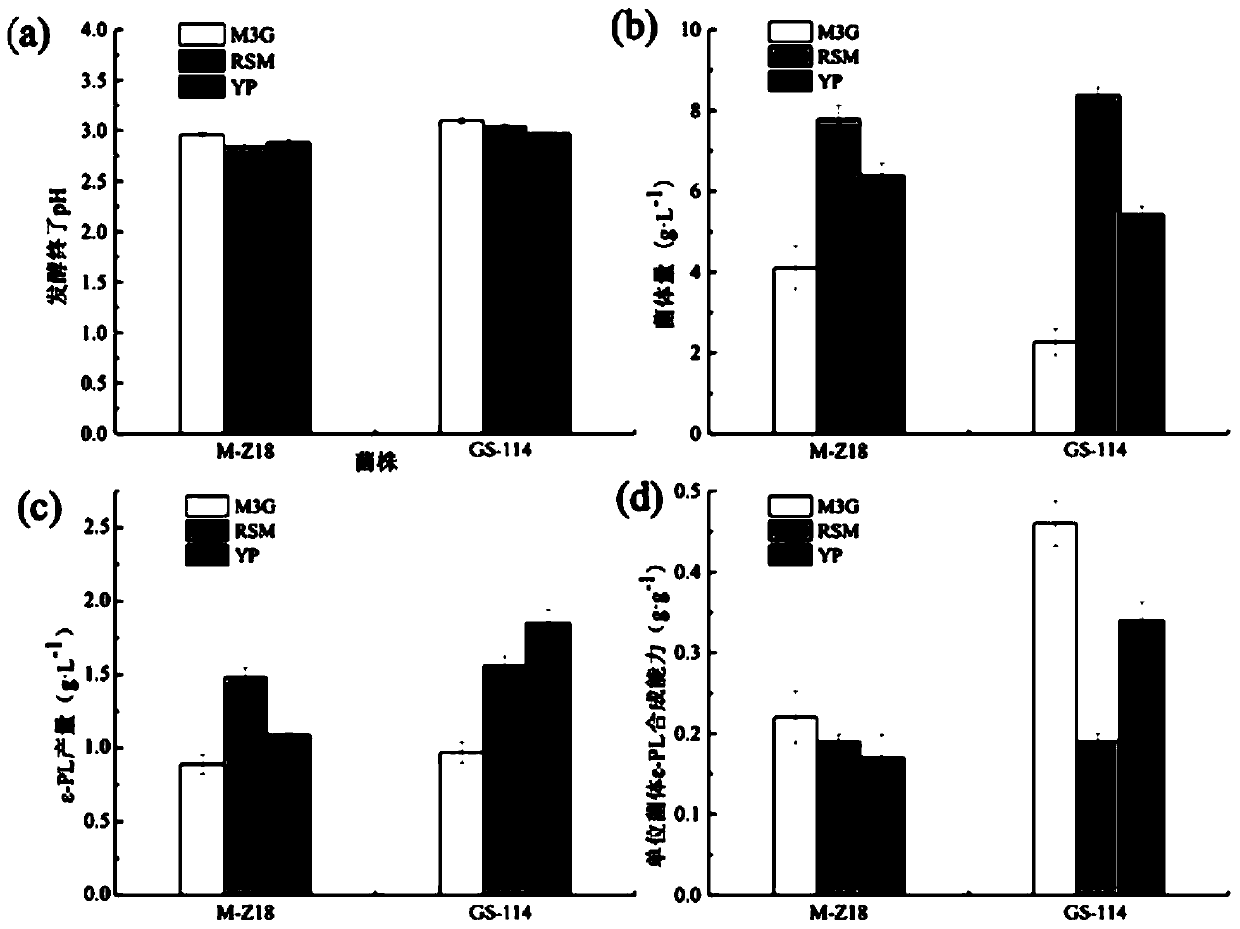
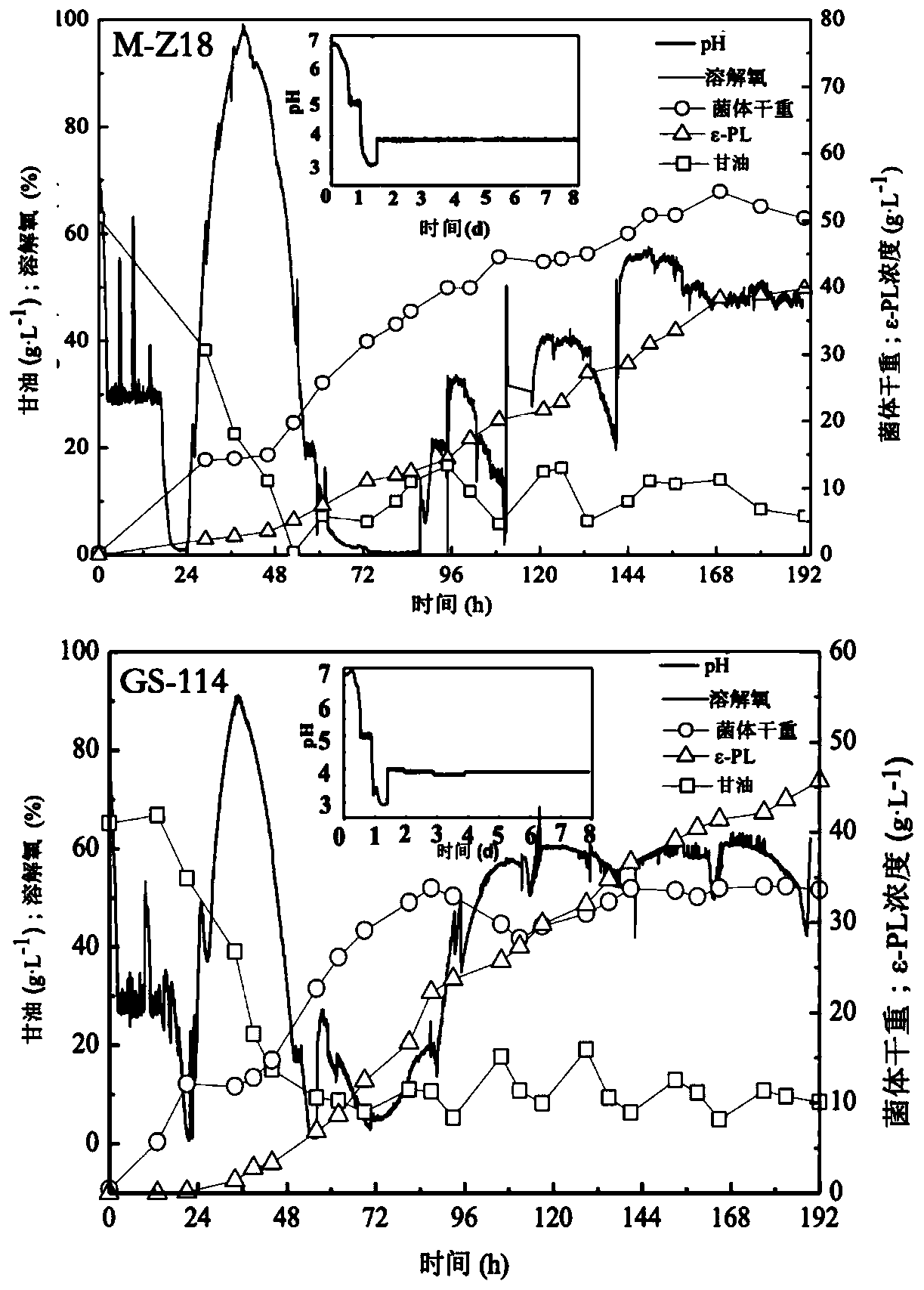
Streptomyces albulus (GS-114) and method of the same to prepare epsilon-polylysine
InactiveCN110804572AStrong synthetic abilityIncrease productivityBacteriaMicroorganism based processesBiotechnologyStreptomyces albulus
The invention discloses streptomyces albulus (GS-114) and a method of the same to prepare epsilon-polylysine, and belongs to the field of microbial fermentation engineering. The provided streptomycesalbulus (GS-114) has resistance to streptomycin, gentamicin and rifamycin with 3 [mu]g / mL, 1 [mu]g / mL and 0.1 [mu]g / mL or higher concentrations, and can efficiently synthesize epsilon-PL in quantity,the yield with 192 h of fermentation can reach 56.3 g / L, and production efficiency can reach up to 7.03 g / L / d. The streptomyces albulus (GS-114) also has good passage stability and has strong potential for the industrial production of the epsilon-PL.
