Preparation method for rifamycin S derivative
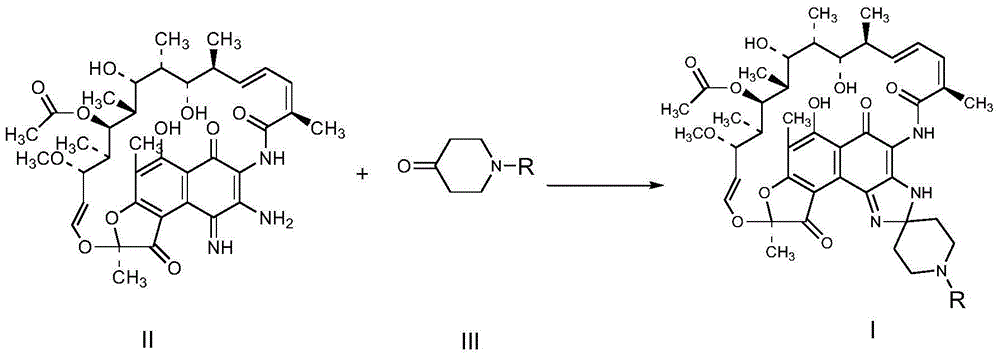
A kind of technology of rifamycin, imino-rifamycin is applied in the field of preparing rifamycin S derivatives shown in formula I, and can solve the problem that the yield of rifamycin S derivatives is low and the reaction time is long. , low production efficiency and other problems, to achieve the effect of overcoming long reaction time, shortening reaction time and improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
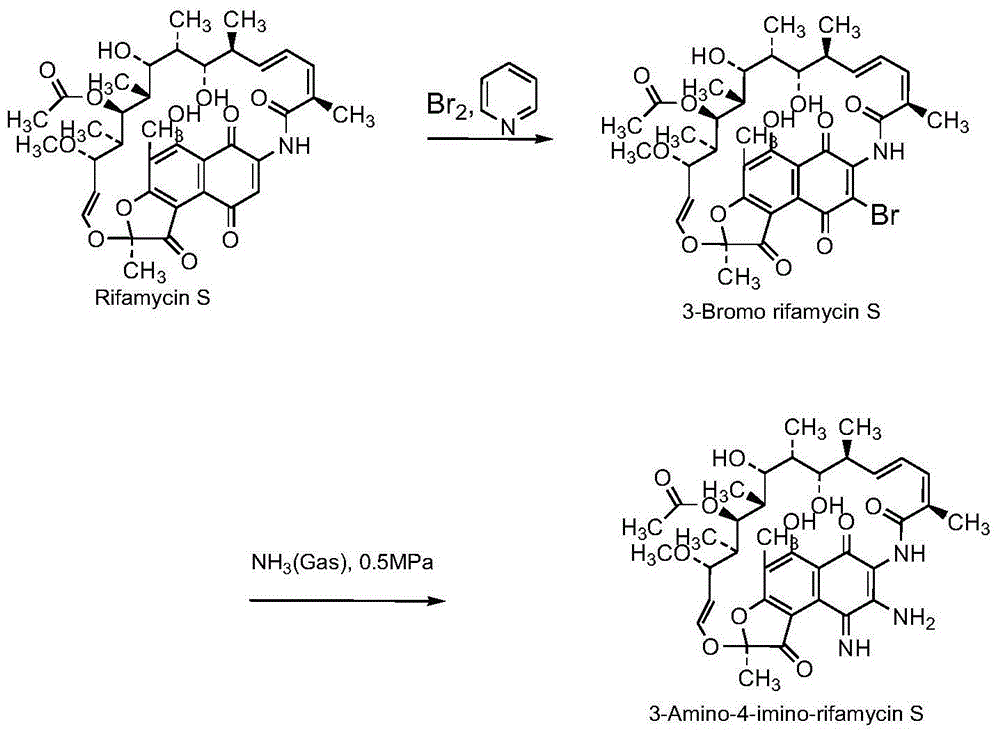
[0044] Example 1 3-amino-4-imine rifamycin S was reduced and hydrolyzed under the action of zinc powder and ammonium acetate
[0045]Add 400ml of ethyl acetate, 2.2g of ammonium acetate, 2.2g of zinc powder, 30g (42.25mmol) of 3-amino-4-imine rifamycin S into the reactor, and react at 50°C-60°C for 24 hours. Filter, wash with 50ml of ethyl acetate, combine filtered solution, wash with 300ml of 15% aqueous hydrochloric acid solution, then wash with 300ml of 5% aqueous sodium carbonate solution, and finally wash with 300ml of water, dry over sodium sulfate, and concentrate the mother liquor to dryness in vacuo. Column chromatographic separation, eluting with a mixture of n-hexane and ethyl acetate (the volume ratio of n-hexane and ethyl acetate is 20:1), to obtain 3,4-diaminorifamycin S and 3-amino - 4-imino-25-hydroxyrifamycin S.
