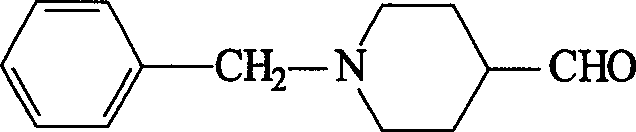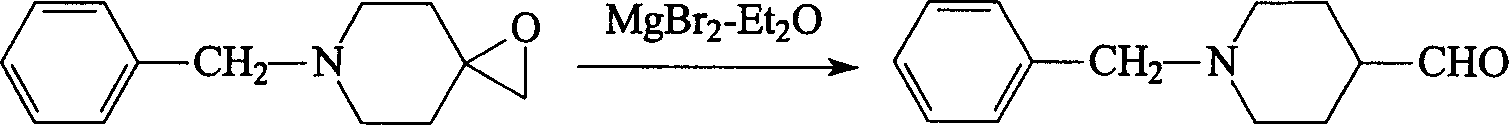Synthesis method of N-benzyl-4-piperidyl formaldehgde
A synthetic method, benzyl technology, applied in the field of pharmaceuticals, can solve the problems of high price, difficulty in industrial production, complex products, etc., and achieve the effect of easy preparation, cheap price and wide source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Put 18.9g (0.10 moles) of N-benzyl-4-piperidone, 24.2g (0.11 moles) of trimethoxysulfonium iodide, and 180ml of toluene into a reaction flask, and add 50ml of 10% NaOH dropwise at room temperature After the addition of the solution, the temperature was slowly raised to 80° C., and the reaction was continued for 4 hours. Cool to room temperature, pour the reaction solution into a separatory funnel, separate the organic layer, extract the aqueous layer with toluene (50ml×3), combine the toluene layers, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, and recover under reduced pressure Toluene, the intermediate product 6-benzyl-1-oxa-6-azaspiro[2.5]octane 18.6g was obtained, and the yield was 91.6%;
[0025] 1 HNMR data: δ: 1.49-1.55 (m, 2H), 1.76-1.83 (m, 2H), 2.50-2.59 (m, 6H), 3.53 (s, 2H), 7.20-7.33 (m, 5H).
[0026] (2) Use 6.0ml (0.12 moles) of bromine, 5.76g of magnesium chips and 200ml of anhydrous ether to prepare magnesium bromide-eth...
Embodiment 2
[0029] (1) In a three-necked flask equipped with 4.4g (0.11 moles) of sodium hydride (60%) and 200ml of anhydrous tetrahydrofuran, add 24.2g (0.11 moles) of trimethoxysulfonium iodide in portions under ice-cooling, add After the reaction was completed, the reaction was continued for 30 minutes, and then a solution of 18.9 g (0.10 moles) of N-benzyl-4-piperidone and 30 ml of tetrahydrofuran was slowly added dropwise. After the addition, the temperature was gradually raised to reflux, and the reaction was continued for 5 hours. After cooling, recover the solvent, add 50ml of ice water and 200ml of ether, separate the ether layer, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, recover the ether to obtain the intermediate product 6-benzyl-1-oxa-6-aza Spiro[2.5]octane 17.5g, the yield is 86.2%;
[0030] (2) Using benzene instead of anhydrous ether as a solvent, the operation method is the same as the second step reaction in Example 1, and the yield...
Embodiment 3
[0032] (1) Operation is the same as in Example 1.
[0033] (2) Use benzene and anhydrous ether (1: 1) mixed solvent to replace anhydrous ether as solvent, other operating methods are the same as the second step reaction of Example 1, and the yield to obtain the target product is 74.8%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com