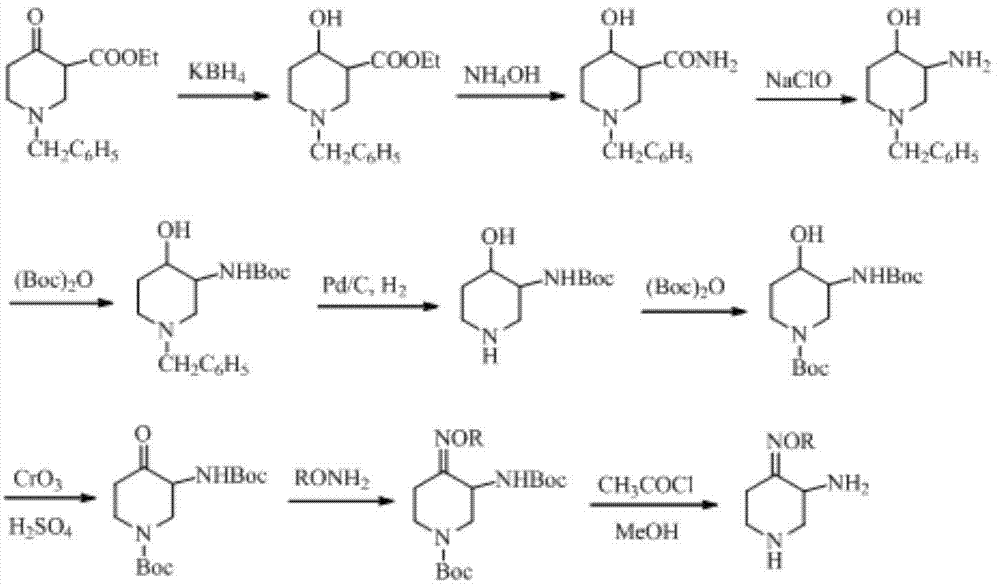
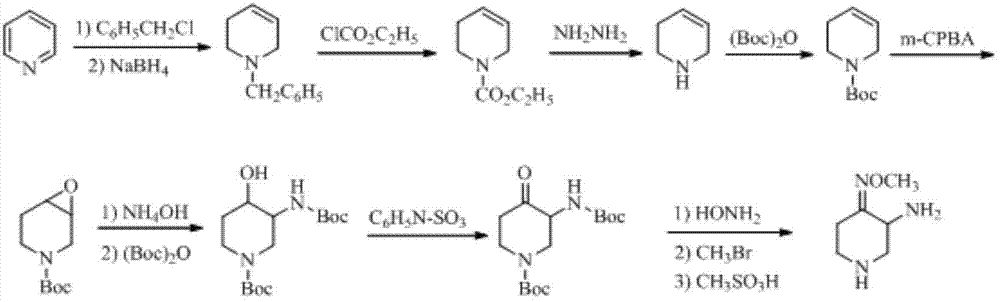
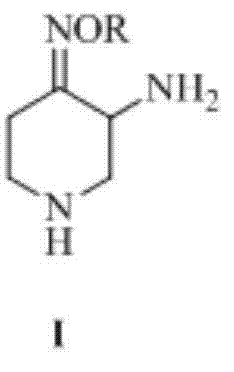
Preparation method of 3-amino-4-alkoxyimino piperidine
A technology of alkoxyiminopiperidine and alkoxyamine hydrochloride, applied in the preparation of 3-amino-4-alkoxyiminopiperidine, 3-amino-4-alkoxyiminopiperidine and In the field of salt, it can solve the problems of being unsuitable for industrial production, odorous dimethyl sulfide, and polluting the environment, and achieve the effects of being suitable for large-scale production, less reaction steps, and reducing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1, 1-N-tert-butoxycarbonyl-3-cyano-4-piperidone
[0031] Add ethyl 3-alanine propionate hydrochloride (153.5g, 1mol) and ethanol (850mL) into a 2000mL three-necked flask, stir it mechanically at room temperature to dissolve, add sodium hydroxide (41.5g, 1mol), and stir at room temperature for 0.5 hours . Heat to 50°C (internal temperature, the same below), and add a solution of acrylonitrile (66 mL, 1 mol) in ethanol (150 mL) dropwise. After dropping (1 hour), the stirring was continued at the same temperature, and the reaction was followed by TLC for about 4 hours.
[0032] After the reaction mixture obtained above was slightly cooled (~40°C), a solution of di-tert-butyl dicarbonate (218.25g, 1mol) in ethanol (150mL) was added dropwise thereto (temperature ≤50°C during the period), and the dropwise completion (~1 Hours), continued stirring at 40-45°C, and TLC followed the reaction to end in about 3 hours. After cooling down to room temperature, the insoluble...
Embodiment 2
[0034] Example 2, 1-N-tert-butoxycarbonyl-3-carbamoyl-4-methoxyiminopiperidine
[0035] Add methoxylamine hydrochloride (50.1g, 0.6mol) and methanol (400mL) to a 2000mL three-necked reaction flask, stir at room temperature to dissolve, add sodium hydroxide (25g, 0.6mol), stir at room temperature for 0.5 hours, add 1- tert-butoxycarbonyl-3-cyano-4-piperidone (112 g, 0.5 mol) in methanol (400 mL) was heated to 50° C. and stirred, and the reaction was followed by TLC for about 3 hours. Cool down to room temperature, filter out the insoluble matter (sodium chloride), concentrate the filtrate under reduced pressure, add distilled water (250mL) to the residue, stir well, extract with ethyl acetate (200mL×3), combine the extracts, and wash with saturated NaCl Washed with aqueous solution (250mL), anhydrous Na 2 SO 4 dry. Filtration, the filtrate was concentrated under reduced pressure, and under full stirring, the resulting residue was slowly poured into diethyl ether (1000 mL), a...
Embodiment 3
[0038] Example 3, N-tert-butoxycarbonyl-3-amino-4-methoxyiminopiperidine
[0039] Add 1-N-tert-butoxycarbonyl-3-carbamoyl-4-methoxyiminopiperidine (27.1g, 0.1mol) and acetonitrile (600mL) into a 1000mL three-necked flask, mechanically stir at room temperature to dissolve, and cool in an ice-water bath To about 0-5 ℃, add the freshly prepared sodium hypobromite solution (253 mL, 0.18mol) dropwise, after the dropwise completion (about 1 hour), stir the reaction at room temperature overnight, and follow the completion of the reaction by TLC. Leave to stand for stratification, and separate the water layer (lower layer). The upper layer (acetonitrile layer) was adjusted to pH 6.5 with acetic acid, concentrated under reduced pressure, distilled water (50 mL) was added to the obtained oily residue, and the pH was adjusted to 3 with 6N HCl solution. Combined, adjust pH9 with 6N NaOH solution, extract with ethyl acetate (150mLx6) and combine extracts, wash with saturated NaCl aqueous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com