Owner:JIANGNAN UNIV
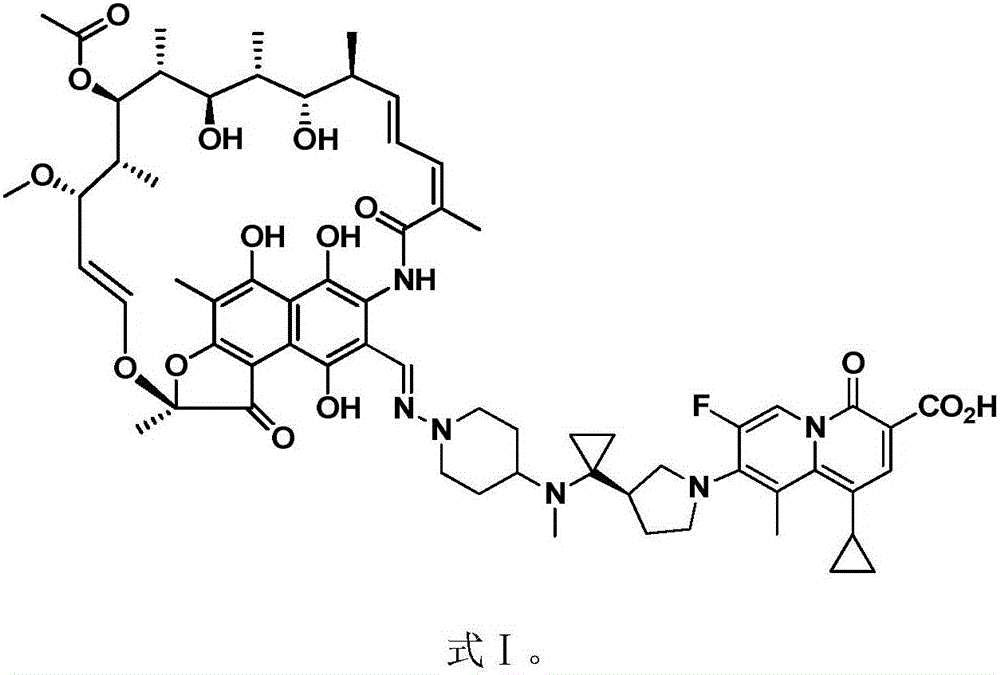
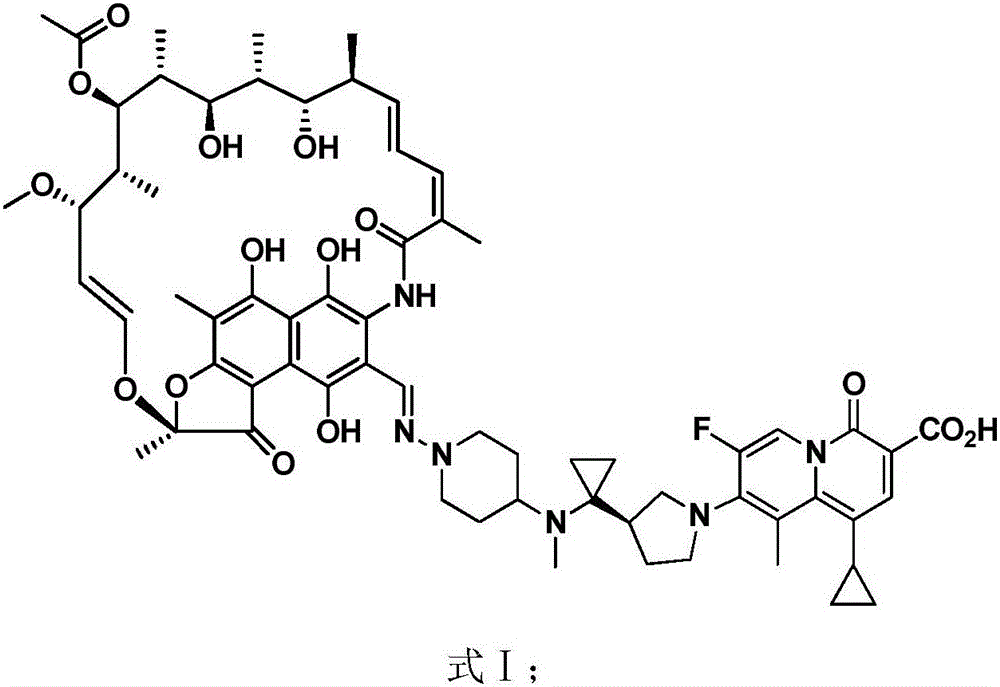
New use of rifamycin-quinolizidone dual-target molecule
InactiveCN106822125AHigh antibacterial activityLow frequency of drug resistanceAntibacterial agentsOrganic active ingredientsAntibacterial activityHepatic encephalopathy
The invention discloses application of a rifamycin-quinolizidone dual-target molecule represented in a formula I in inhibition of ammonia producing floras in gastrointestinal tracts. The rifamycin-quinolizidone dual-target molecule represented in the formula I is similar to an antibacterial spectrum of rifaximin and has relatively strong antibacterial activity to common ammonia producing floras in the gastrointestinal tracts; and meanwhile, the rifamycin-quinolizidone dual-target molecule has the property of low drug resistance frequency and has application prospects in prevention and treatment of infection of hepatic encephalopathy and relevant bacterial genera (types). The formula I is as shown in the specification.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
Viscous soda type saline-alkali soil modifier and process for applying modifier to modification of saline-alkali soil
ActiveCN107739620AImprove textureLower pHAgriculture tools and machinesOther chemical processesPorosityAlkali soil
The invention discloses a viscous soda type saline-alkali soil modifier and a process for applying the modifier to modification of saline-alkali soil. The modifier is prepared from the following materials in parts by weight: coarse sand, rifamycin fermented filter residue obtained after innocent treatment, superphosphate, ferrous sulfate, aluminum sulfate and glucosamine hydrochloride. The viscoussoda type saline-alkali soil modifier is suitable for viscous soda type saline-alkali soil, can effectively reduce the pH value and degree of alkalization of soil and improve the character of the viscous soil, and is rich in material source; crops after modification of 2-3 years can reach the level of middle-output fields; especially by using rifamycin fermented filter residue, waste is changed into wealth, so that the increasingly severe environment problem can be solved. The viscous soda type saline-alkali soil modifier fundamentally improves the character of the viscous soil by adopting the coarse sand, and can improve the porosity and permeability and increase water penetration.
