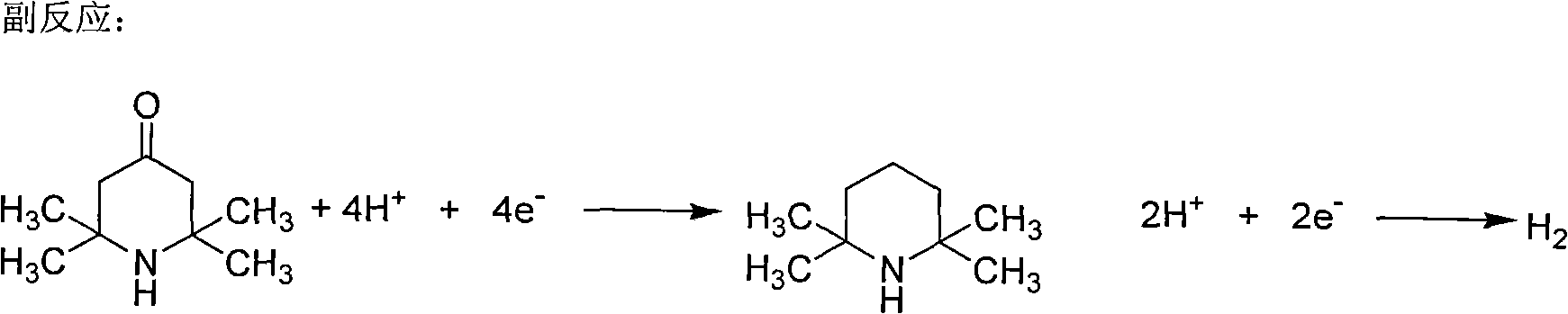
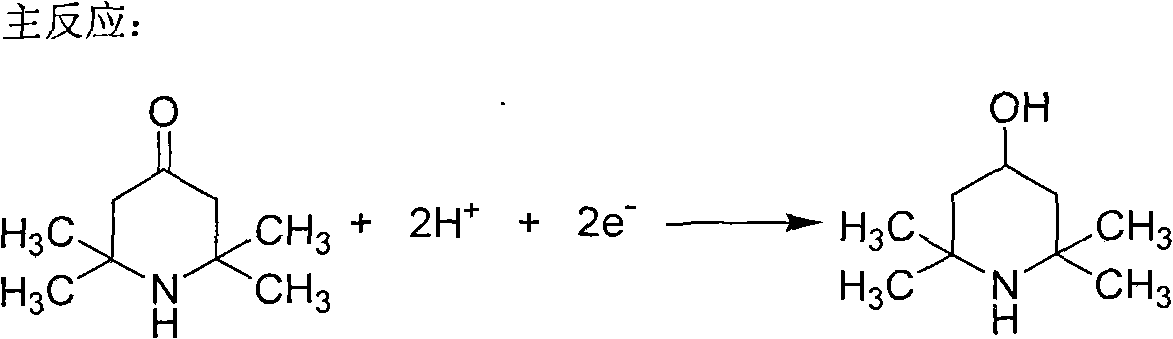Method for non-membrane electrochemical synthesis of 2,2,6,6-tetramethyl-piperidinol
A non-diaphragm, piperidinol technology, applied in the field of electrochemical synthesis, can solve the problems of difficult to realize industrialization, short service life, high equipment requirements, and achieve the effects of easy availability of equipment, short service life and low investment cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Make a simple electrolytic cell without a diaphragm, use a Pb plate as an anode, and a Zn plate as a cathode;
[0020] (2) Configure the electrolyte solution:
[0021] The composition and content of the electrolyte solution are: sodium hydroxide 0.50mol / L, anhydrous sodium sulfate 0.35mol / L, piperidone 1.0mol / L, co-solvent is methanol or ethanol, and the mass fraction of co-solvent is 10%-40 %;
[0022] (3) Electrolysis: Under normal temperature and pressure, use a potentiostat to control the electric quantity to 1.0F / mol and the current density to 100A / m 2 , calculate the amount of time for electrolyzing the electrolyte in the electrolytic cell;
[0023] (4) Extraction of the product
[0024] a. Extraction: Take out the electrolyte in the diaphragmless electrolytic cell, neutralize it to pH=7 with sulfuric acid (50% by mass fraction, the same as in the following examples), extract with chloroform three times, discard the upper layer, and keep the lower layer . ...
Embodiment 2
[0027] (1) Make a simple electrolytic cell without a diaphragm, use a Pb plate as an anode, and a Zn plate as a cathode;
[0028] (2) Configure the electrolyte solution:
[0029] The composition and content of the electrolyte solution are: sodium hydroxide 0.50mol / L, anhydrous sodium sulfate 0.35mol / L, piperidone 1.0mol / L, co-solvent is methanol or ethanol, and the mass fraction of co-solvent is 10%-40 %;
[0030] (3) Electrolysis: under normal temperature and pressure, use a potentiostat to control the electric quantity to 1.5F / mol and the current density to 300A / m 2 , calculate the amount of time for electrolyzing the electrolyte in the electrolytic cell;
[0031] (4) Extraction of the product
[0032] a. Extraction: Take out the electrolyte solution in the electrolytic cell without diaphragm, neutralize it with sulfuric acid (50%) to pH=7, extract it three times with chloroform, discard the upper layer, and keep the lower layer. The solvent was distilled off under reduc...
Embodiment 3
[0035] (1) Make a simple electrolytic cell without a diaphragm, use a Pb plate as an anode, and a Zn plate as a cathode;
[0036] (2) Configure the electrolyte solution:
[0037] The composition and content of the electrolyte solution are: sodium hydroxide 0.50mol / L, anhydrous sodium sulfate 0.35mol / L, piperidone 1.0mol / L, co-solvent is methanol or ethanol, and the mass fraction of co-solvent is 10%-40 %;
[0038] (3) Electrolysis: under normal temperature and pressure, use a potentiostat to control the electric quantity to 1.1F / mol and the current density to 200A / m 2 , calculate the amount of time for electrolyzing the electrolyte in the electrolytic cell;
[0039] (4) Extraction of the product
[0040] a. Extraction: Take out the electrolyte solution in the diaphragmless electrolytic cell, neutralize it with sulfuric acid (50% by mass) to pH=7, extract it three times with chloroform, discard the upper layer, and keep the lower layer. The solvent was distilled off under r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com