Patents
Literature
66 results about "4-Piperidinone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
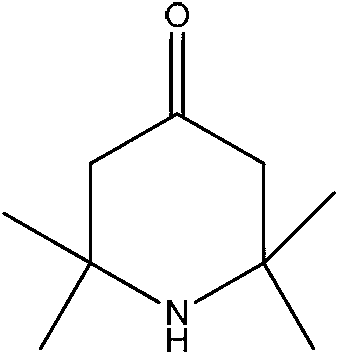
4-Piperidinone is a derivative of piperidine with the molecular formula C₅H₉NO. 4-Piperidone is used as an intermediate in the manufacture of chemicals and pharmaceutical drugs.
Semi-synthetic metal cutting fluid
InactiveCN104830513AImprove cooling effectGood anti-corrosion and anti-rustAdditivesCarboxylic saltEngineering
The invention relates to cutting fluid, in particular to semi-synthetic metal cutting fluid. The semi-synthetic metal cutting fluid is prepared by methyl levulinate, (R)-(+)-2,4-dyhydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutyramide, 2-oxo-1,5-disoidum glutarate dihydrate, 4-piperidone-3-carboxylate hydrochloride and trimethylsilylketene. The semi-synthetic metal cutting fluid has good cooling, anticorrosion, antirust and lubricating performance, service life of a knife can be prolonged effectively, and excellent machining accuracy can be realized; the semi-synthetic metal cutting fluid can play a role in safely and reliably protecting and inhibiting corrosion of nonferrous metal like aluminum and steel and ferrous metal, so that time and cost for subsequent treatment can be omitted; the semi-synthetic metal cutting fluid is suitable for being widely popularized and applied in the field of the cutting fluid.
Owner:烟台顺隆化工科技有限公司
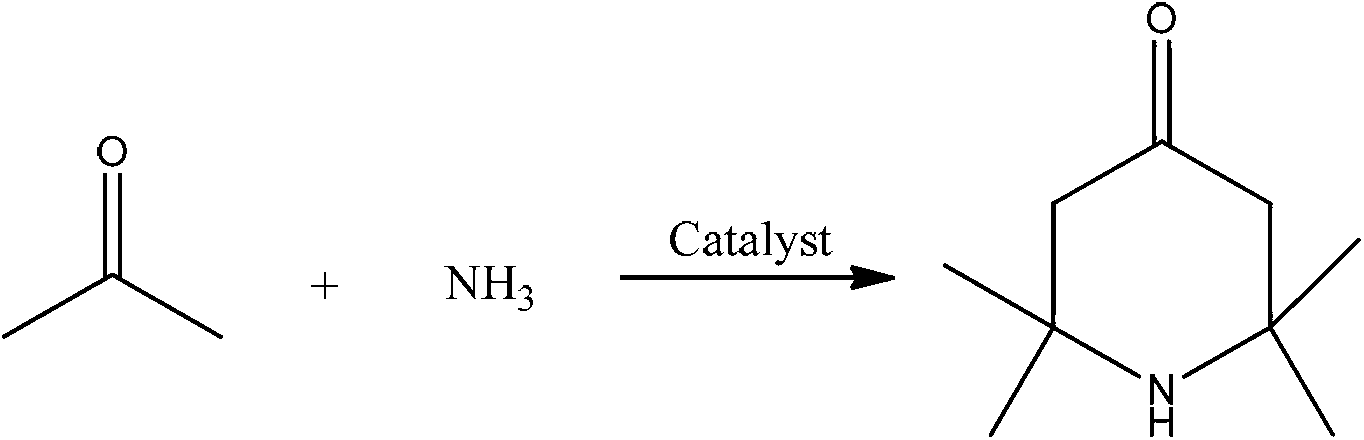
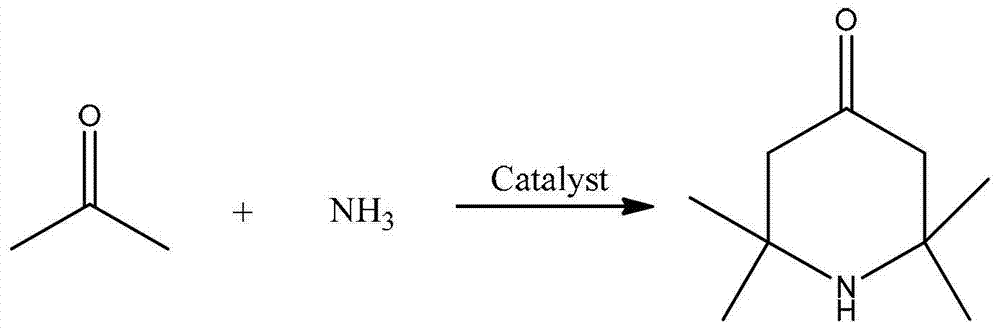
2,2,6,6,-tetramethyl-4-piperidone continuous synthesis method
ActiveCN103224465ASubsequent separation is simpleAvoid pollutionOrganic chemistrySynthesis methodsFixed bed
The invention discloses a 2,2,6,6,-tetramethyl-4-piperidone continuous synthesis method. The 2,2,6,6,-tetramethyl-4-piperidone continuous synthesis method comprises the following steps of 1, filling an acidic resin into a fixed bed reactor, and carrying out heating so that a temperature of the fixed bed reactor is in a range of 40 to 70 DEG C, and 2, feeding acetone and ammonia gas into the fixed bed reactor according to a mole ratio of 3-9: 1 under the conditions of acetone hourly space velocity of 0.15 to 1.17h<-1> and ammonia gas hourly space velocity of 5.25 to 124.20h<-1>, and cooling the product to obtain a 2,2,6,6,-tetramethyl-4-piperidone crude product. The 2,2,6,6,-tetramethyl-4-piperidone continuous synthesis method has simple processes and a low cost, is suitable for industrial continuous production, does not adopt an organic solvent or water as a solvent, avoids the pollution produced by the organic solvent on the environment, has mild reaction conditions, can be carried out at a temperature of 40 to 70 DEG C, has low energy consumption, and avoids the influence caused by impurities produced at a high temperature on the product.
Owner:安徽兴欣新材料有限公司
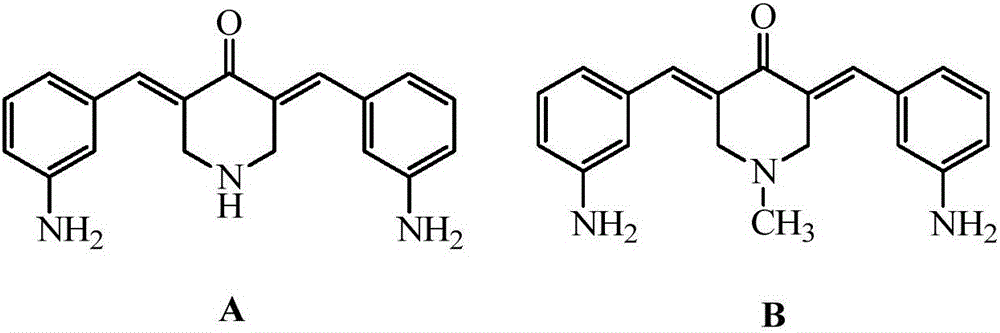
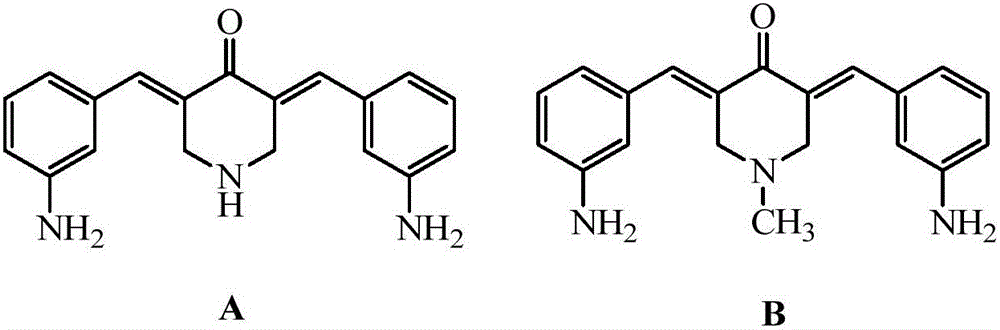
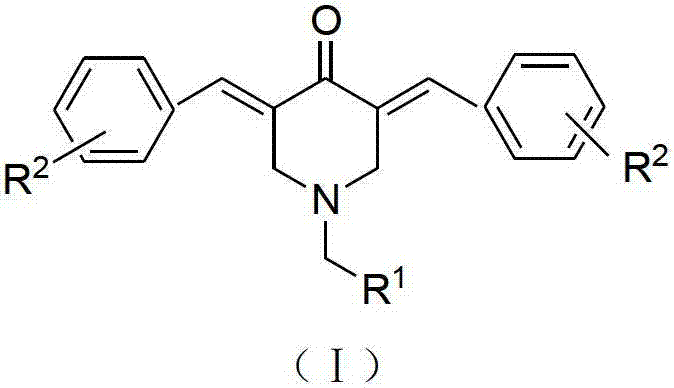
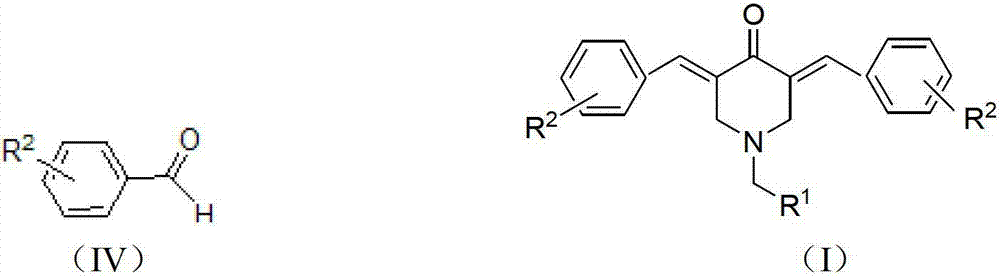
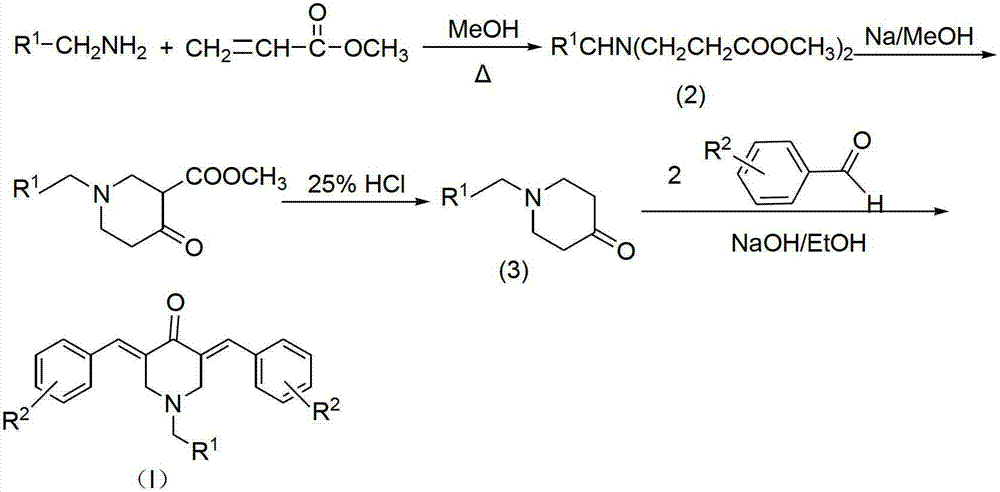
N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone and preparation method and application thereof
InactiveCN102863376AInhibit biological activitySmall side effectsOrganic chemistryAntineoplastic agentsCarcinoma cell lineCancer cell
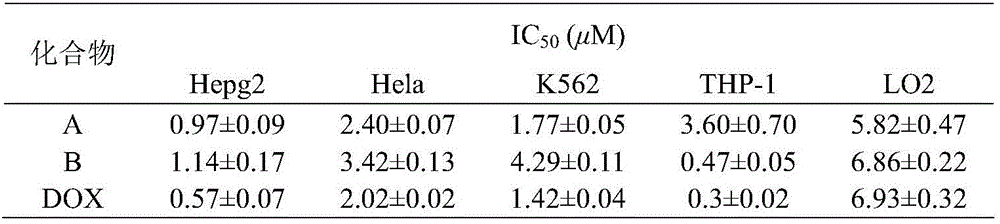
The invention relates to the field of organic synthesis and medicine, and discloses a preparation method for N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone and biological activity for efficiently inhibiting cell line proliferation such as leukemia, ovarian cancer, breast cancer, liver cancer and esophagus cancer. The method includes: starting from various substituted methylamine and methyl acrylate, sequentially going through Michael addition, Dieckmann condensation, acidolysis and decarboxylation to obtain N-substituted methyl-4-piperidone, and subjecting the N-substituted methyl-4-piperidone to aldol reaction with substituted benzaldehyde to obtain a target compound N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone. The target compound can selectively and efficiently inhibit cell line proliferation such as leukemia, ovarian cancer, breast cancer, liver cancer and esophagus cancer, and activity of inhibiting carcinoma cell line proliferation is obviously higher than conventional chemotherapeutic 5-fluorouracil.