Owner:内蒙古漫瀚农业科技有限公司
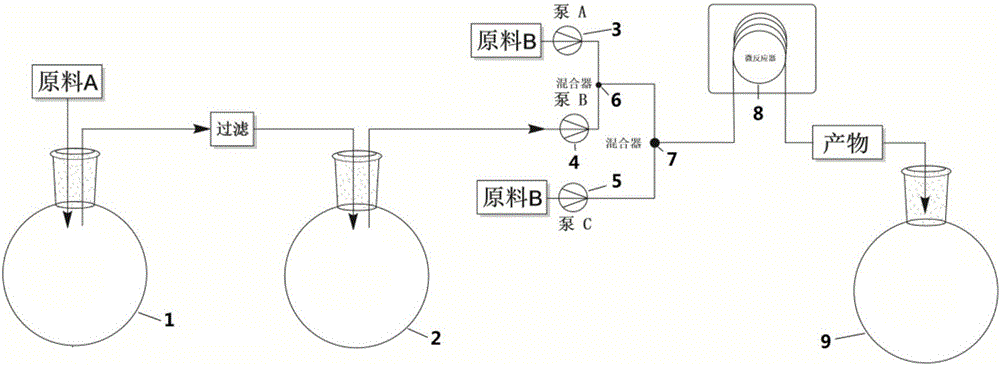
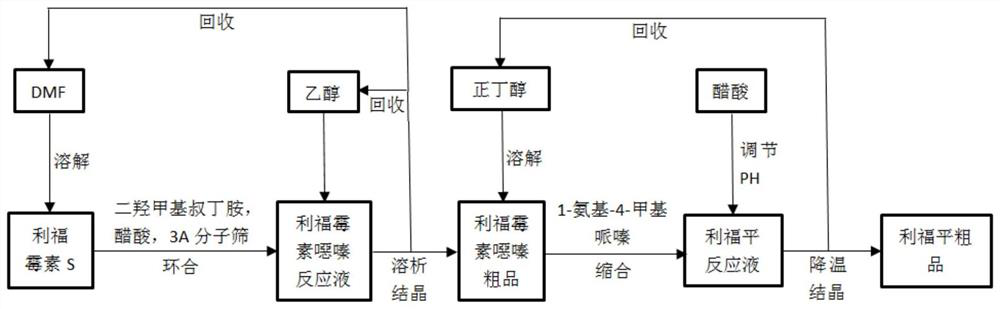
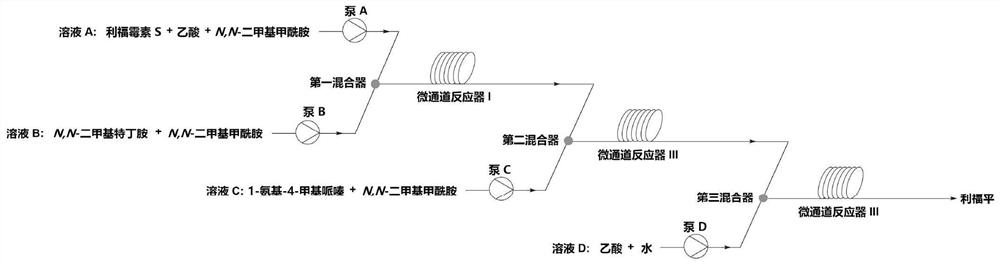
Method for preparing rifampicin by utilizing cascade reaction of kettle type reaction device and microchannel reaction device
InactiveCN106632394ASave heatLow costOrganic chemistryChemical/physical/physico-chemical microreactorsSodium saltPiperazine
The invention provides a method for preparing rifampicin by utilizing cascade reaction of a kettle type reaction device and a microchannel reaction device. In the kettle type reaction device, rifamycin S sodium salt is used as a raw material to obtain N-tedin-1,3-oxazine (5,6-C) rifamycin through reaction, and the N-tedin-1,3-oxazine (5,6-C) rifamycin reacts with 1-methyl-4-amino-piperazine in the microchannel reaction device to obtain the rifampicin. Continuous production of the rifampin is achieved, the product quality is good, the cost is low, the profit is high, and the method is green, environmentally friendly, capable of saving energy, efficient and suitable for industrial application.
Owner:NANJING UNIV OF TECH
Treatment for leukemia and idiopathic aplastic anemia
A process for treating a patient with leukemia or an aplastic anemia having cells with inclusions that stain with anti-E. canis antibodies or antibodies to other Ehrlichia or Anaplasma is disclosed. That process comprises administering to the patient (i) an antibacterial amount of a rifamycin, (ii) an antibacterial amount of a quinolone, or a mixture of (i) and (ii).
Owner:SPHINGOMONAS RES PARTNERS
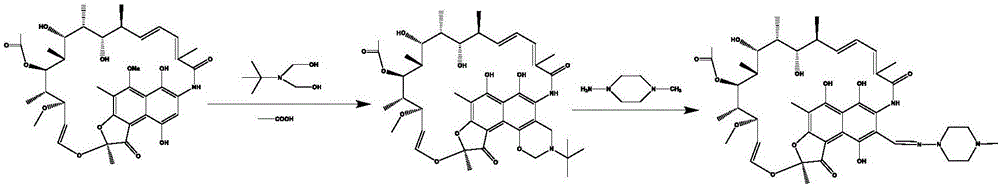
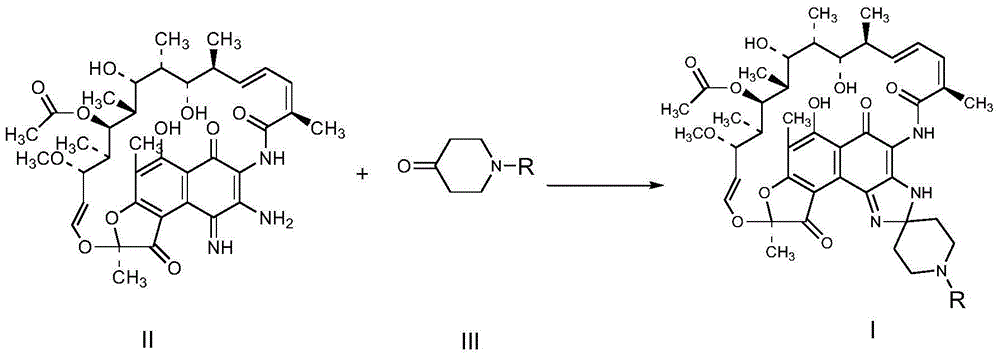
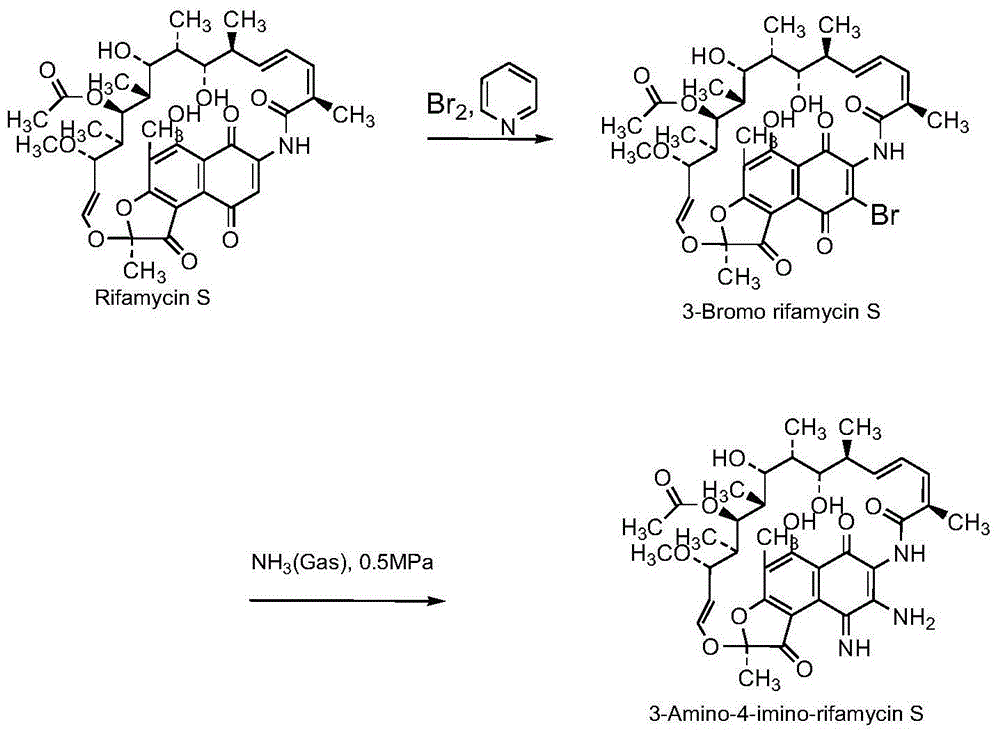
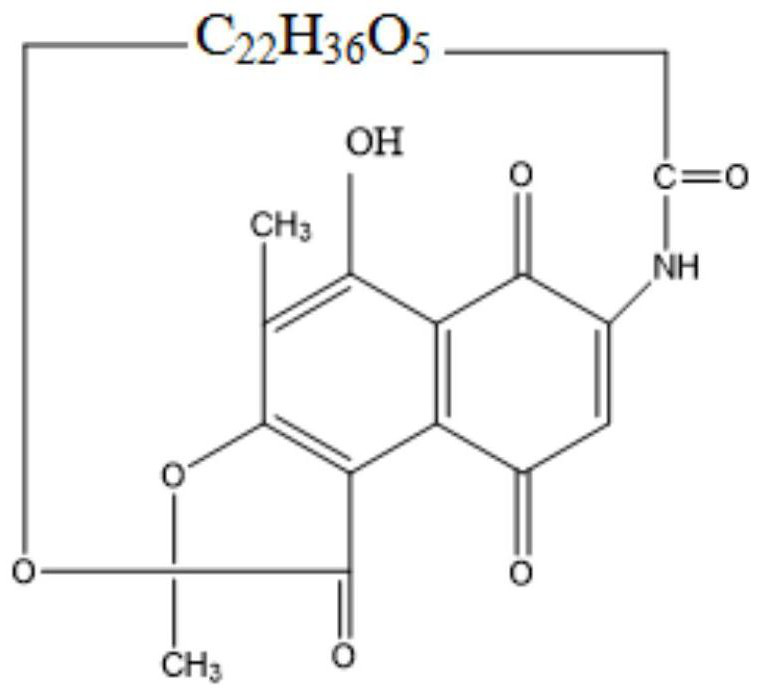
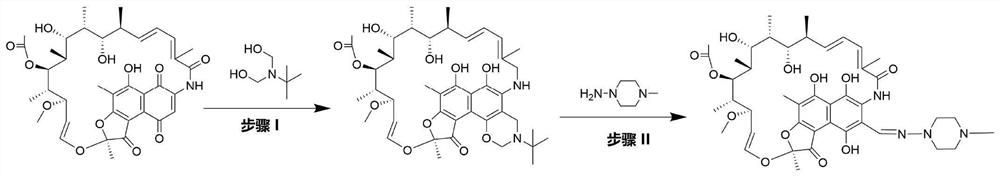
Preparation method for rifamycin S derivative
The invention relates to a preparation method for a rifamycin S derivative. According to the preparation method, 3-amino-4-imine rifamycin S as shown in a formula II which is described in the specification and a 4-piperidone derivative as shown in a formula III which is described in the specification are added according to a certain feeding mode and subjected to a condensation reaction in the presence of an organic solvent, ammonium acetate and zinc dust so as to produce the rifamycin S derivative as shown in a formula I which is described in the specification. The feeding mode is that the 3-amino-4-imine rifamycin S is added into a reaction system in a final step, so 3-amino-4-imine rifamycin S is prevented from reduction and hydrolysis. The preparation method increases the utilization rate of materials and is shortened in reaction time, so high-efficiency high-yield preparation of the rifamycin S derivative is realized, and the yield and content of the rifamycin S derivative reach 80% or above and 95% or above, respectively; and the preparation method overcomes the problems of long reaction time, low yield, many impurities and high cost, and is simple to operate, highly efficient, environment friendly and beneficial for large-scale production.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Slow released antituberculotic preparation
The slow released antituberculotic preparation is implanting agent or injection with slow releasing of medicine in the local tuberculosis focus to maintain the local effective medicine concentration while lowing the systemic toxicity. The slow released injection consists of slow released microsphere and solvent. The slow released microsphere consists of slow releasing supplementary material and antituberculotic selected from rifampicin, rifamycin, rifapentine and / or rifabutine. The solvent is special solvent containing suspending agent of carboxymethyl cellulose sodium, etc. and has viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is selected from EVAc, PLA, PLGA, etc. The slow released preparation may be prepared with slow released microsphere. The present invention has obvious and unique treating effect on various kinds of intractable tuberculosis.
