Method for producing hydrochloride of dopamine D4 receptor agonist A-412997
A technology of A-412997 and receptor agonist, which is applied in the field of medicine and chemical industry, can solve the problems of expensive coupling reagents, unfavorable scale-up production, difficult purification, etc., and achieve the effect of low reagent price, favorable scale-up production, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
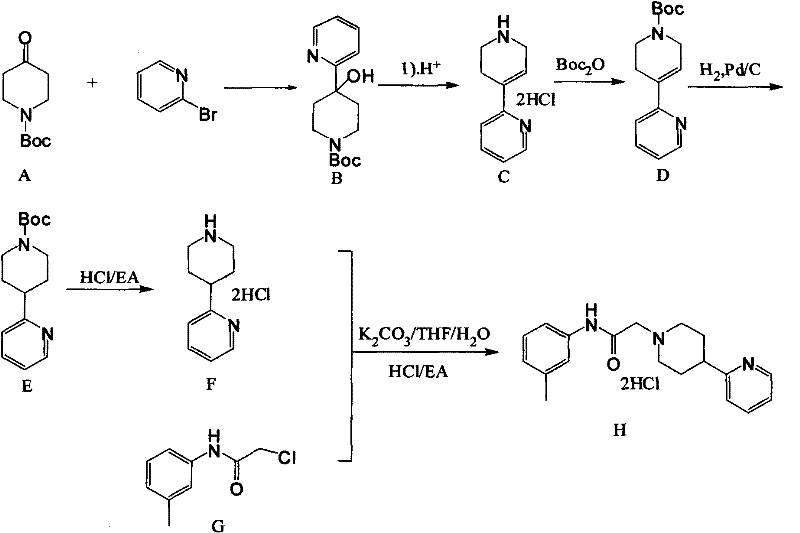
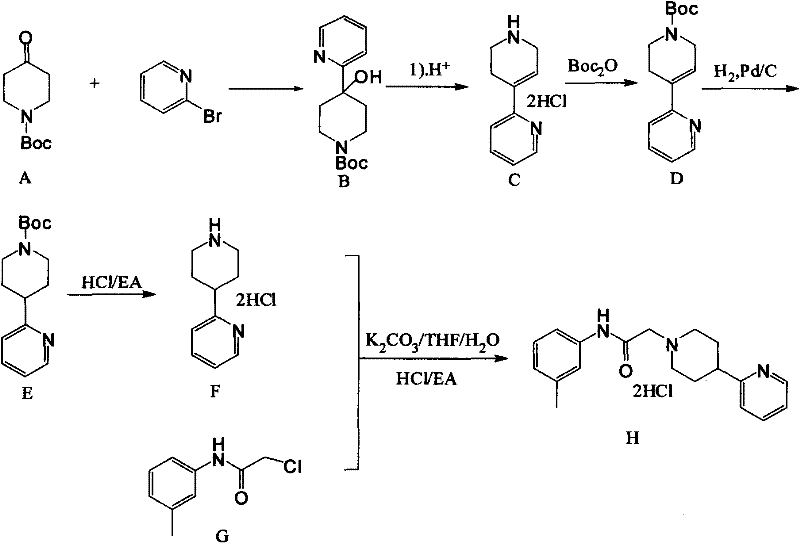
[0022] The preferred embodiments of the present invention will be described in detail below in conjunction with the chemical reaction equations of each step of the present invention.
[0023] Such as figure 1 As shown, first add 2-bromopyridine (950g, 6.01mol) and dissolve it in 6.6LTHF. Keep warm for 2 hours, drop the compound 1-N-BOC-4-piperidone (945g dissolved in 2L of THF) into the reaction flask, after the drop, naturally warm up to room temperature, monitor the reaction by TLC, after the reaction is complete, add 3L Water, liquid separation, the aqueous phase was extracted 3 times with 4L ethyl acetate, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate for 2 hours, concentrated, petroleum ether: ethyl acetate (5:1) crystallized to obtain 1.4 kg yellow solid compound 1-N-Boc-4-(2-pyridyl)-4-hydroxypiperidine, purity>90%, yield 93.5%. .
[0024] Then 2.4kg of thionyl chloride was added to the reaction flask, and the comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com