Owner:SHANGHAI NORMAL UNIVERSITY
Novel 3, 5-bis(aryl-methyne)-1-methylpiperidine-4-ketone compound as well as synthetic method and application thereof in preparation of anti-cancer medicines
ActiveCN103936667AHigh anticancer activitySignificant inhibitory effectOrganic chemistryAntineoplastic agentsSide effectKetone
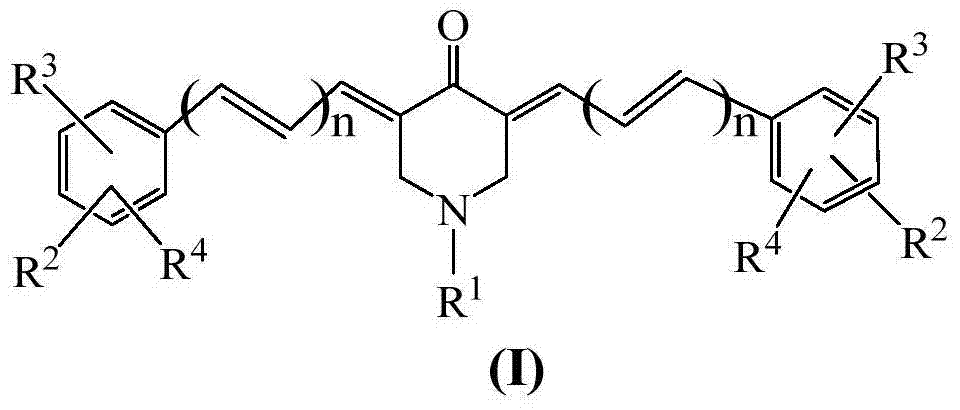
The invention provides a novel 3,5-bis(aryl-methyne)-1-substituted-4-piperidone compound as well as a synthetic method and application thereof in the preparation of anti-cancer medicines. The structural formula of the novel 3,5-bis(aryl-methyne)-1-substituted-4-piperidone compound is as shown in a formula (I), wherein R1 is selected from hydrogen, propyl, benzyl, 2-phenethyl, acetyl and ethyoxyl carbonyl, R2, R3 and R4 are respectively and independently selected from fluorine, chlorine, bromine, C1-C12 alkenyl, C1-C12 alkyl, C1-C12 alkoxy, C1-C12 carboxylic acids, C1-C12 carboxylic esters and amide to which the C1-C12 carboxylic acids are corresponding, and n is 0 or 1. The compound has good anticancer activity and no toxic or side effects, is simple in synthetic method and suitable for preparation of the anti-cancer medicines.
Owner:SUN YAT SEN UNIV
Method for non-membrane electrochemical synthesis of 2,2,6,6-tetramethyl-piperidinol
InactiveCN101886269ASolve the problem of longevitySolve the costElectrolysis componentsElectrolytic organic productionSupporting electrolyteSolvent
The invention discloses a method for non-membrane electrochemical synthesis of 2,2,6,6-tetramethyl-piperidinol. A non-membrane electrolytic cell is adopted, a Pb plate is taken as an anode, a Zn plate is taken as a cathode, sodium sulfate is taken as supporting electrolyte, a certain amount of sodium hydroxide and raw material of piperidone are added, two different organic latent solvents of methanol and ethanol are respectively added, and distilled water is added for preparing electrolyte. The electrochemical synthesis of piperidinol is carried out under a certain current density and electric quantity. The results show that the 2,2,6,6-tetramethyl-piperidinol can be directly obtained by taking 2,2,6,6-tetramethyl-4-piperidone as the raw material and carrying out the electrochemical synthesis under the condition of not using a membrane. The method can solve the technical defects of short service life of the membrane, high cost, difficult industrialization and the like caused by using the membrane. On the whole, the method is characterized by simple operation, easy obtainment of equipment, simple process flow, low investment cost and the like, and can carry out industrial production.
Owner:HEBEI NORMAL UNIV
Preparation method of homopiperazine and derivative thereof
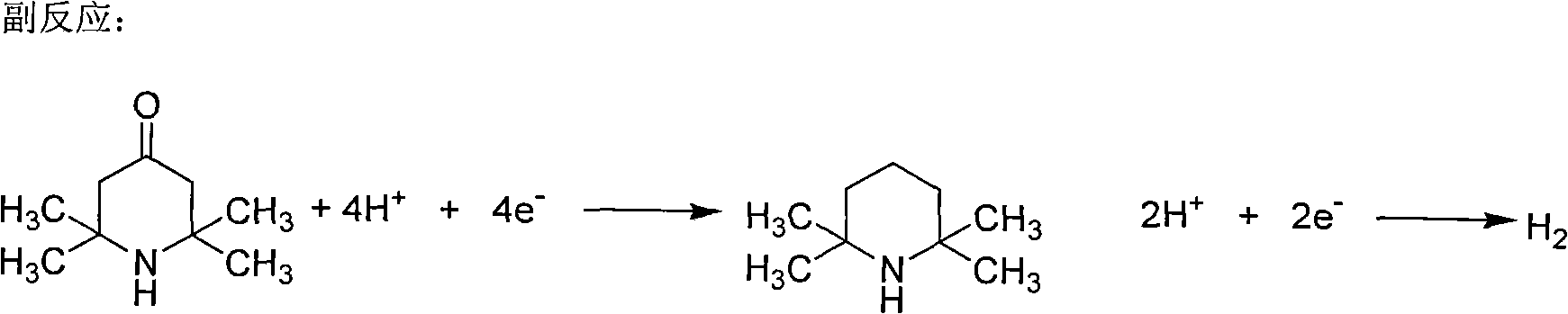
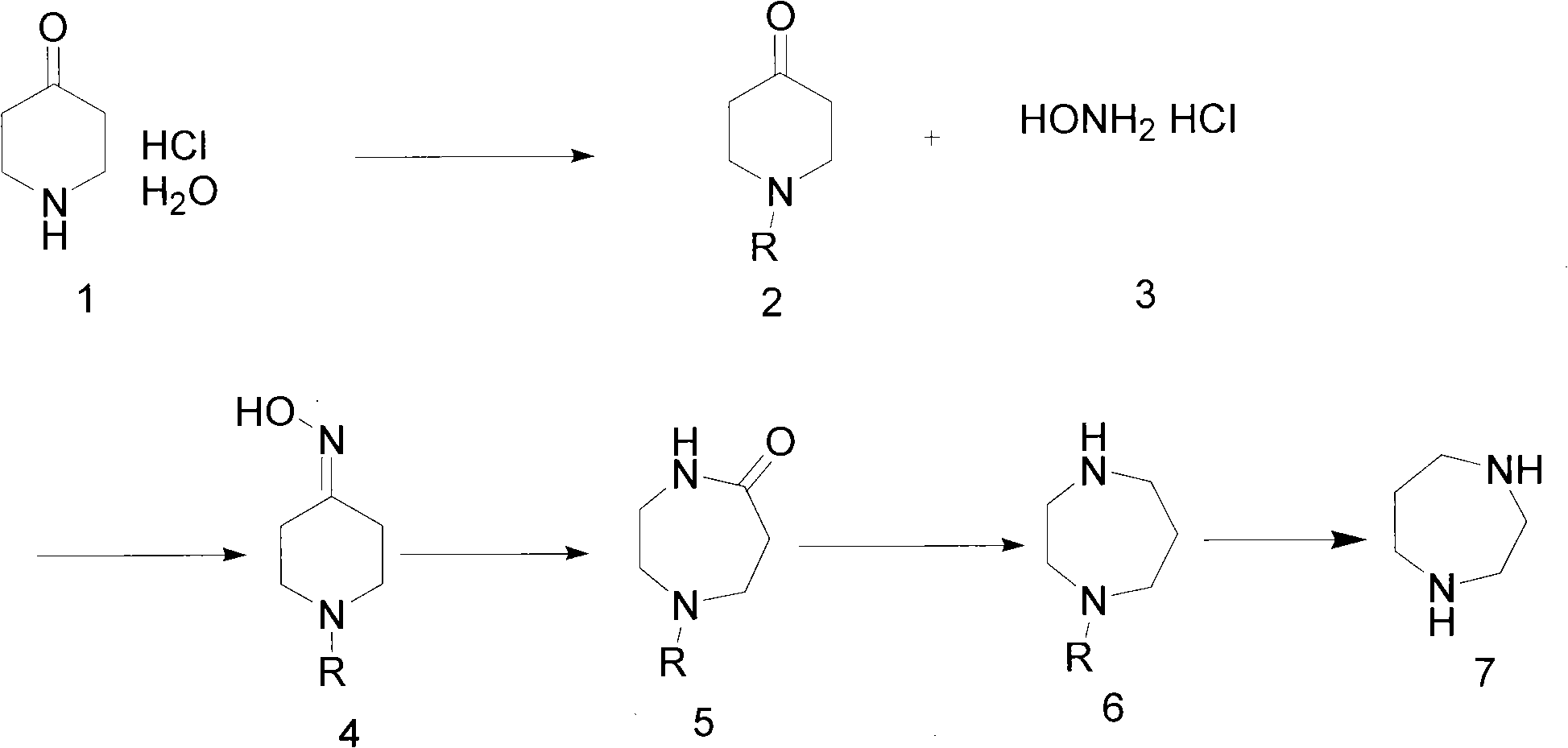
The invention discloses a preparation method of a homopiperazine derivative. The preparation method comprises the following steps of: (1) reacting 4-piperidone hydrochloride hydrate with an amino protective agent to prepare amino-protected 4-piperidone; (2) performing oximation reaction on the amino-protected 4-piperidone to prepare amino-protected 4-oxime piperidone; (3) performing molecular rearrangement on the amino-protected 4-oxime piperidone to prepare amino-protected 5-carbonyl homopiperazine; and (4) performing reduction reaction on the amino-protected 5-carbonyl homopiperazine to prepare amino-protected homopiperazine. The 4-piperidone hydrochloride hydrate is taken as a raw material, and is subjected to amino protection, oximation, rearrangement, reduction and deprotection to prepare the homopiperazine, and the amino-protected homopiperazine is reduced by sodium borohydride in the presence of an adjuvant to prepare the homopiperazine, so that lithium aluminum hydride which has high safety risk and cost in a preparation process of the conventional method is avoided. The whole process is easy to control, reaction conditions of each step are mild, the method is suitable forindustrial production, the yield is 75 percent, the raw material with low cost is used, and production cost is effectively reduced.
Owner:CHENGDU XINJIE HIGH TECH DEV CO LTD
Method for preparing 4-(N-phenylpropionamide)-4-methoxymethyl-piperidine hydrochloride
InactiveCN102127007AShort production processImprove securityOrganic chemistrySilanesPotassium cyanide
The invention discloses a method for preparing 4-(N-phenylpropionamide)-4-methoxymethyl-piperidine hydrochloride, which belongs to the technical field of preparation of medicinal intermediates. The 4-(N-phenylpropionamide)-4-methoxymethyl-piperidine hydrochloride is synthesized by using 1-phenyl-4-piperidone and aniline as initiative raw materials and by seven reactions. In raw materials used in the invention, trimethyl cyanato silane is used in place of potassium cyanide or sodium cyanide which is a highly toxic raw material to make the operation and management more convenient; potassium tert-butoxide and other alkalis are used in place of sodium hydride which is a dangerous and flammable reagent to improve the safety of scale-up production; propionic anhydride is used in place of highly irritant propionyl chloride to improve the safety, purity and yield of experiments; dimethyl sulfate is used in place of expensive methyl iodide to further control the production cost; and the high-pressure liquid chromatography (HPLC) purity of the obtained 4-(N-phenylpropionamide)-4-methoxymethyl-piperidine hydrochloride product can reach 99.7 percent, the single impurity content is less than 0.1 percent, the total yield can reach 23 percent, and the product is a hydrochloride which is favorable for storage and transport.