Owner:SHANDONG LANJIN PHARMA
Diagnosis and management of infection caused by Chlamydia
InactiveUS20050143386A1Prevent reinfection/reactivationChlamydial pathogen can be eliminated more rapidlyBiocideCarbohydrate active ingredientsDiseaseCoronary artery disease
The present invention provides a method of treating coronary artery disease in a patient in need thereof by administering to the patient an antichlamydial amount of a rifamycin for a duration to treat said coronary artery disease.
Owner:VANDERBILT UNIV
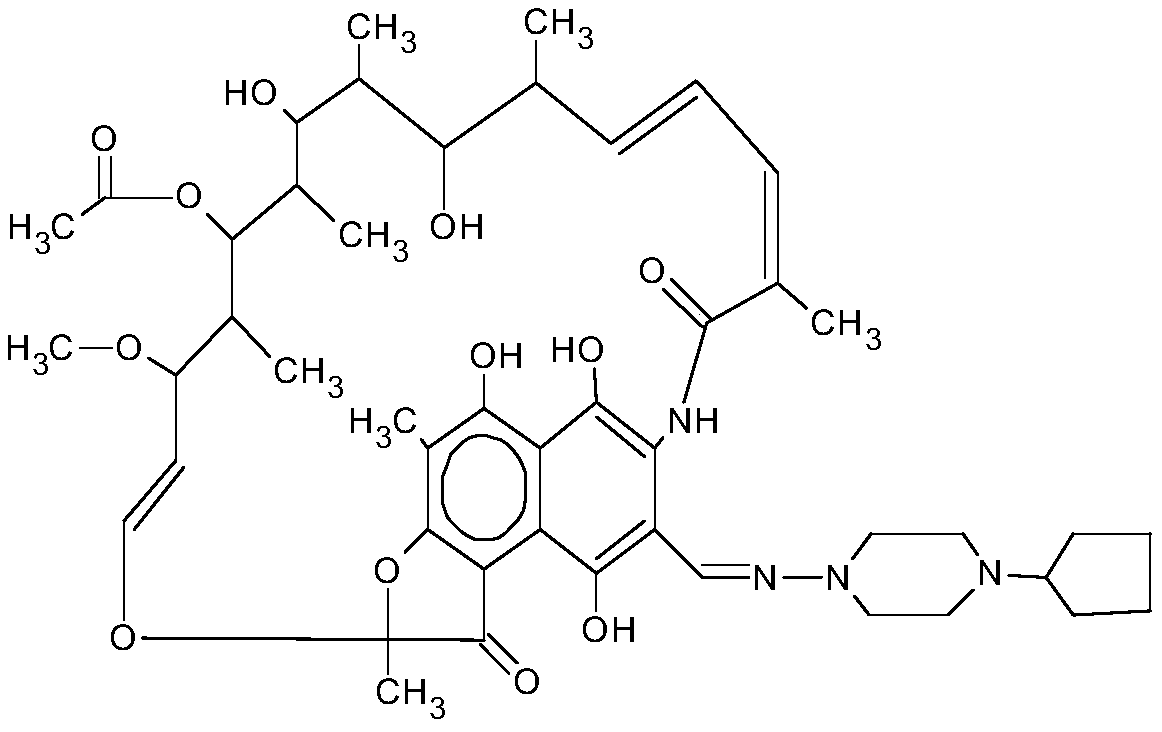
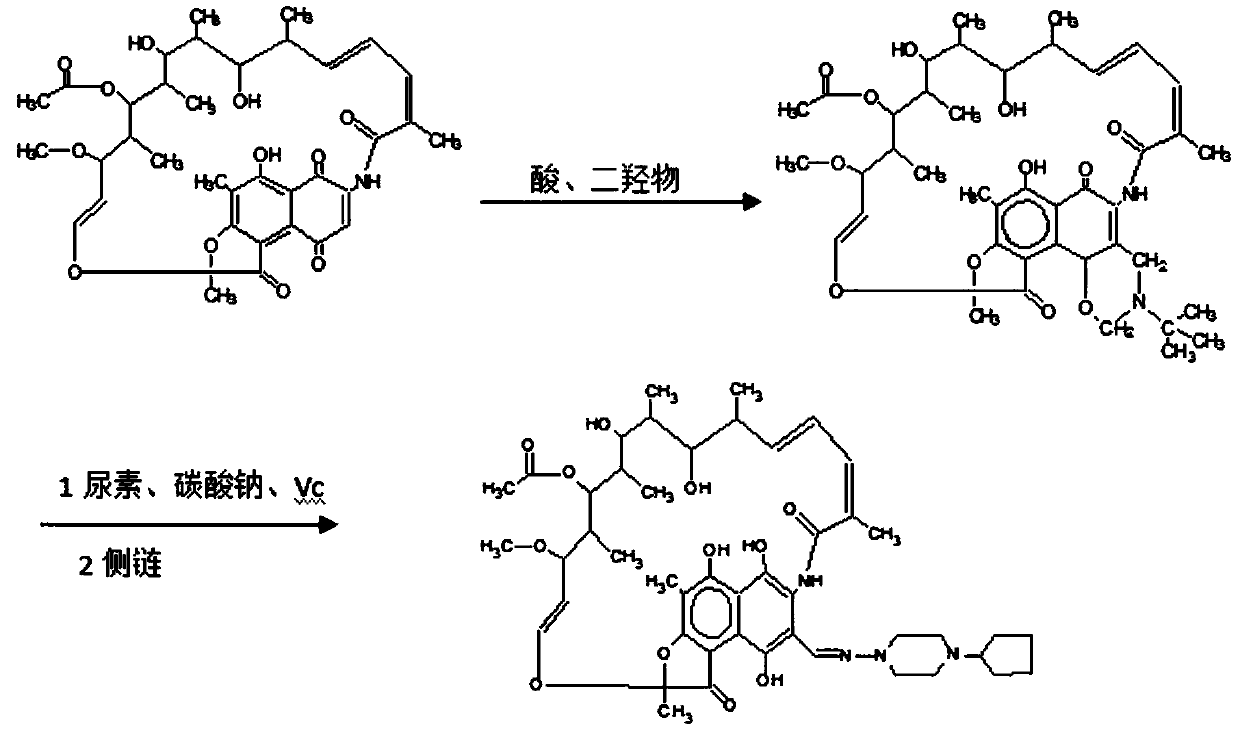
Preparation method of high-purity rifapentine
The invention provides a preparation method of high-purity rifapentine, which is characterized by comprising the following steps: 1. carrying out a cyclization reaction on rifamycin S acid, acid and dimethyloltert-butylamine in an N, N-dimethylformamide solvent; 2, pouring the reaction solution into acid water, standing, and filtering to obtain a rifaxizine filter cake; 3, adding urea, n-butyl alcohol, Vc and sodium carbonate into the rifaxizine filter cake for a ring-opening reaction; 4, after the reaction is completed, increasing the purity to 1-amino-4-cyclopentylpiperazine for a condensation reaction until the reaction is completed so as to obtain a rifapentine reaction solution; and step 5, dropwise adding acid water into the rifapentine reaction solution, stirring, stopping heating after crystallization, lowering the rotating speed after turning red, cooling after dropwise adding is finished, filtering to obtain the filter cake, and carrying out secondary recrystallization on thefilter cake. According to the preparation method of the high-purity rifapentine, provided by the invention, the maximum single impurity of the rifapentine can be controlled within 0.1%, and the residual n-butyl alcohol and the residual ethanol can be controlled within 0.5%.