[0046] The imine at the 4-position of 3-amino-4-imine rifamycin S is reduced to 3,4-diamino rifamycin S, 3-amino-4-imine rifamycin under the action...
Embodiment 2
[0048] The preparation of embodiment 2 N-methyl-4-piperidone rifamycin S
[0049] Add 400ml of ethyl acetate, 2.2g of ammonium acetate, 2.2g of zinc powder, 7.3g (64.52mmol) of N-methyl-4-piperidone into the reactor, stir at 10°C-20°C for 10 minutes, then slowly add 3 -Amino-4-imine rifamycin S 30g (42.25mmol), after adding for 40 minutes, react at 20°C-30°C for 5 hours, filter, wash with 50ml of ethyl acetate, combine filter solution, and use 15% hydrochloric acid aqueous solution Wash with 300ml, then wash with 300ml of 5% aqueous sodium carbonate solution, and finally wash with 300ml of water, dry over sodium sulfate, concentrate and dry in vacuo, and recrystallize from isooctane to obtain 32.3g of N-methyl-4-piperidone rifamycin S , the calculated yield was 93.2%, and the HPLC detection content was 98.5%.
[0050] The chemical reaction is as follows:
[0051]
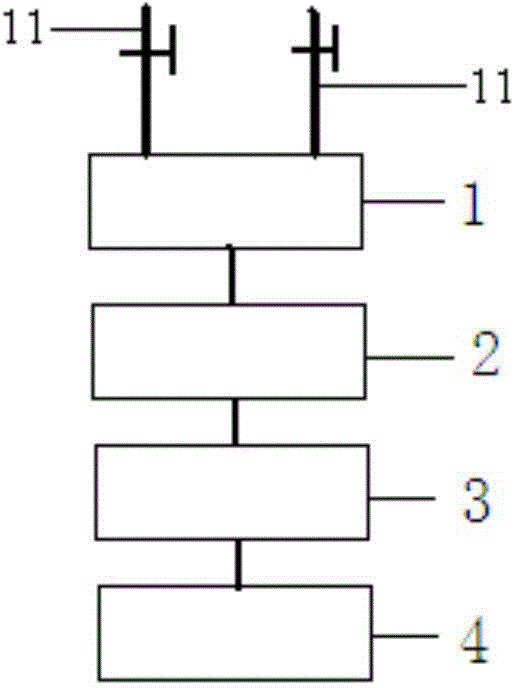
[0052] As industrialized production, in the production system (as shown in Figure 1), the production is magn...
Embodiment 34
[0054] Example 3 Preparation of 4-piperidone rifamycin S
[0055] Add 200ml of tetrahydrofuran, 6.6g of ammonium acetate, and 6.6g of zinc powder into the reactor, stir at 10°C-20°C for 10 minutes, slowly add 8.4g (84.50mmol) of 4-piperidone, 3-amino-4-imine Rifamycin S30g (42.25mmol), tetrahydrofuran 200ml solution, after 120 minutes, react at 10°C-20°C for 10 hours, filter, add 300ml of dichloromethane, wash with 300ml of 15% hydrochloric acid aqueous solution, and then wash with 5% Wash with 300ml of sodium carbonate aqueous solution, and finally wash with 300ml of water, dry over sodium sulfate, concentrate to dryness in vacuo, recrystallize from n-hexane to obtain 28.5g of 4-piperidone rifamycin S, the calculated yield is 85.3%, and the content detected by HPLC is 98.8 %.
[0056] The chemical reaction is as follows:
[0057]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com