Owner:ZHEJIANG LANGHUA PHARMA
Tetrahydropyridopyridone derivative as well as preparation method and application thereof
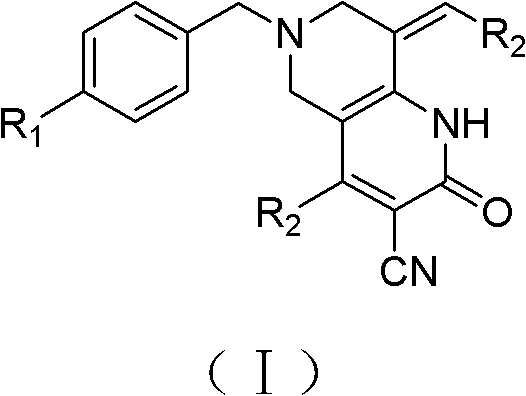
ActiveCN103288824APrevent proliferationHigh activityOrganic active ingredientsOrganic chemistryKetoneHydrolysis
The invention provides a tetrahydropyridopyridone derivative as shown in a general formula (I), and a preparation method thereof. The method comprises the following steps of: preparing N,N-bi(methoxycarbonyl group) substituted benzylamine (b) by carrying out Michael addition on substituted benzylamine (a) and methyl acrylate, carrying out Dieckmann condensation on (b) under the action of sodium alcoholate, subsequently carrying out hydrolysis and decarboxylation under the action of acid so as to obtain N-substituted benzyl-4-piperidone (d), carrying out an aldol condensation reaction on (d) and bimolecular aromatic aldehyde so as to obtain N-substituted-3,5-bi(substituted benzylidene piperidine-4-ketone (e), and refluxing and heating (e) malononitrile and ammonium acetate in ethanol so as to obtain a final product as shown in the general formula (I). The tetrahydropyridopyridone derivative is simple in process and convenient to produce in scale, and the compound (I) has a good inhibition effect on multiplication of leukemia K562 cells, ovarian cancer HO-8910 cells and liver cancer SMMC-7721 cells. Therefore, the invention further provides an application of the compound as shown in the general formula (I) in preparing medicaments for preventing multiplication of the leukemia K562 cells, the ovarian cancer HO-8910 cells and the liver cancer SMMC-7721 cells.
Owner:SHANGHAI NORMAL UNIVERSITY +1
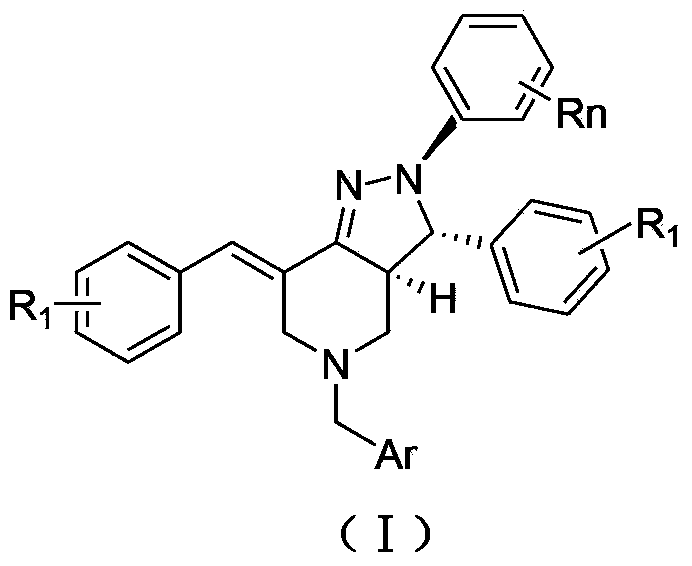
2, 3, 5, 7-tetrasubstituted dihydro-pyrazolo piperidine derivative and preparation method and application thereof
The invention provides 2, 3, 5, 7-tetrasubstituted dihydro-pyrazolo piperidine derivative and a preparation method and application thereof. The derivative is 2, 3-bis(substituted phenyl)-5-subsituted arylmethyl-7-substituted benzylidene dihydro-pyrazolo piperidine derivative, having the following formula (I). The preparation method includes using substituted arylmethyl amine and methyl acrylate as raw materials; subjecting the materials to Michael addition, Dieckmann condensation and hydrolysis-decarboxylation sequentially; allowing for Aldol reaction with substituted benzaldehyde to obtain intermediate N-substituted arylmethyl-3, 5-bis(substituted benzylidene)-4-piperidone; allowing for condensation with substituted phenylhydrazine to obtain a compound according to the formula (I). The derivative is efficient in inhibiting multiplication of various carcinoma cell lines such as leukemia, esophagus cancer, ovarian cancer and breast cancer in human, is well stably metabolic in liver microsomes of human and rat, is free of direct and competitive inhibition on five enzymes of liver microsomes, such as CYP3A4, CYP2D6, CYP2C9, CYP1A2 and CYP2C19, is highly bioavailable, is low in toxicity to normal cells, and is available for the preparation of drugs for the cancers.
Owner:SHANGHAI NORMAL UNIVERSITY
Antitumor N-methyl-3,5-diarylmethylene-4-piperidone and quaternary ammonium derivatives thereof
ActiveCN104592098AAvoid genotoxicityToxicOrganic chemistryAntineoplastic agentsQuaternary ammonium cationMethyl group
The invention relates to antitumor drugs and particularly relates to an anti-tumor N-methyl-3,5-diarylmethylene-4-piperidone and quaternary ammonium derivatives thereof. The preparation method comprises the following steps of carrying out Claisen-Schmidt condensation reaction on N-methyl-4-piperidinone and an aryl formaldehyde derivative to obtain an intermediate and carrying out quaternization reaction on the intermediate and benzyl halide to obtain the N-methyl-3,5-diarylmethylene-4-piperidone quaternary ammonium derivatives. N-methyl-3,5-diarylmethylene-4-piperidone and the quaternary ammonium derivatives thereof are novel in structures and have the advantages of both multi-molecular target binding and multi-drug resistance reversal activity and the genotoxicity of the currently used anticancer drugs can be avoided. N-methyl-3,5-diarylmethylene-4-piperidone and quaternary ammonium derivatives thereof have greater toxicity to tumor cells compared to normal cells in anti-tumor activity and have very great application prospects in the field of anticancer drugs.
Owner:BINZHOU MEDICAL COLLEGE
A preparing method of 2,2,6,6-tetramethyl-4-piperidylamine by a catalytic amination method
A preparing method of 2,2,6,6-tetramethyl-4-piperidylamine by a catalytic amination method is disclosed. The preparing method includes following steps of: (1) adding 2,2,6,6-tetramethyl-4-piperidone, sodium hydroxide, ammonia water and a skeleton nickel catalyst into a high-pressure kettle having a condenser, a pressure gage, a stirrer and a thermocouple, feeding nitrogen to replace air in the kettle, feeding hydrogen until the pressure is 2.5 MPa, stirring, heating to 110 DEG C, and reacting for 30 min; and (2) after the reaction is completed, cooling to room temperature, stopping stirring, venting, taking reaction materials out, filtering, subjecting filtrate to distillation under atmospheric pressure to distill ammonia gas and water, subjecting the residue in the kettle to vacuum distillation, and collecting a fraction that is the 2,2,6,6-tetramethyl-4-piperidylamine. The preparing method adopts the skeleton nickel as the catalyst, synthesizes the 2,2,6,6-tetramethyl-4-piperidylamine by hydrogenation and amination, and is high in yield and purity of products.
Owner:青岛欧美亚橡胶工业有限公司
Synthesis method and application of AEN-structured Si-P-Al molecular sieve
ActiveCN107915233ADispersed particle separationMolecular-sieve and base-exchange phosphatesSynthesis methods4-Piperidinone
The invention relates to the field of synthesis of catalytic materials and discloses a synthesis method and an application of an AEN-structured Si-P-Al molecular sieve. The synthesis method comprisesa mode one: a mixed solution containing a P source, an Al source, a Si source, a structure-directing agent and water is subjected to hydrothermal crystallization with a hydrothermal method and then issubjected to solid-liquid separation and dried; a mode two: a mixed solution A containing the P source, the Al source and water is aged and dried with a P-Al dry glue liquid phase conversion method,and dry P-Al glue is prepared; a raw material mixture B containing the dry P-Al glue, the Si source, the structure-directing agent and water is subjected to hydrothermal crystallization, solid-liquidseparation and drying, wherein the structure-directing agent is 1-methyl-4-piperidinone and / or 1,3-dimethyl-4-piperidone. According to the synthesis system, the AEN-structured Si-P-Al molecular sieveis easy to synthesize. The AEN-structured Si-P-Al molecular sieve synthesized with the method can be applied to storage of small molecule gases such as hydrogen, methane and the like, can also be usedfor gas absorption separation and has good application prospect.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of hexamethylenediamine piperidine
InactiveCN104592097ALow reaction pressureHigh purityOrganic chemistrySide effectHexamethylenediamine
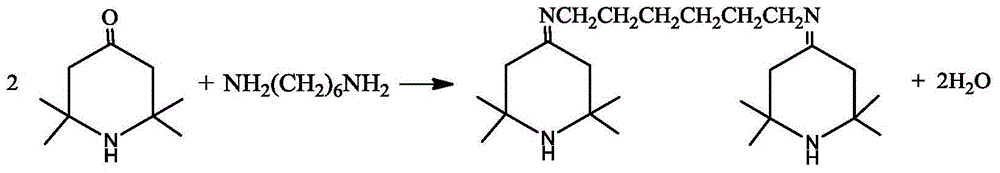
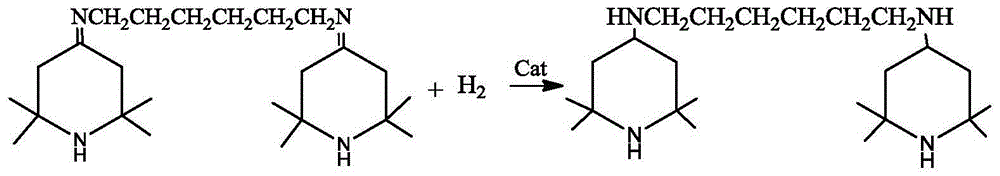
The invention discloses a preparation method of N,N-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6-hexamethylenediamine (called hexamethylenediamine piperidine for short). The preparation method comprises the following steps: 1) carrying out a dehydration reaction on 2,2,6,6-tetramethyl-4-piperidone (called tetramethyl piperidone for short) and 1,6-hexanediamine to prepare a Schiff base intermediate; 2) carrying out a hydrogenation reaction on the Schiff base intermediate in the presence of a catalyst to obtain hexamethylenediamine piperidine. The preparation method disclosed by the application is low in reaction pressure, and the requirements of the bearing pressure of equipment are reduced, so that the production safety is greatly improved and the cost is reduced; a non-polar solvent is used, so that side effects are reduced, and a post-treatment method is simple and convenient, so that the production efficiency is increased, industrialized mass production can be adapted better, and the obtained target compound is high in purity and slight in colour.
Owner:JIANGSU FEIXIANG CHEM
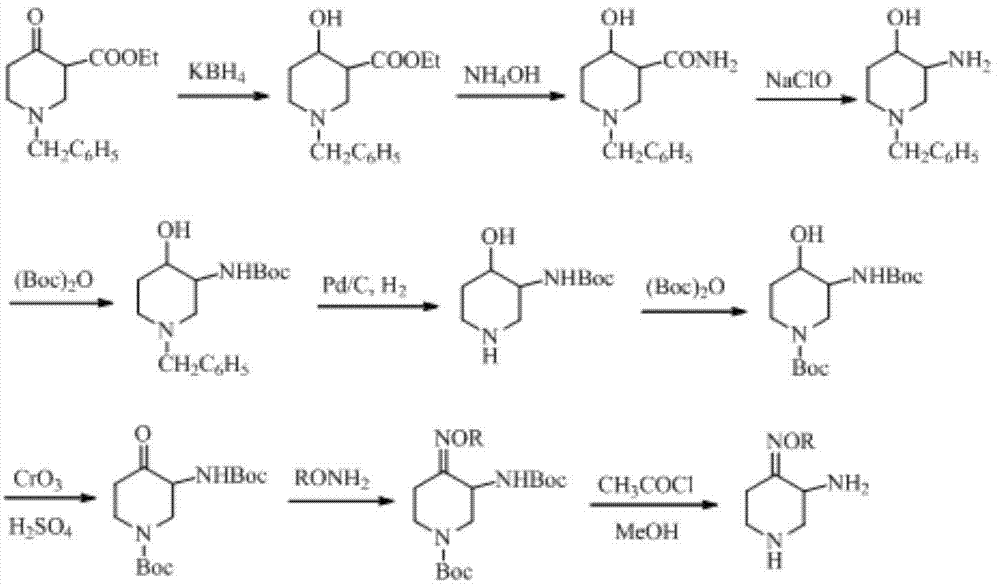
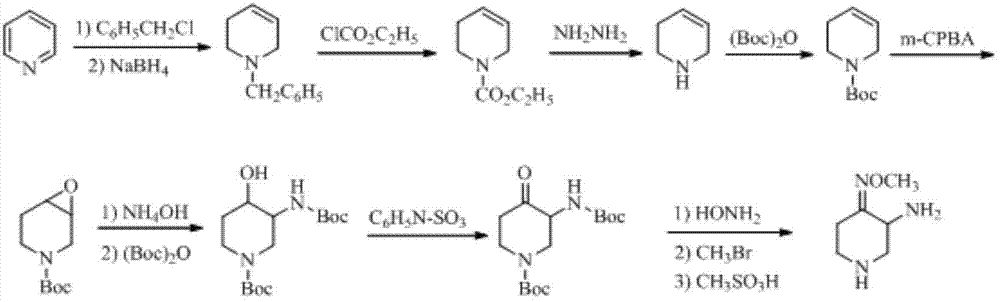
Preparation method of 3-amino-4-alkoxyimino piperidine
InactiveCN103086955AAvoid detachmentSolve pollutionOrganic chemistryTert-Butyloxycarbonyl protecting groupAcrylonitrile
The invention relates to a preparation method of 3-amino-4-alkoxyimino piperidine. More particularly, the preparation method provided by the invention comprises the following steps: performing nucleophilic addition reaction on ethyl 3-aminopropionate hydrochloride and acrylonitrile in the presence of alkali; treating with di-tert-butyl dicarbonate to obtain ethyl N-tert-butoxycarbonyl-3-(2-cyanoethyl)aminopropionate; performing cyclization in the presence of strong alkali to obtain 1-N-tert-butoxycarbonyl-3-cyano-4-piperidone, and performing oximation to obtain 1-N-tert-butoxycarbonyl-3-cyano-4-alkoxyimino piperidine; sequentially performing cyano hydrolysis and Hofmann degradation reaction on the latter to obtain 1-N-tert-butoxycarbonyl-3-amino-4-alkoxyimino piperidine; and finally, performing deprotection to obtain the 3-amino-4-alkoxyimino piperidine and salt thereof.