Owner:WUXI FORTUNE PHARMA
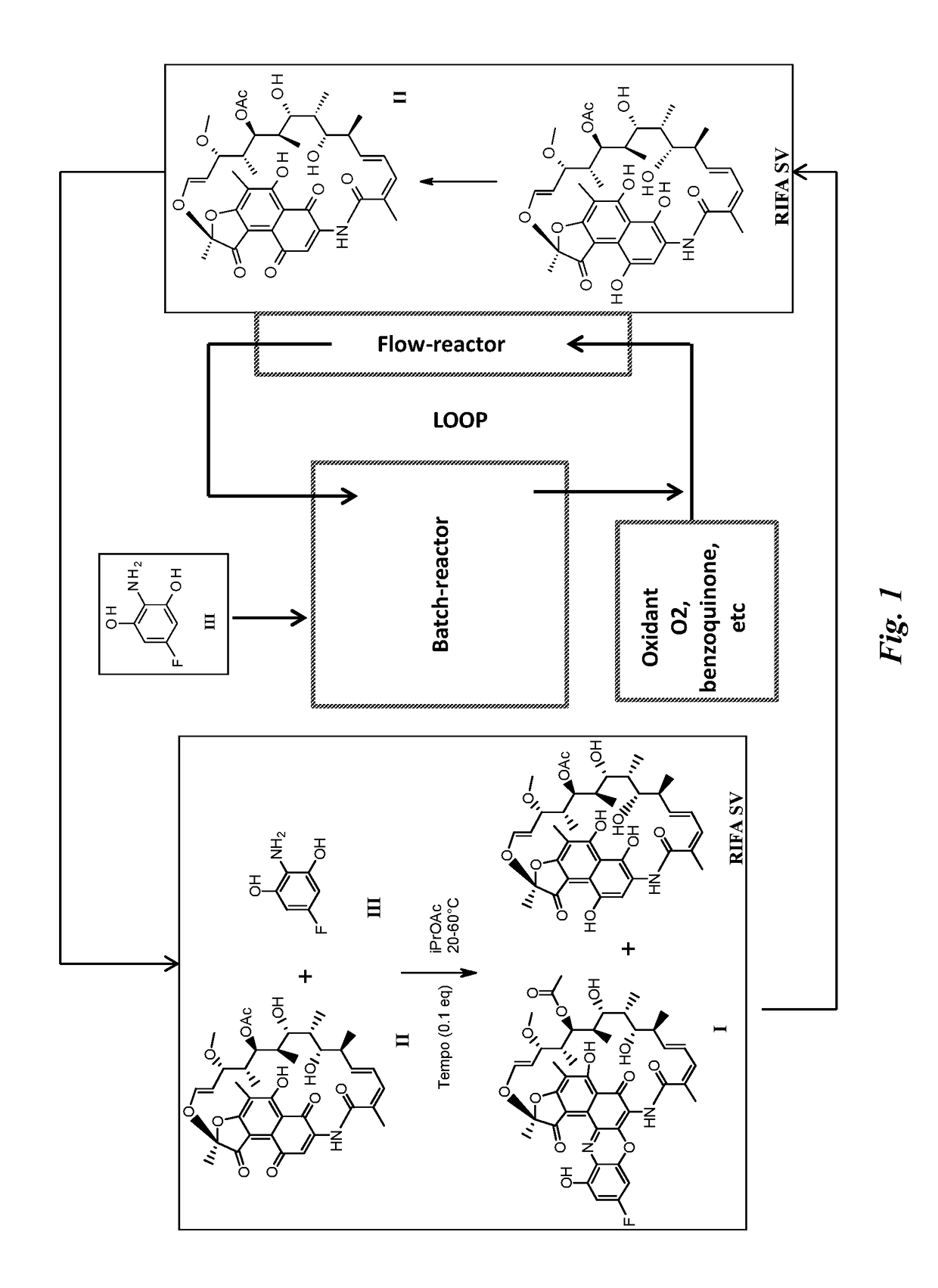
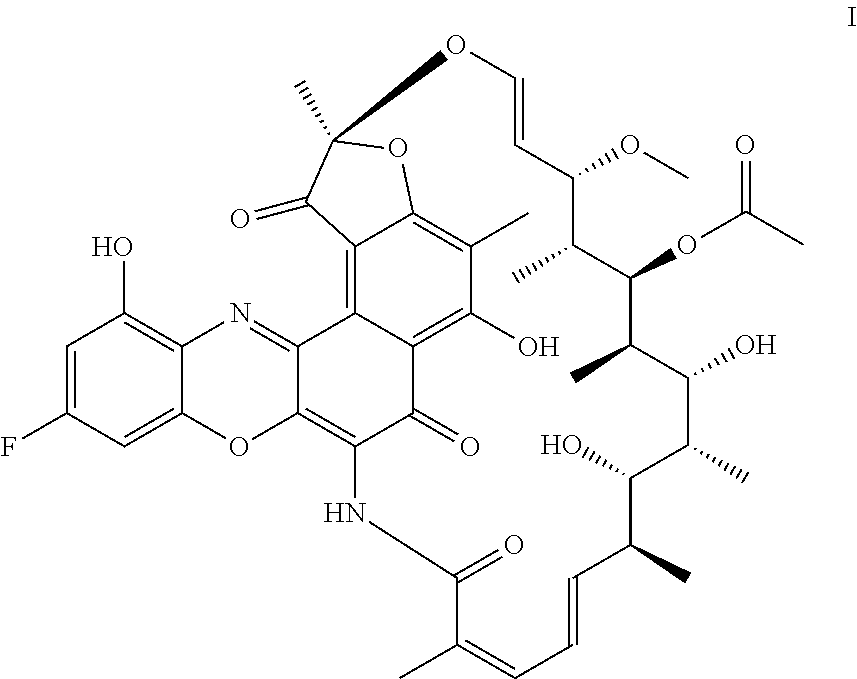
Process for the preparation of an antibody-rifamycin conjugate
ActiveUS20170252457A1Organic compound preparationImmunoglobulins against bacteriaAntibodyPhotochemistry
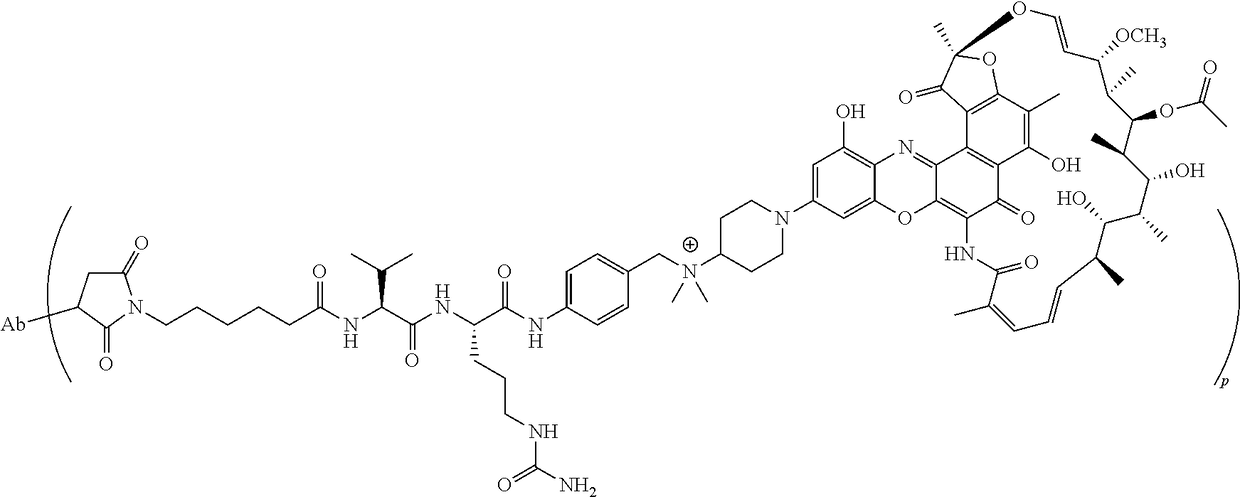
Processes are described for the preparation of F-benzoxazinorifamycin I:and intermediates for conjugation with an antibody.