Owner:浙江宏康医药化工股份有限公司
4-acetylamino benzene sulfonyl substituted 3,5-bis(arylidene)-4-piperidinone compounds and preparation method thereof
ActiveCN108191742ASimple and fast operationMild reaction conditionsOrganic chemistryAntipyretic4-PiperidinoneAnti-inflammatory analgesics
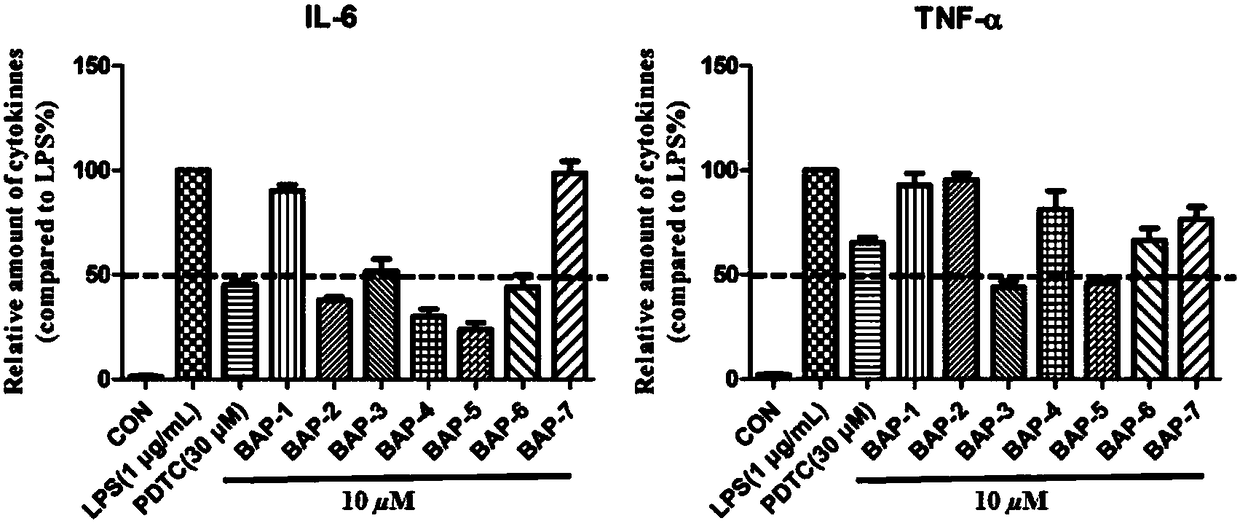
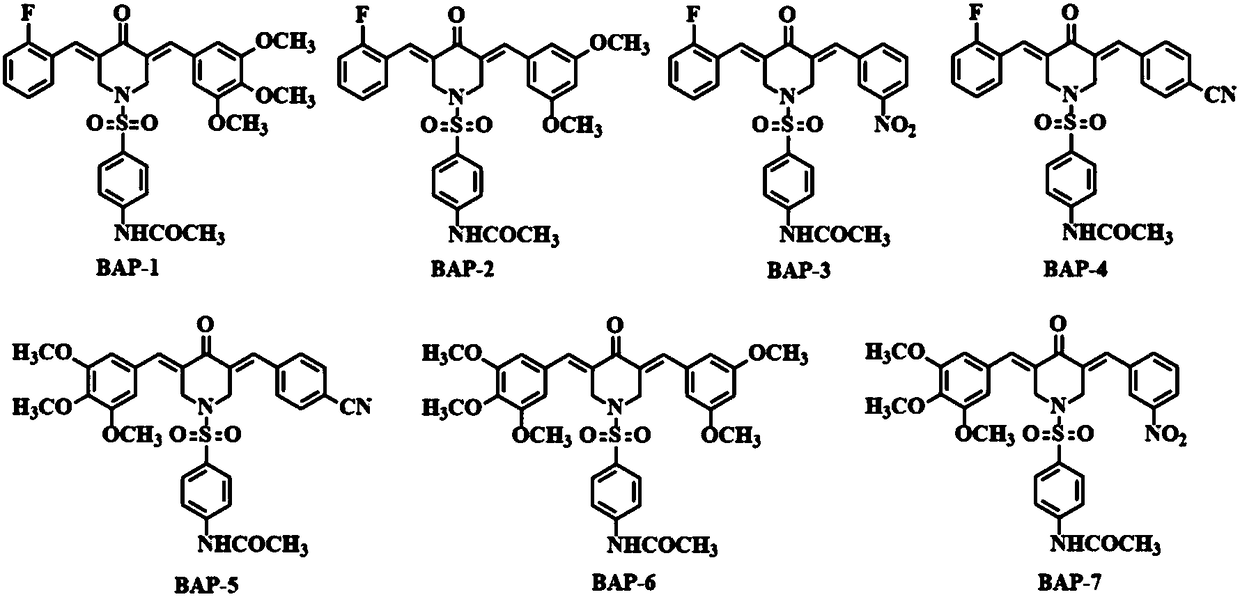
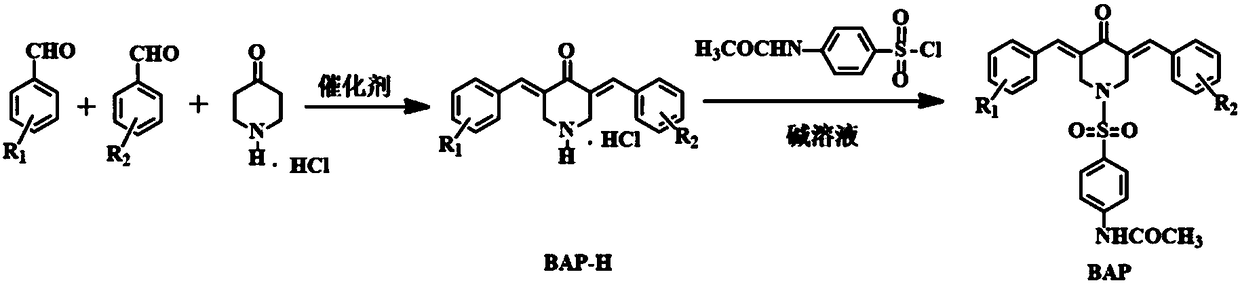
The invention relates to seven 4-acetylamino benzene sulfonyl substituted 3,5-bis(arylidene)-4-piperidinone compounds having anti-tumor and anti-inflammatory activities, and belongs to the technical field of anti-tumor and anti-inflammatory medicines. A preparation method of the 4-acetylamino benzene sulfonyl substituted 3,5-bis(arylidene)-4-piperidinone compounds comprises the following steps: firstly, respectively performing claisen-schmidt condensation reaction on 4-piperidinone hydrochloride and two aromatic aldehyde and performing column chromatography to obtain a 3,5-bis(arylidene)-N-H-4-piperidinone hydrochloride intermediate product (BAP-H) having different substituents; then performing benzene sulfonylation on the 3,5-bis(arylidene)-N-H-4-piperidinone hydrochloride intermediate product and 4-acetylsulphanilyl chloride to obtain the 4-acetylamino benzene sulfonyl substituted 3,5-bis(arylidene)-4-piperidinone compounds (BAP). The compounds have good anti-tumor and anti-inflammatory activities, can avoid the genetic toxicity of currently-used anti-tumor medicines, has little toxicity to normal cells, and also has good anti-inflammatory activity. The preparation method is simple and convenient to operate, is mild in reaction conditions, is high in synthetic yield, and is beneficial for being widely popularized in anti-tumor and anti-inflammatory fields.
Owner:BINZHOU MEDICAL COLLEGE
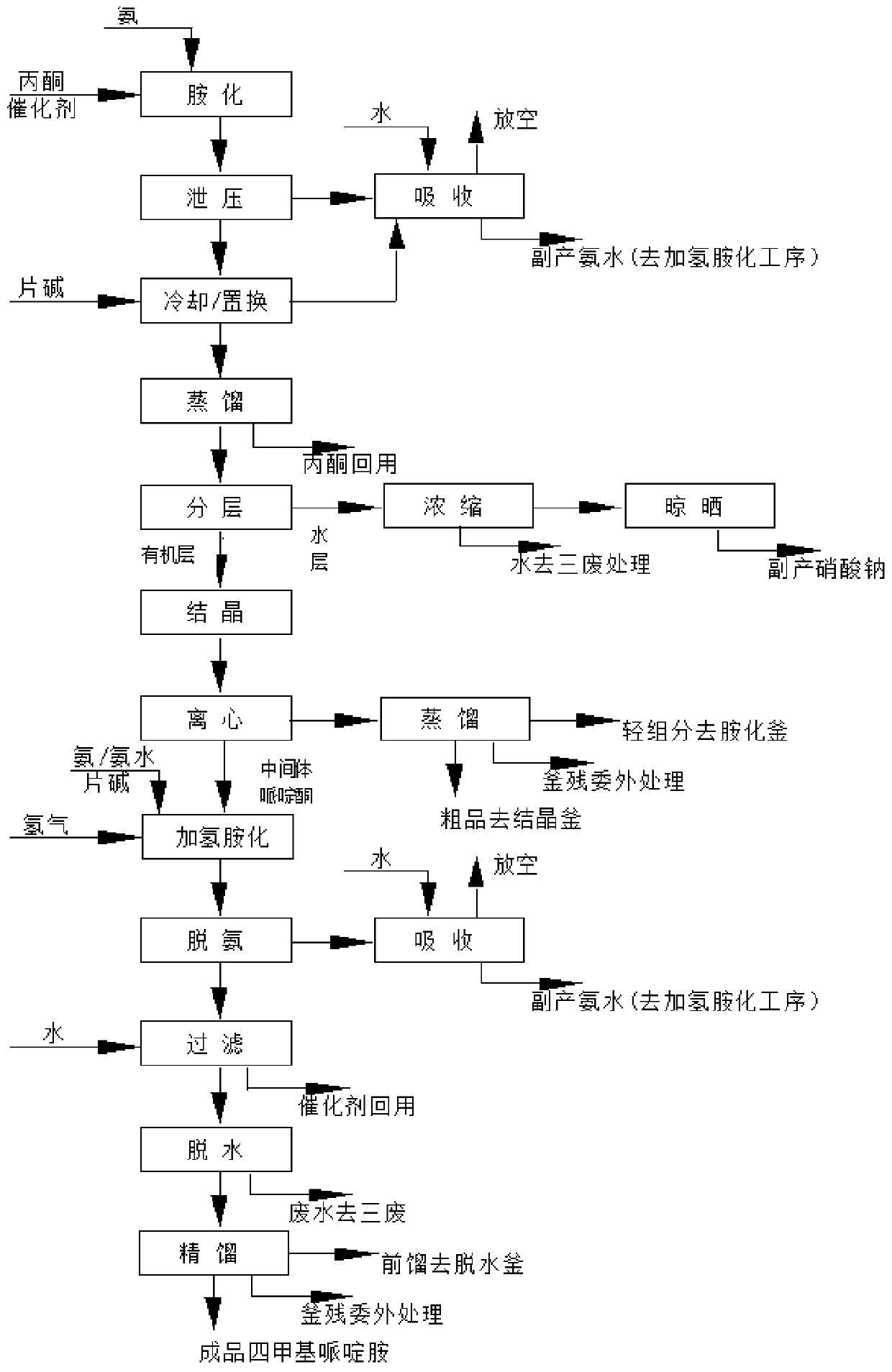
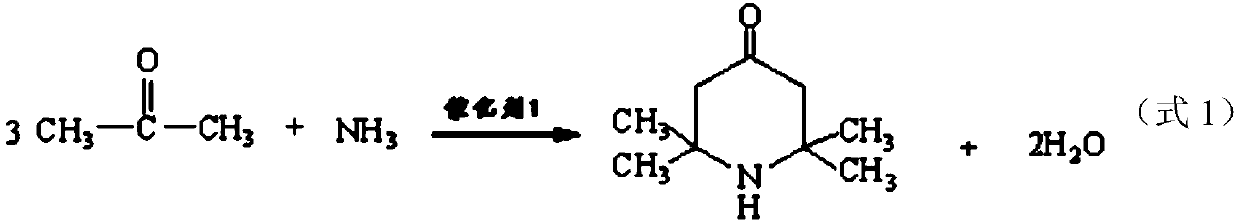
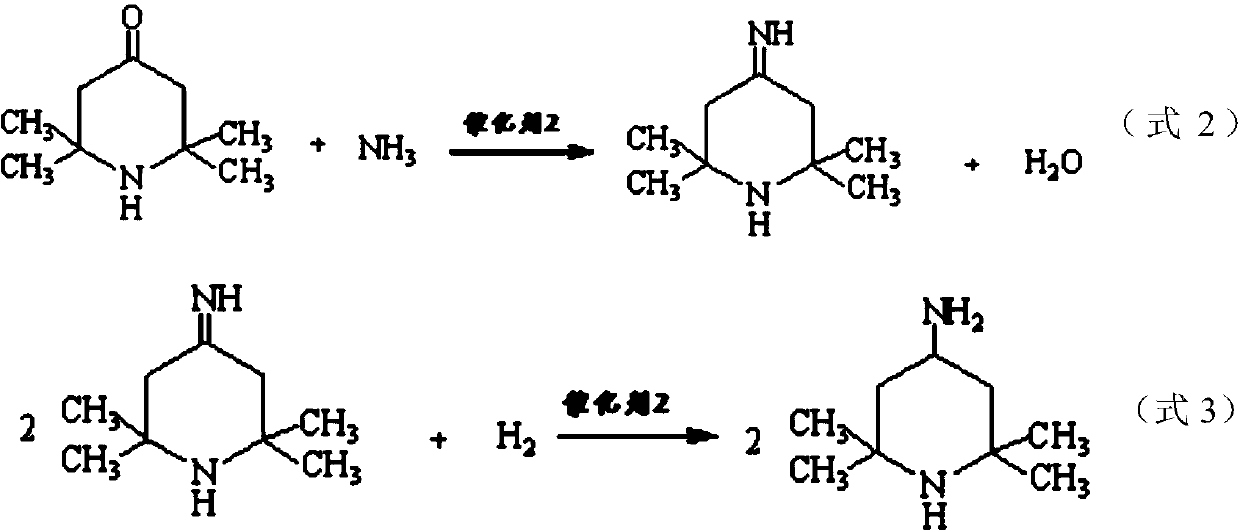
Method for preparing intermediate 2,2,6,6-tetramethyl-4-piperidylamine
ActiveCN110526860AGood technical effectImprove conversion rateOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsNickel catalystHydrogen
The invention relates to a method for preparing intermediate 2,2,6,6-tetramethyl-4-piperidylamine. The method is characterized by comprising the following steps: step 1), in the presence of a catalyst1, reacting acetone with ammonia gas to produce 2,2,6,6-tetramethyl-4-piperidinone and water; and step 2), in the presence of a catalyst 2, reacting the 2,2,6,6-tetramethyl-4-piperidinone with ammonia gas to produce 2,2,6,6-tetramethyl-4-piperidylimine and water, and reacting the 2,2,6,6-tetramethyl-4-piperidylimine with hydrogen gas to produce the 2,2,6,6-tetramethyl-4-piperidylamine, wherein the catalyst 1 is ammonium nitrate, and the catalyst 2 is a sodium hydroxyphenyl phosphate modified framework nickel catalyst. The method provided by the invention has the advantages of short synthetictime, a high conversion rate of the acetone and a high repeating utilization rate of the raw materials.
Owner:宁夏沃凯珑新材料有限公司
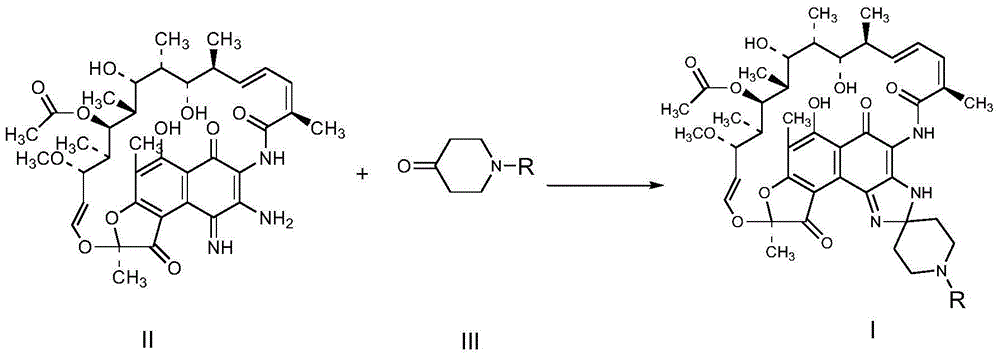
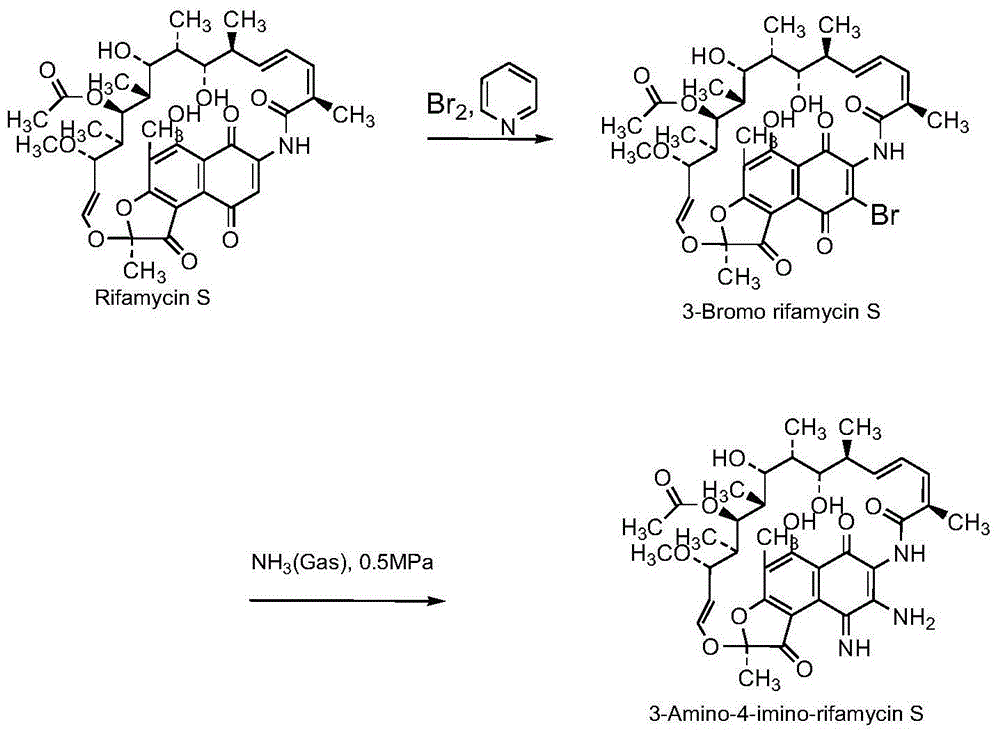
Preparation method for rifamycin S derivative
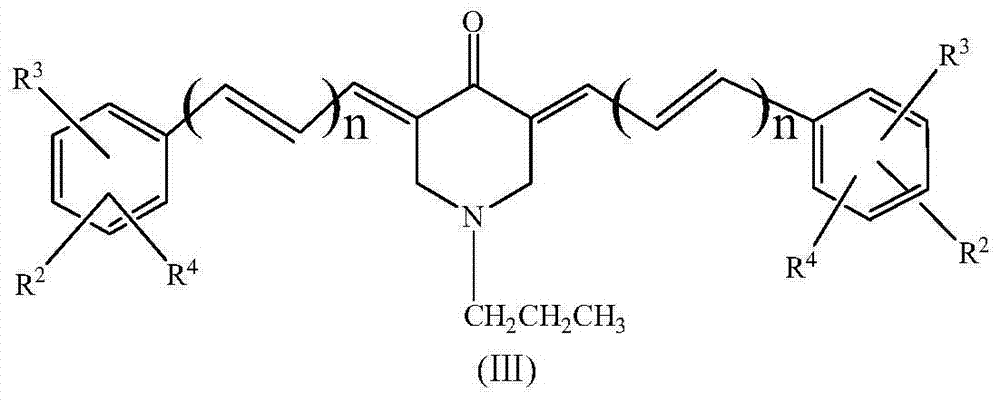
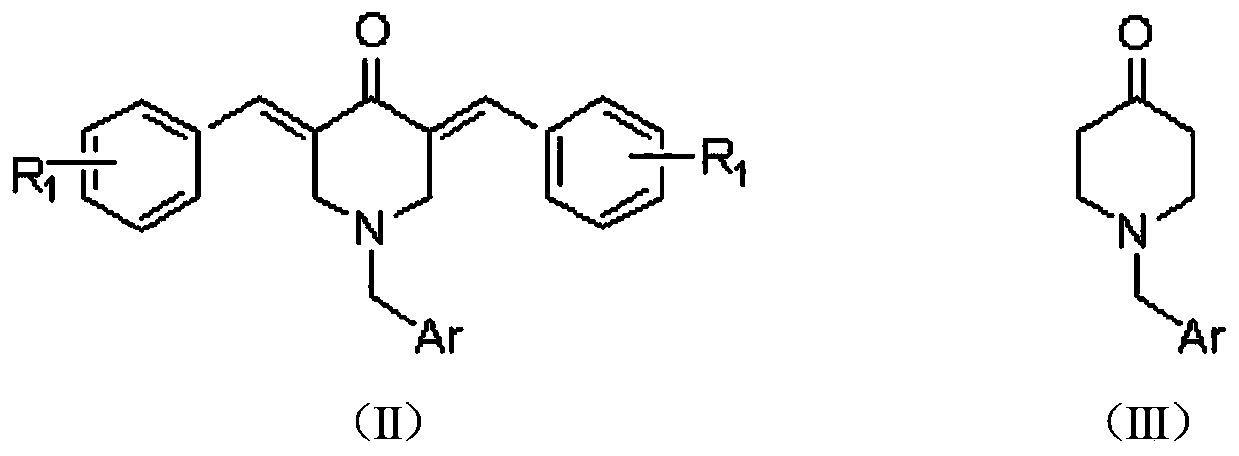
The invention relates to a preparation method for a rifamycin S derivative. According to the preparation method, 3-amino-4-imine rifamycin S as shown in a formula II which is described in the specification and a 4-piperidone derivative as shown in a formula III which is described in the specification are added according to a certain feeding mode and subjected to a condensation reaction in the presence of an organic solvent, ammonium acetate and zinc dust so as to produce the rifamycin S derivative as shown in a formula I which is described in the specification. The feeding mode is that the 3-amino-4-imine rifamycin S is added into a reaction system in a final step, so 3-amino-4-imine rifamycin S is prevented from reduction and hydrolysis. The preparation method increases the utilization rate of materials and is shortened in reaction time, so high-efficiency high-yield preparation of the rifamycin S derivative is realized, and the yield and content of the rifamycin S derivative reach 80% or above and 95% or above, respectively; and the preparation method overcomes the problems of long reaction time, low yield, many impurities and high cost, and is simple to operate, highly efficient, environment friendly and beneficial for large-scale production.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Tert-butyl substituted asymmetrical piperidone compounds with anti-tumor activities and preparation method thereof
InactiveCN105646337AAvoid genotoxicityLow toxicityOrganic chemistryAntineoplastic agentsMethyl groupTert butyl
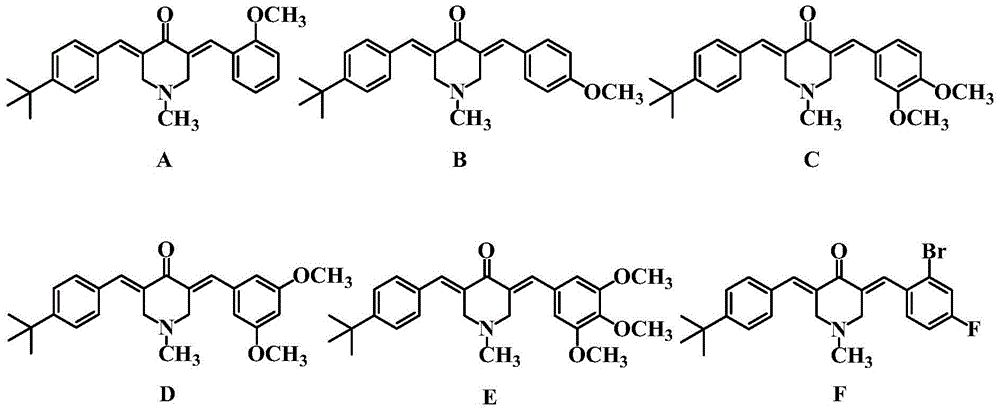
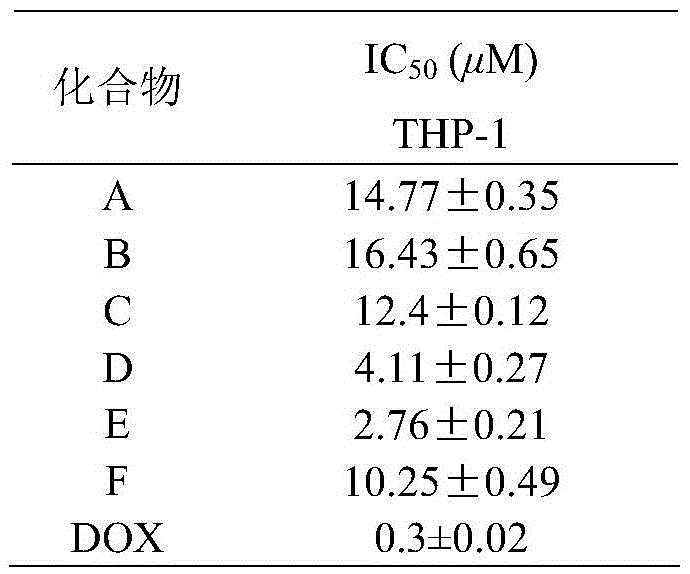
The invention relates to six tert-butyl substituted asymmetrical piperidone compounds with anti-tumor activities and a preparation method thereof, and belongs to the technical field of anti-tumor medicaments and a preparation method thereof. According to the preparation method, a Claisen-Schmidt condensation reaction is performed on N-methyl-4-piperidone, 4-tert-butylbenzaldehyde and another aromatic aldehyde to obtain products A-F. The compounds have high anti-tumor activities, genotoxicity of the conventional anti-tumor medicaments can be avoided, and the toxicity to normal cells is low. The preparation method is easy and convenient to operate, is mild in the reaction condition, is high in synthesis yield, and can be widely popularized in the anti-tumor field.