Owner:GENENTECH INC
Use of tigecycline, alone, or in combination with rifampin to treat osteomyelitis and/or septic arthritis
InactiveCN1889957AHigh serum levelsLow inhibitory concentrationAntibacterial agentsTetracycline active ingredientsTigecyclineSeptic osteoarthritis
The present invention is directed to a method for treating bone or bone marrow infections, joint infection or infection of the tissues surrounding the joint by administration of the antibiotic tigecycline alone or in combination with a rifamycin antibiotic. In a preferred embodiment the bone or bone marrow infection causes osteomyelitis. In another embodiment the joint infection or infection of the tissues surrounding the joint causes septic arthritis. The invention is also directed to manufacture of a medicament for treatment of bone and / or bone marrow infections, or joint infections and / or infections in tissues surrounding the joint with tigecycline alone or in combination with rifampin.
Owner:WYETH LLC +1
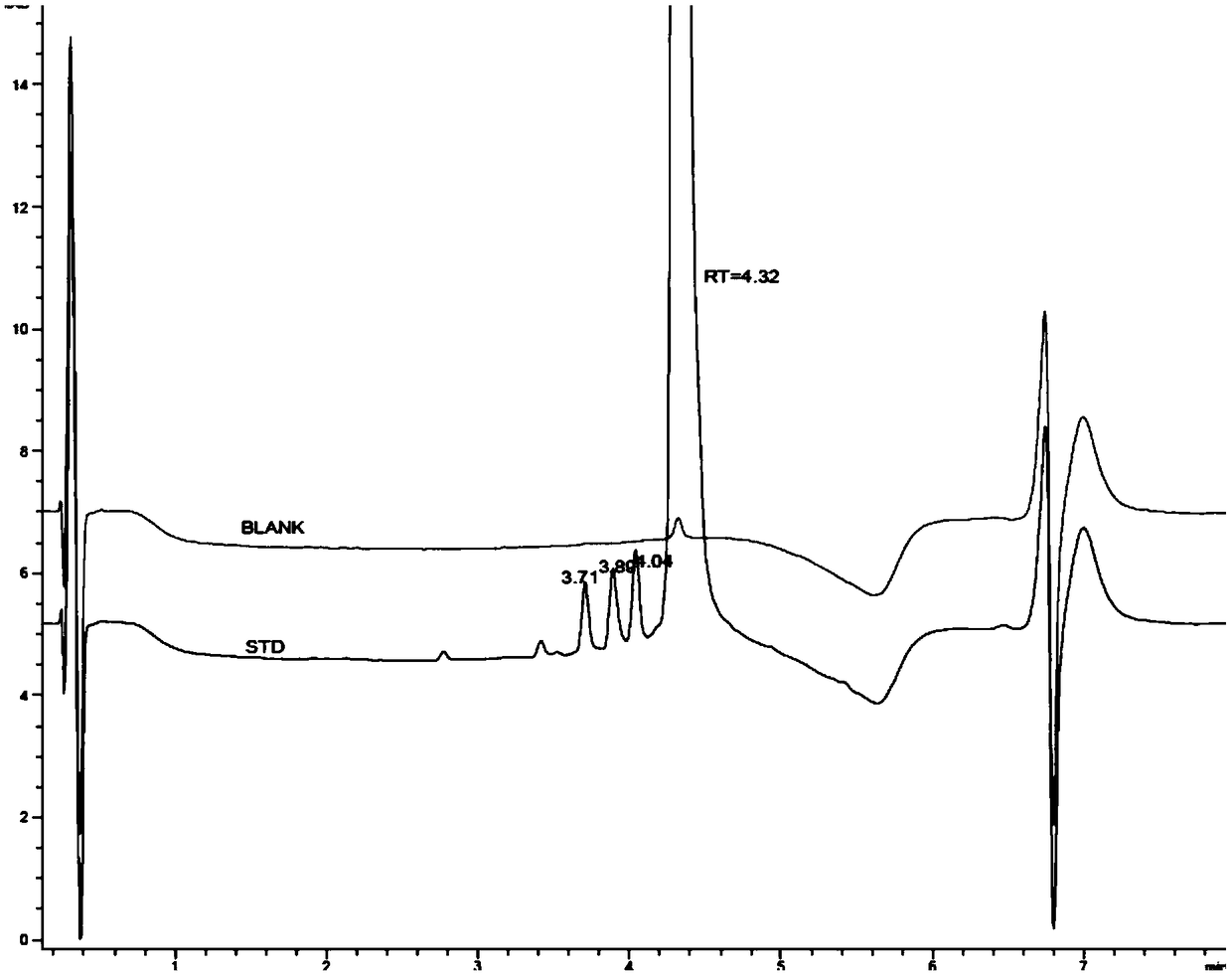
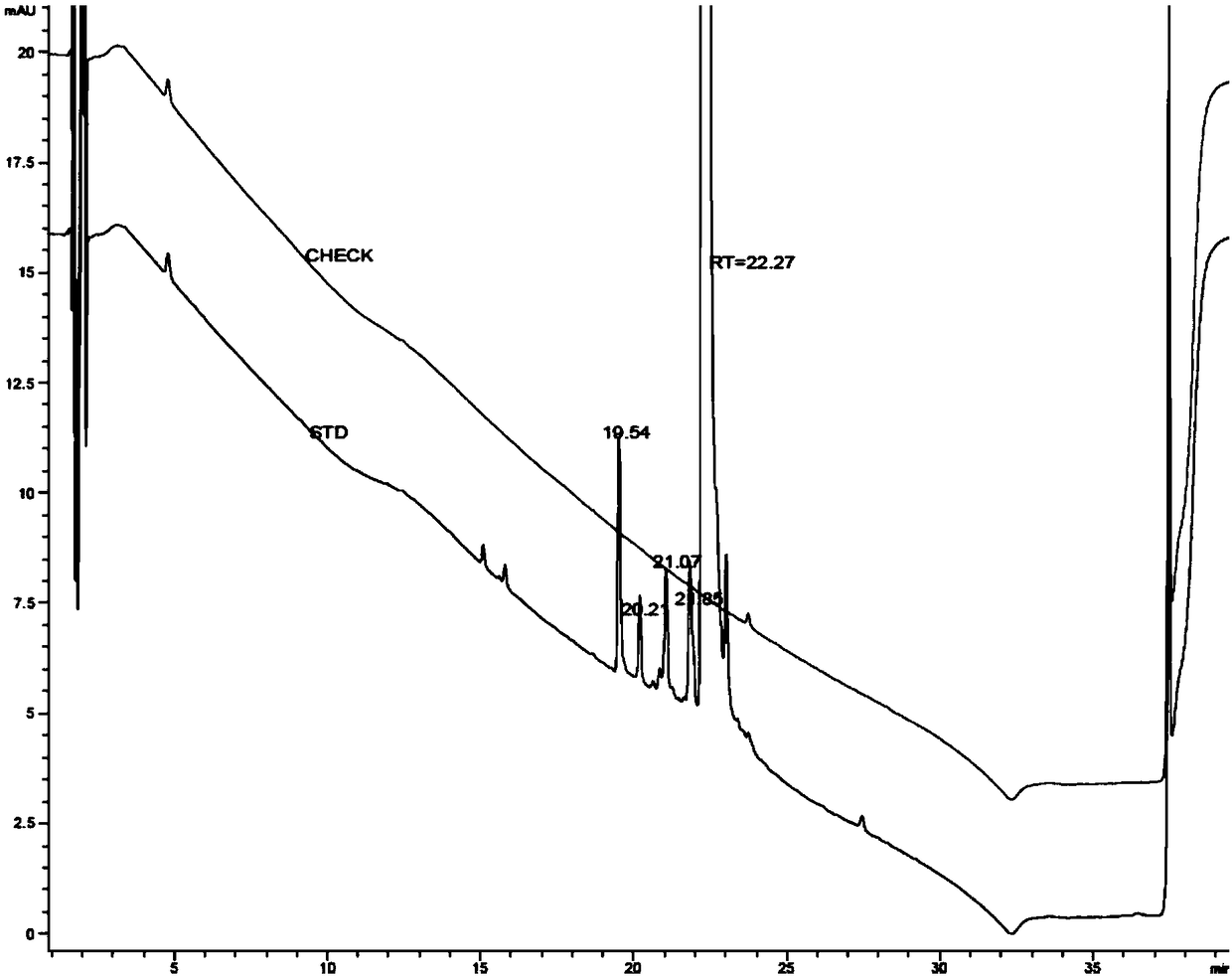
Method for separating and determining 3-amino-4-imino rifamycin S and related impurities
PendingCN112946135AEfficient separationEfficient methodComponent separationChromatography columnOctadecane
The invention belongs to the field of analytical chemistry, and particularly relates to a method for separating and determining 3-amino-4-imino rifamycin S and related impurities. A chromatographic column adopted in the method takes octadecylsilane chemically bonded silica as a filler, a mobile phase A and a mobile phase B are adopted for gradient elution, and a detector is entered for detection; the related impurities comprise one or more of 3-amino rifamycin S, 3-bromo rifamycin S, rifamycin S, an impurity A, an impurity B, an impurity C and an impurity D; the mobile phase A is acetonitrile and methanol; and the mobile phase B is a phosphate buffer solution. According to the method disclosed by the invention, the 3-amino rifamycin S, the 3-bromo rifamycin S, the rifamycin S, the impurity A, the impurity B, the impurity C and the impurity D can be simultaneously separated and determined; and the quality of the 3-amino-4-imino rifamycin S bulk drug can be accurately controlled, and the safety and effectiveness of the product are finally ensured.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Solid dispersoid of rifamycin-quinazone coupling molecule and application thereof
The invention provides a solid dispersoid of a rifamycin-quinazone coupling molecule. Components of the solid dispersoid comprise the rifamycin-quinazone coupling molecule in structure as shown in a formula I, a high-molecular carrier, a functional auxiliary material and a solvent. The high-molecular carrier comprises one or combination of povidone K30, povidone VA64, hydroxy propyl cellulose L, apolyvinyl caprolactam-polyvinyl acetate-polyethylene glycol grafted copolymer and polymethacrylate. The functional auxiliary material comprises one or combination of vitamin E polyethylene glycol succinate, lauryl sodium sulfate, meglumine and Tween 80. The formula is as shown in the description. The solid dispersoid of the rifamycin-quinazone coupling molecule can be used as a preparation of a drug for treating bacterial infection.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