Owner:BINZHOU MEDICAL COLLEGE
Synthesis method of N-benzyl-4-piperidyl formaldehgde
A process for synthesizing N-benzyl-4-piperidyl formaldehyde as the intermediate for preparing donepezil to treat senile dementia includes reacting between N-benzyl-4-piperidinone and Liuyelide to generate 6-benzyl-1-oxo-6-azaspiro[2,5] octane, and catalytic reforming with magnesium bromide-ether. Its advantage is high output rate.
Owner:ZHEJIANG UNIV
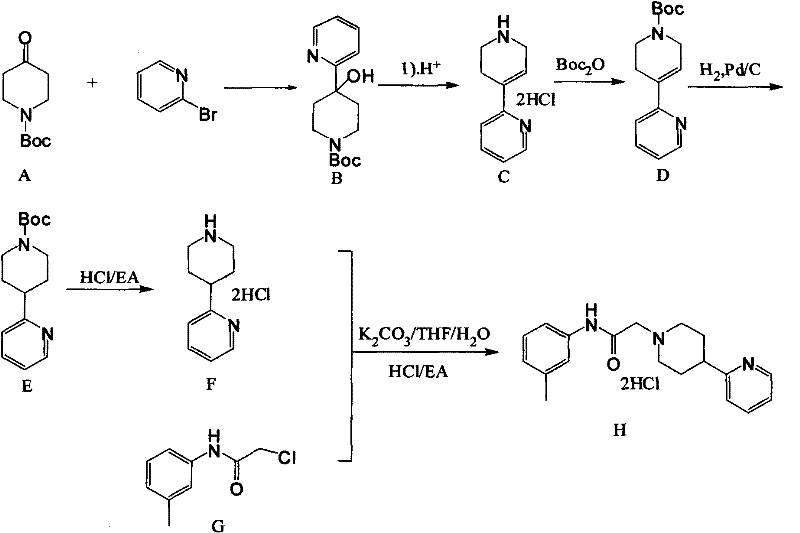
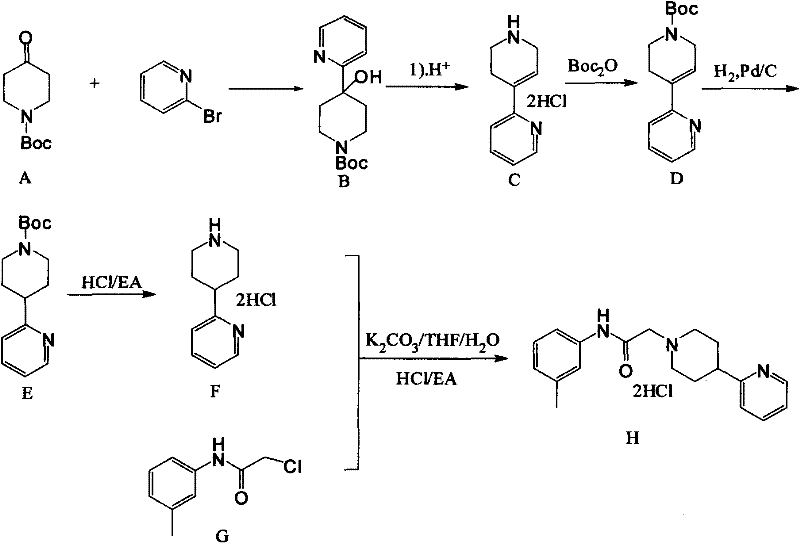
Method for producing hydrochloride of dopamine D4 receptor agonist A-412997
The invention discloses a method for producing hydrochloride of a dopamine D4 receptor agonist A-412997. The method comprises the following steps of: obtaining lithium pyridine by using a cheap reagent of butyl lithium as an exchange reagent; reacting with N-Boc-4-piperidone to obtain a compound of 1-N-Boc-4-(2-pyridyl)-4-hydroxypiperidine; removing water molecules, adding Boc, hydrogenating, removing the Boc and performing other steps to obtain a compound of 4-(2-pyridyl)piperidine dihydrochloride; and performing substitution on the 4-(2-pyridyl)piperidine dihydrochloride and a compound of chloracetyl m-toluidine to obtain the target product, namely the hydrochloride of the dopamine D4 receptor agonist A-412997. The process flow is simple, the method is suitable for industrial production, the reagent has low price, the yield is high, the total yield is 44.5 percent, and the method is an effective method for producing the hydrochloride of A-412997.
Owner:ITIC MEDCHEM CO LTD
Catalyst and application thereof to preparing 2, 2, 6, 6-tetramythl-4-piperidone
ActiveCN109746004AHigh catalytic activityExtended service lifeOrganic chemistryCatalyst activation/preparation4-PiperidinoneZirconium
The invention provides a catalyst and application thereof to preparing 2, 2, 6, 6-tetramythl-4-piperidone. The catalyst comprises SO4<2->-supported zirconium-aluminum compound oxides, which are composed of ZrO and Al2O3. When applied to preparing the 2, 2, 6, 6-tetramythl-4-piperidone, the catalyst has the advantages of being safe, efficient, easy to recycle and the like.
Owner:WANHUA CHEM GRP CO LTD
Synthesis method of N-boc-4-hydroxypiperidine
InactiveCN104628625AMeet the requirementsRaw materials are easy to getOrganic chemistrySynthesis methodsFiltration
The invention discloses a synthesis method of N-boc-4-hydroxypiperidine, which comprises the following steps: taking 4-piperidone hydrochloride hydrate, adding distilled water, introducing liquid ammonia to alkalinity, extracting with toluene, drying with anhydrous magnesium sulfate, and carrying out vacuum filtration to obtain 4-piperidone; dissolving in methanol, adding sodium borohydride, refluxing, concentrating, adding dilute hydrochloric acid to regulate the pH value, adding dichloromethane to separate out the water layer, maintaining the organic phase, drying with anhydrous magnesium sulfate over night, carrying out vacuum filtration, maintaining the organic phase, concentrating, adding n-hexane, refrigerating to crystallize, carrying out vacuum filtration, adding methanol, potassium carbonate and di-tert-butyl dicarbonate, refluxing, filtering, concentrating, adding petroleum ether, and refrigerating to crystallize, thereby obtaining the final white crystal product. The method has the advantages of accessible raw materials, high reaction yield, low cost, favorable selectivity and the like, is simple to operate and can easily implement industrialization. The product has the advantages of high purity and stable properties, and completely conforms to the operating requirements as a drug intermediate.
Owner:ANHUI DEXINJIA BIOPHARM
2,2,6,6,-tetramethyl-4-piperidone continuous synthesis method
ActiveCN103224465BSubsequent separation is simpleAvoid pollutionOrganic chemistrySynthesis methodsFixed bed
The invention discloses a 2,2,6,6,-tetramethyl-4-piperidone continuous synthesis method. The 2,2,6,6,-tetramethyl-4-piperidone continuous synthesis method comprises the following steps of 1, filling an acidic resin into a fixed bed reactor, and carrying out heating so that a temperature of the fixed bed reactor is in a range of 40 to 70 DEG C, and 2, feeding acetone and ammonia gas into the fixed bed reactor according to a mole ratio of 3-9: 1 under the conditions of acetone hourly space velocity of 0.15 to 1.17h<-1> and ammonia gas hourly space velocity of 5.25 to 124.20h<-1>, and cooling the product to obtain a 2,2,6,6,-tetramethyl-4-piperidone crude product. The 2,2,6,6,-tetramethyl-4-piperidone continuous synthesis method has simple processes and a low cost, is suitable for industrial continuous production, does not adopt an organic solvent or water as a solvent, avoids the pollution produced by the organic solvent on the environment, has mild reaction conditions, can be carried out at a temperature of 40 to 70 DEG C, has low energy consumption, and avoids the influence caused by impurities produced at a high temperature on the product.
Owner:安徽兴欣新材料有限公司
A ceritinib preparing method
The invention relates to a ceritinib preparing method. The method includes (1) reacting 4-bromo-2-isopropoxy-5-methylaniline and acetic anhydride to generate N-(4-bromo-2-isopropoxy-5-cresyl) acetamide, (2) reacting 4-piperidone to generate N-benzyl-4-piperidone, (3) subjecting the N-(4-bromo-2-isopropoxy-5-cresyl) acetamide and the N-benzyl-4-piperidone to a docking reaction, (4) subjecting a product of the former step to dehydroxylation and (5) generating a finally product that is ceritinib from N-(4-(1-phenyl-1,2,3,6-tetrahydropyridin-4-yl)-2-isopropoxy-5-cresyl) acetamide under the existence of a catalyst. The novel ceritinib preparing method is provided. The synthesis route is low in cost, reaction conditions are mild and easy to control, and the method is suitable for large-scale industrial production.
Owner:常州市勇毅生物药业有限公司
Synthesis process of N-sustituent-4-piperidyl alcohol
The synthesis process of preparing N-substituent-4-piperidyl alcohol includes the following steps: 1. adding primary amine into ethyl acrylate to react to obtain the secondary addition product; 2. adding cyclizing agent into the secondary addition product and adding concentrated hydrochloric acid after finishing cyclization to obtain N-substituent-4-piperidone hydrochloride; 3. reducing N-substituent-4-piperidone hydrochloride with NaBH4-Amberlyst-15(H+) system and regulating pH value to extract N-substituent-4-piperidyl alcohol; and 4. recovering and treating resin for reuse. The present invention has wide raw material resource, mild reaction condition, simple operation, low production cost, high yields in different steps, and excellent industrial application foreground.
Owner:SOUTHEAST UNIV
Electrophotographic photosensitive member, process cartridge, electrophotographic apparatus and phthalocyanine crystal
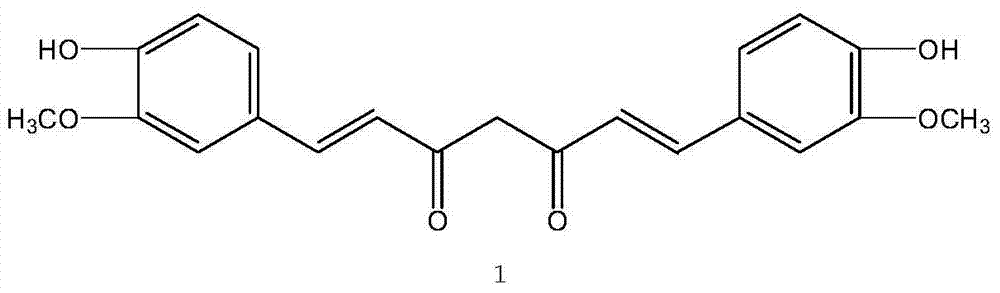
InactiveCN105247416AReduce outputImprove performancePorphines/azaporphinesElectrography/magnetographyPhthalocyanine4-Piperidinone
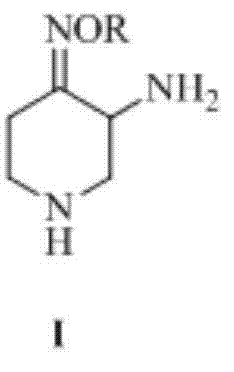
The present invention provides an electrophotographic photosensitive member comprising a support, and a photosensitive layer formed on the support, wherein the photosensitive layer contains a phthalocyanine crystal in which a 4-piperidone compound represented by the following formula (1) is contained:
Owner:CANON KK
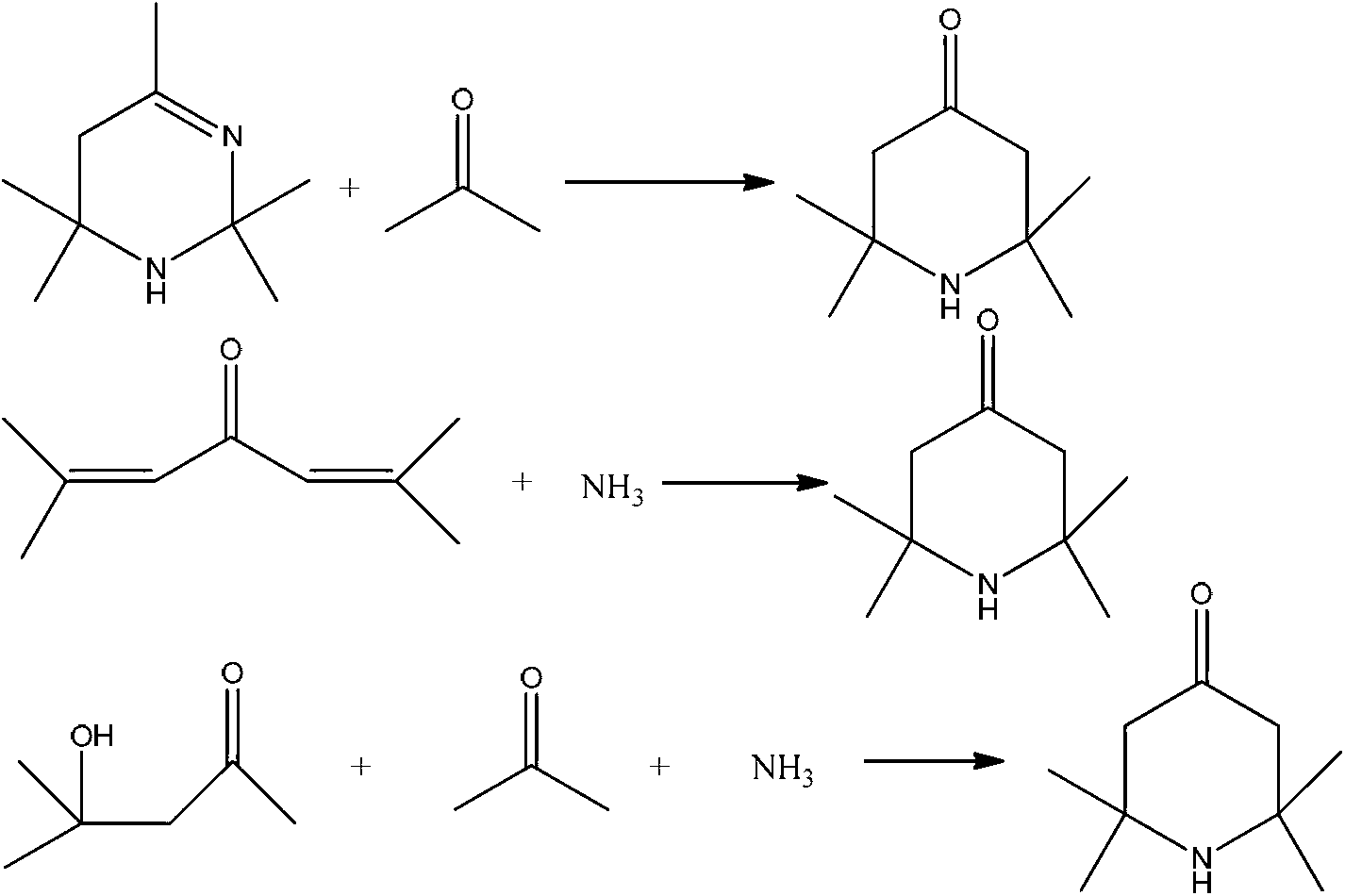
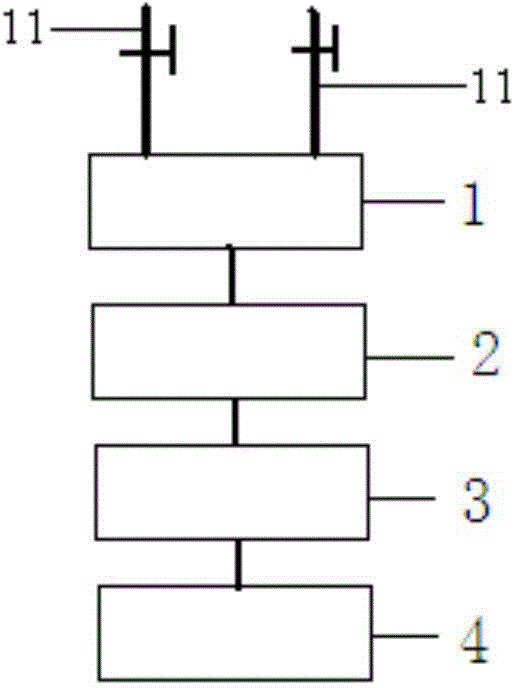
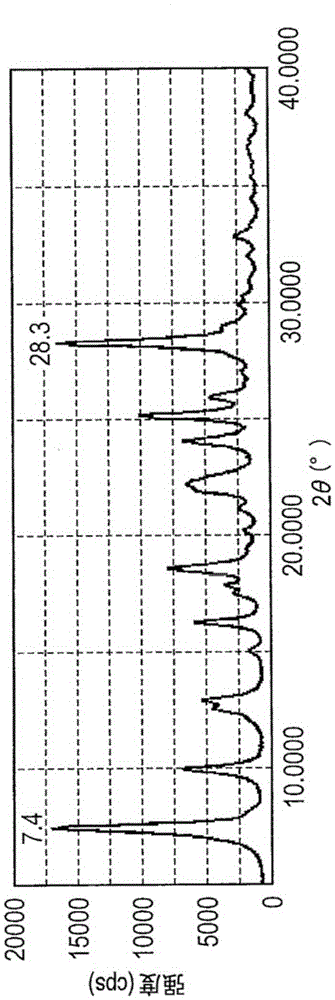
Method for synthesis of 1,8-diazaspiro [4,5] decane-3-hydroxy-1-benzyl-8-carboxylic acid tert-butyl ester
The invention discloses a method for synthesis of the bicyclic compound 1,8-diazaspiro [4,5] decane-3-hydroxy-1-benzyl-8-carboxylic acid tert-butyl ester. The raw material N-Boc-4-piperidone easy to obtain is adopted, and the 1,8-diazaspiro [4,5] decane-3-hydroxy-1-benzyl-8-carboxylic acid tert-butyl ester is obtained through dehydration condensation reaction, allyl Grignard addition reaction, epoxidation reaction and proton abstraction ring-closing reaction which are conducted continuously.
Owner:SHANGHAI TBBMED CO LTD
3,5-bis(3-aminobenzylidene)-4-piperidone derivatives with antitumor activity and preparation method thereof
InactiveCN105669537AAvoid genotoxicityLow toxicityOrganic chemistryAntineoplastic agents4-PiperidinoneAntitumor activity
The invention relates to two 3,5-bis(3-aminophenyl)methylene-4-piperidone compounds with antitumor activity and a preparation method thereof, and belongs to the technical field of antitumor drugs and preparation methods thereof.The preparation method of the compounds includes the steps that 4-piperidone and m-nitrobenzaldehyde are subjected to a Clayson-Schmidt condensation reaction to obtain an intermediate, and then the intermediate is subjected to reduction with a reducing agent to obtain the products A and B.The compounds are good in antitumor activity, can avoid the genotoxicity of antitumor drugs used at present and have little toxicity on normal cells.The preparation method is easy and convenient to implement, reaction conditions are mild, the synthesis yield is high, and thus the preparation method can be widely popularized in the antitumor field easily.
Owner:BINZHOU MEDICAL COLLEGE
Method for synthesis of 1-(N-Boc-4-piperidine)-4-pyrazoleboronic acid pinaol ester
ActiveCN105153211AHigh purityEasy to operateGroup 3/13 element organic compoundsBromineEthyl Chloride
The invention discloses a method for synthesis of 1-(N-Boc-4-piperidine)-4-pyrazoleboronic acid pinaol ester. According to the method, N-Boc-4-piperidone serves as the raw material and reacts with hydrazine hydrate, N-Boc-4-piperidine hydrazine is generated through reduction, then dehydration condensation reaction is conducted between N-Boc-4-piperidine hydrazine and 2-halogenate malonaldehyde so that a 1-(N-Boc-4-piperidine)-4-chlorine / bromine pyrazol midbody can be obtained effectively, and in the presence of metallic nickel, metallic nickel catalytic coupling reaction ester forming is conducted on the midbody under a mild condition to obtain the 1-(N-Boc-4-piperidine)-4-pyrazoleboronic acid pinaol ester. The whole process is easy to operate, conditions are mild, and untralow-temperature reaction is not needed; meanwhile, required raw materials are cheap and easy to obtain, reproducibility is high, and the method is suitable for industrialized large-scale production.