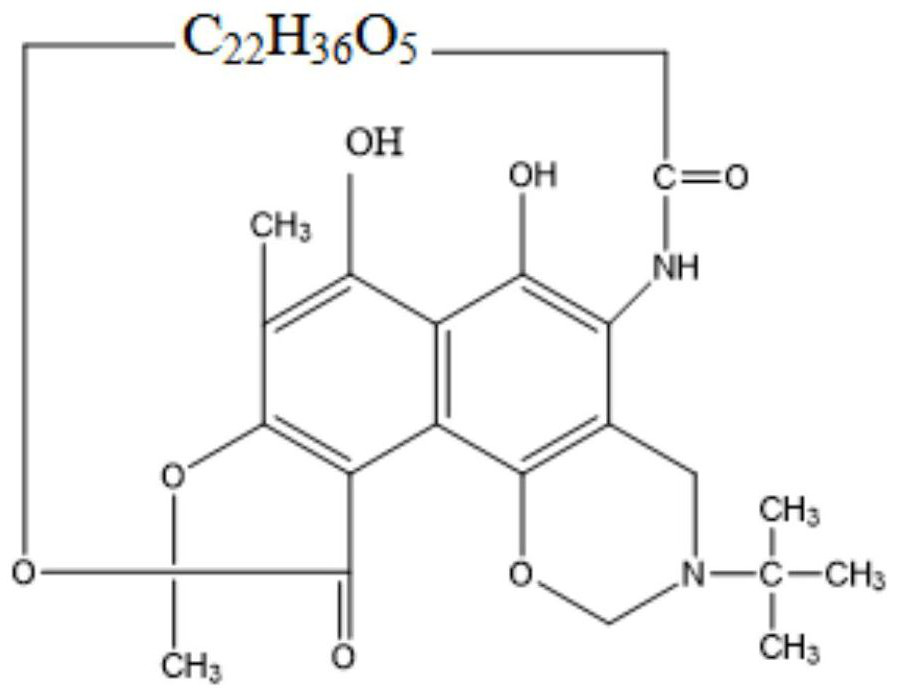
Method for continuously preparing benzoxazine rifamycin
InactiveCN110684037AShort reaction timeImprove efficiencyOrganic chemistryMicroreactorTert-Butylamine
The invention discloses a method for continuously preparing benzoxazine rifamycin. The method comprises the following steps: 1) dissolving rifamycin S into an organic solvent, performing filtering soas to obtain a homogeneous solution of rifamycin S; 2) dissolving dihydroxymethyl tert-butylamine into an organic solvent, and performing sufficient mixing so as to obtain a homogeneous solution of dihydroxymethyl tert-butylamine; 3) continuously pumping the two homogeneous solutions obtained in steps 1) and 2) into a micro-reactor for mixing, enabling the mixed materials to react in a second reactor of the micro-reactor so as to obtain a product benzoxazine rifamycin; and 4) performing aftertreatment on samples, and performing external standard quantitative analysis on the samples by using ahigh performance liquid chromatography method, so as to obtain the content of benzoxazine rifamycin in the samples. By adopting the method disclosed by the invention, the characteristics of efficientmass transfer and heat transfer of the micro-reactor are utilized, and with optimized reaction parameters, the reaction time can be shortened to 30 minutes, and the product yield is 95% or greater.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Treatment for leukemia and idiopathic aplastic anemia
A process for treating a patient with leukemia or an aplastic anemia having cells with inclusions that stain with anti-E. canis antibodies or antibodies to other Ehrlichia or Anaplasma is disclosed. That process comprises administering to the patient (i) an antibacterial amount of a rifamycin, (ii) an antibacterial amount of a quinolone, or a mixture of (i) and (ii).
Owner:SPHINGOMONAS RES PARTNERS
Process method for synthesizing rifampicin
The invention relates to a process method for synthesizing rifampicin, wherein the process method mainly comprises the steps: by using rifamycin S as a raw material, carrying out cyclization reactionto obtain a rifamycin oxazine-containing solution, and carrying out solventing-out crystallization on the rifamycin oxazine-containing solution to obtain a rifamycin oxazine crude product; carrying out condensation reaction on the crude rifamycin oxazine product and 1-amino-4-methylpiperazine to obtain a reaction solution containing rifampicin; and cooling and crystallizing the reaction solution containing rifampicin to obtain a rifampicin product. Compared with the prior art, the method has the advantages that the reaction steps are few, the product yield is high, the reaction raw material cost is low, meanwhile, the production is more environmentally friendly, the consumption of raw materials is reduced by 30% (the consumption of dimethylol tert-butylamine required by every 1 kg of rifamycin S is reduced by about 28%, and the consumption of 1-amino-4-methylpiperazine is reduced by about 30%). The method overcomes the defect that a large amount of sewage is generated in production byadopting an elution method, and reduces the influence of impure reaction products on the yield due to the fact that rifamycin S-Na is used as a raw material.