Owner:SHANGHAI TBBMED CO LTD
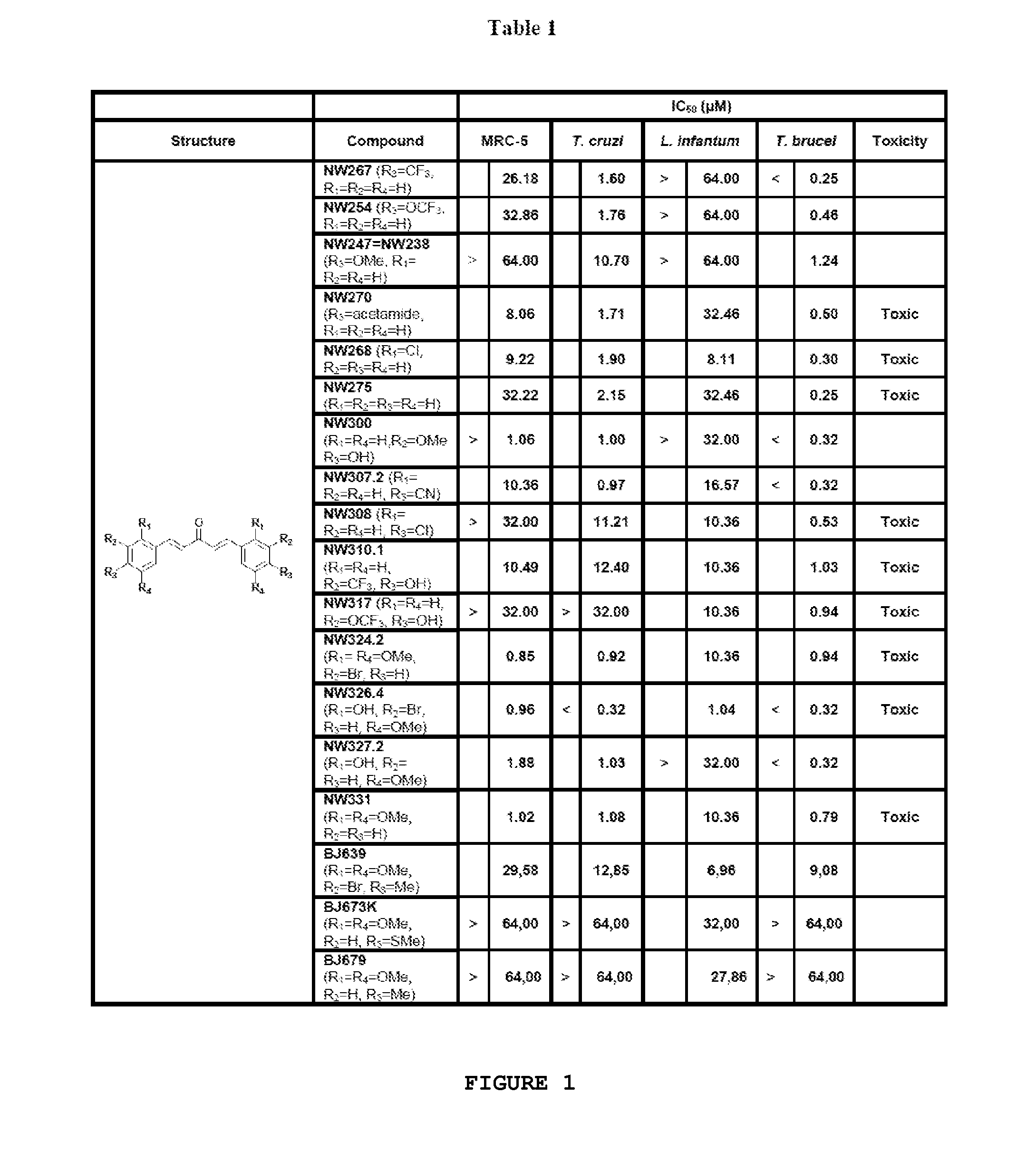
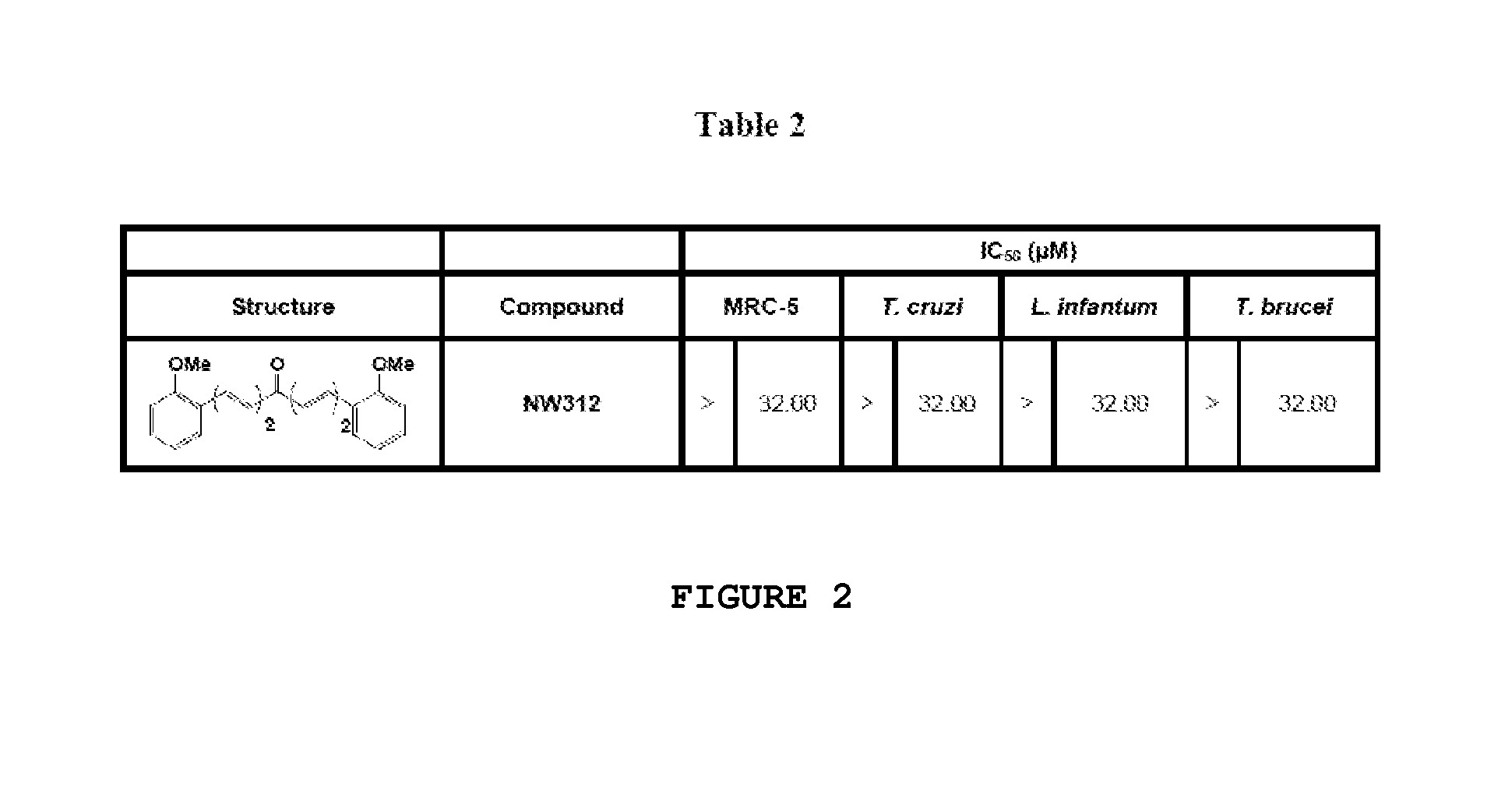
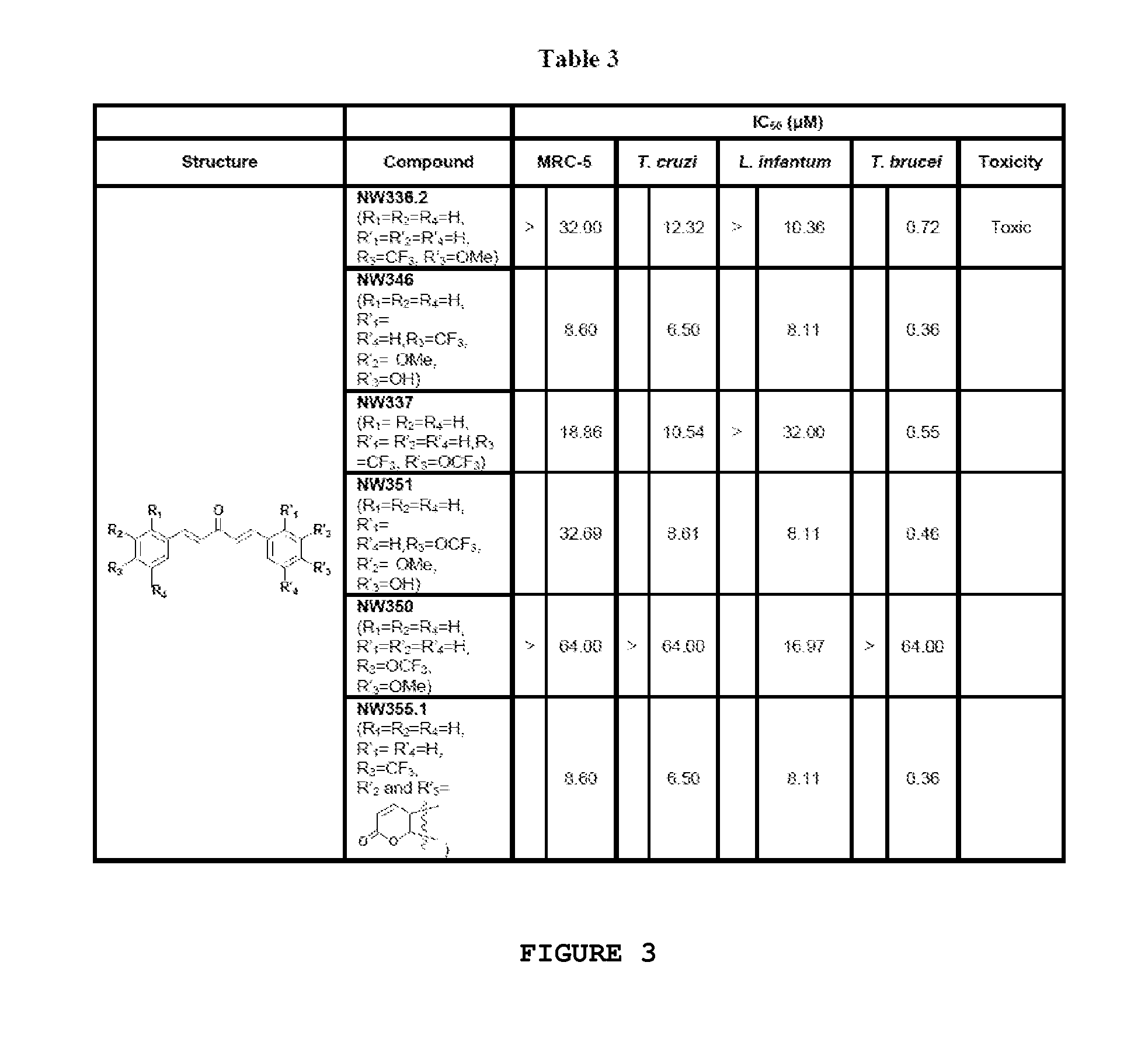
Compounds useful against kinetoplastideae parasites
Dibenzylidene and heterobenzylideneacetone derivatives, related 4-piperidones, related 4-thiopyranones and the corresponding sulfinyl- and sulfonyl-analogues for their use for prophylaxis or treatment of trypanosomiasis and leishmaniasis.
Owner:CENT NAT DE LA RECHERCHE SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
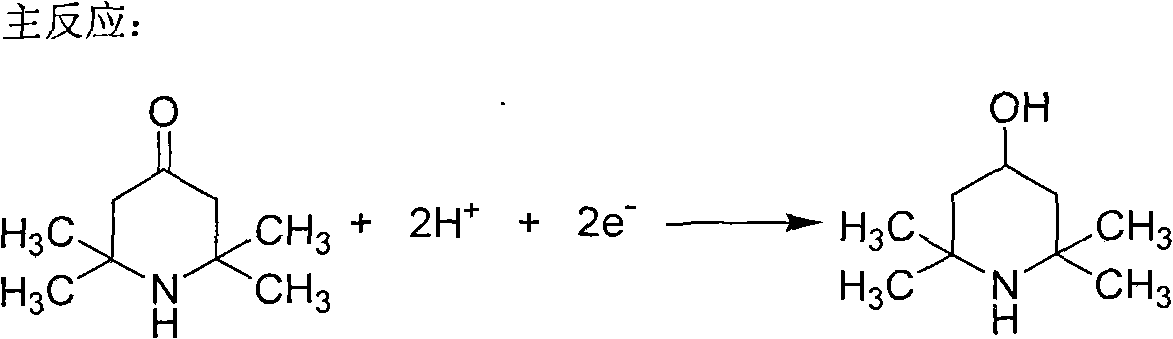

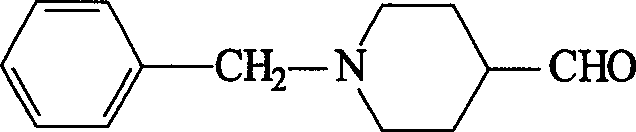

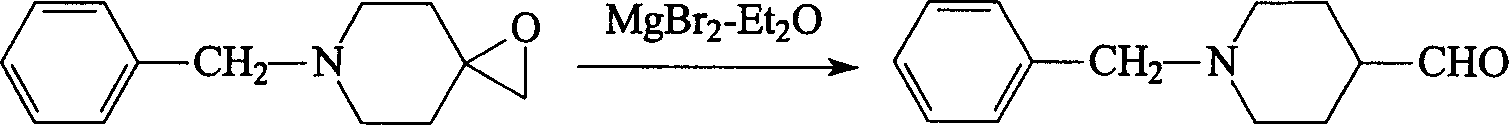

![Method for synthesis of 1,8-diazaspiro [4,5] decane-3-hydroxy-1-benzyl-8-carboxylic acid tert-butyl ester Method for synthesis of 1,8-diazaspiro [4,5] decane-3-hydroxy-1-benzyl-8-carboxylic acid tert-butyl ester](https://images-eureka.patsnap.com/patent_img/503ecb3a-1fa5-42d7-9a18-1821d89d2173/BDA0000809880310000021.PNG)
![Method for synthesis of 1,8-diazaspiro [4,5] decane-3-hydroxy-1-benzyl-8-carboxylic acid tert-butyl ester Method for synthesis of 1,8-diazaspiro [4,5] decane-3-hydroxy-1-benzyl-8-carboxylic acid tert-butyl ester](https://images-eureka.patsnap.com/patent_img/503ecb3a-1fa5-42d7-9a18-1821d89d2173/FDA0000809880300000011.PNG)