Owner:EAST CHINA UNIV OF SCI & TECH
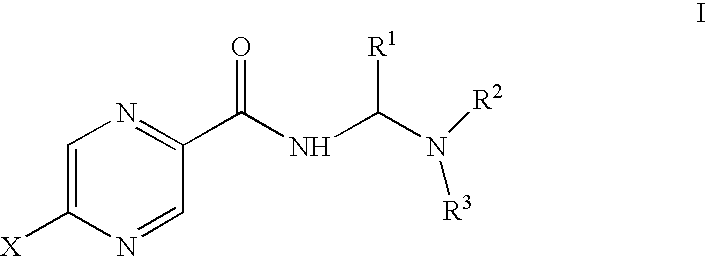
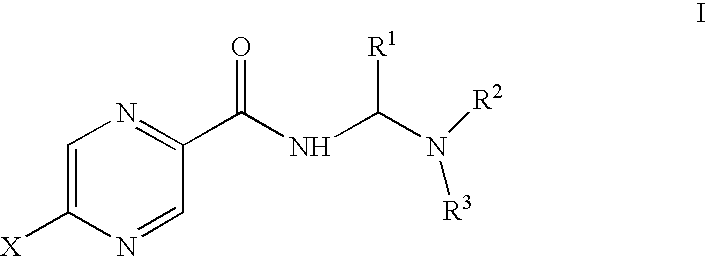
C-25 carbamate rifamycin derivatives with activity against drug-resistant microbes
Compounds of the current invention relate to rifamycin derivatives having antimicrobial activities, including activities against drug-resistant microorganisms. More specifically, compounds of the current invention relate to C-25 carbamate derivatives of rifamycin having another functional group or pharmacophore covalently attached to this position through a carbamate linkage. The resulting compounds exert their antimicrobial activity through a dual-function mechanism and therefore exhibit reduced frequency of resistance.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
Synthesis process of rifaximin-D6
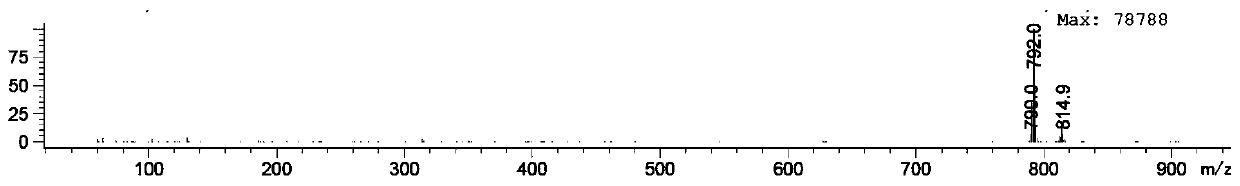
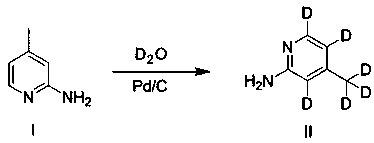
InactiveCN111423456AShort stepsEasy to operateIsotope introduction to heterocyclic compoundsPalladium on carbonMethyl palmoxirate
The invention provides a synthesis process of rifaximine-D6, which comprises the following steps: 1) by using compounds I2-amino-4-methylpyridine and D2O as raw materials, reacting in the presence of5wt% palladium on carbon and under the protection of nitrogen to obtain a deuterated intermediate, i.e., a compound II 4-methyl-2-aminopyridine-D6; step 2), dissolving rifamycin S and the deuterated intermediate compound II 4-methyl-2-aminopyridine-D6 prepared in the step 1) into dichloromethane; under the protection of nitrogen, stirring at room temperature, dropwise adding a dichloromethane solution dissolved with elemental iodine, reacting at room temperature overnight for 18 hours, dropwise adding an L-ascorbic acid aqueous solution, stirring, and after the reaction is completed, washing and purifying to obtain the product rifaximin-D6. The synthesis method has the following technical effects that the synthesis method of the intermediate II of rifaximin-D6 is simple and convenient, andraw materials are easy to obtain; the steps for synthesizing rifaximin-D6 are simple and short, and the operation is easy.
Owner:南京昊绿生物科技有限公司
A kind of purposes of rifamycin-nitroimidazole coupling molecule
ActiveCN104971061BHigh antibacterial activityImprove antibacterial propertiesAntibacterial agentsOrganic active ingredientsNitroimidazoleDisease
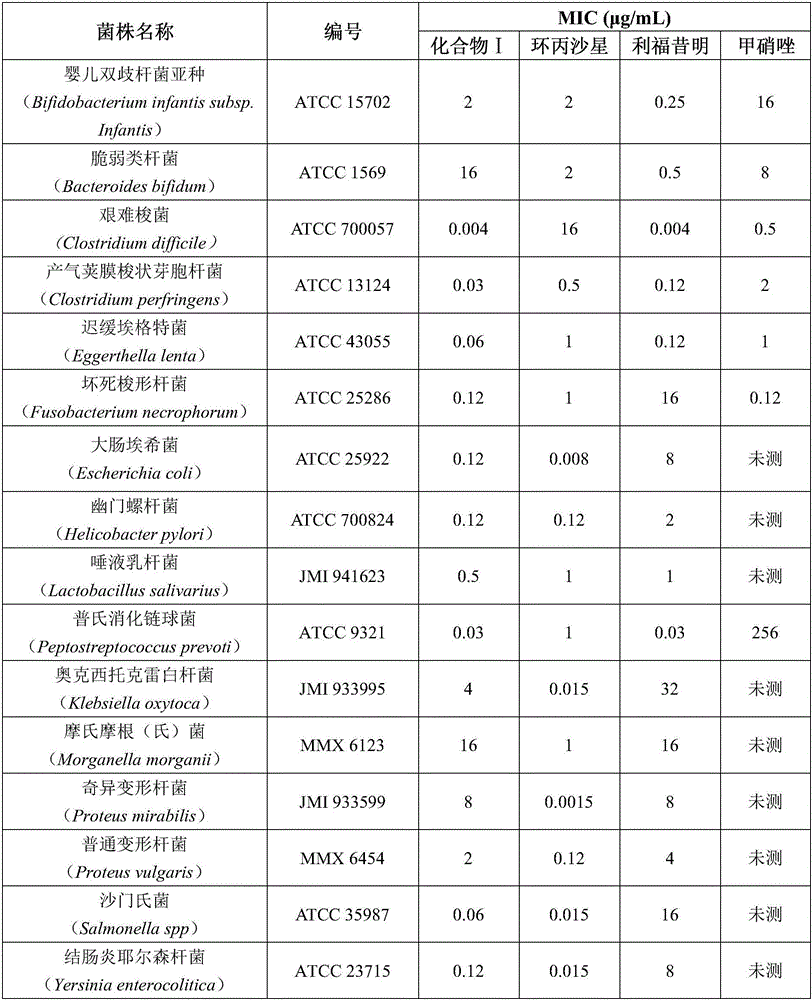
The invention discloses a new application of a rifamycin-nitroimidazole coupling molecule, which belongs to the field of medicinal chemistry. The rifamycin-nitroimidazole conjugate molecule of the present invention exhibits high activity to drug-resistant strains in the treatment of infection-related diseases such as Clostridium difficile and Helicobacter pylori, except for single-drug resistance to rifamycin Both bacteria and metronidazole mono-resistant bacteria have antibacterial activity, and have antibacterial effect on rifamycin and metronidazole double-resistant bacteria, and its activity is better than the 1:1 molar ratio combination of rifampicin and metronidazole.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
A kind of method utilizing microchannel reaction device to prepare rifampicin
The invention discloses a method for preparing rifampicin by using a microchannel reaction device. Through a microchannel reactor, rifamycin S is used as a raw material to undergo cyclization, hydrolysis, condensation and crystallization processes without post-treatment process, and directly obtains Rifampicin crude. The invention realizes complete continuous production of rifampicin, has high yield, low cost, high profit, environmental protection, energy saving and high efficiency, and is suitable for industrial application.
Owner:NANJING TECH UNIV
Selective enrichment media and uses thereof
ActiveUS10619130B1Preventing undesirable false positive responseBacteriaMicrobiological testing/measurementBiotechnologyBenzoic acid
Selective enrichment media and methods for selectively growing and detecting Salmonella spp. and / or Shiga toxin-producing E. coli. The media may comprise a carbon and nitrogen source, an inorganic salt, a fermentable sugar, one or more selective agents, and an efflux pump inhibitor. Various selective agents include sulfa drugs, surfactants, aminocoumarins, cycloheximide, supravital stains, ascorbic acid, bromobenzoic acid, myricetin, nitrofurantoin, rifamycins, polyketides, and oxazolidinones. Various efflux pump inhibitors include arylpiperazines, such as 1-(1-naphthylmethyl)piperazine, and quinoline derivatives, such as 4-chloroquinoline. Methods of selectively growing and detecting Salmonella and / or Shiga toxin-producing E. coli are provided.
Owner:PARADIGM DIAGNOSTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com