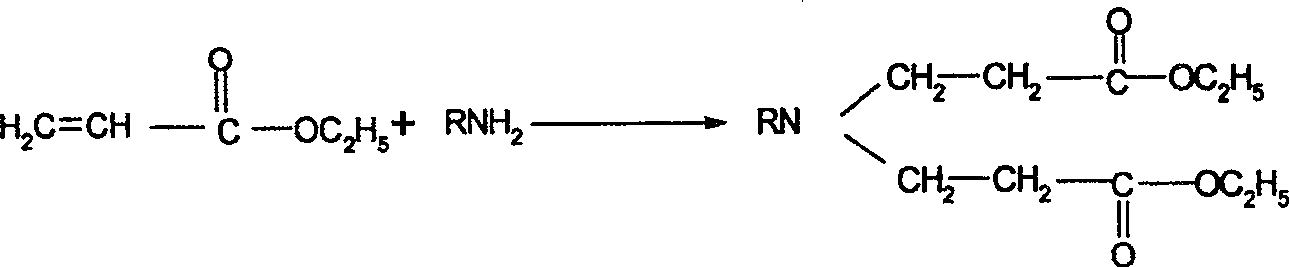
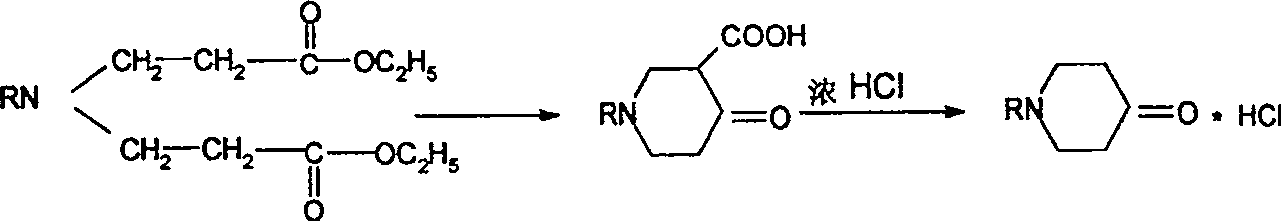
Synthesis process of N-sustituent-4-piperidyl alcohol
A synthesis method and a substituent technology are applied in the field of preparation of N-substituent-4-piperidinol, can solve the problems of high equipment requirements, increased production cost, many reaction steps, etc., and can reduce production costs and save production costs. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Add 200g of ethyl acrylate (containing a polymerization inhibitor) and 100ml of triethylamine into the reaction flask, heat up to 70°C, feed dry methylamine gas under constant temperature stirring, ventilate for 3 hours, stir for a while, and end reaction. Recover low boilers by distillation under reduced pressure, steam to an internal temperature of 120°C / 3mmHg, there is no distillate, cool to room temperature, take a sample for GC analysis, the diester content is 92.3%, and orange liquid N,N-bis(β-acrylic acid Ethyl ester) methylamine 212.4g, yield 84.6%.
[0027] (2) Add 5ml of ethanol and 3.5g of sodium into the reaction flask, add 70ml of toluene after 10 minutes of reaction, heat to reflux, and slowly drop the mixture of 25g of diester and 30ml of toluene prepared above into the reaction system under stirring During the reaction process, the mixture of toluene and ethanol is continuously separated by a water separator, and the reaction is terminated when the ...
Embodiment 2
[0030] (1) Add 200g of ethyl acrylate (containing polymerization inhibitor) and 100ml of 1,4-dioxane into the reaction flask, heat up to 70°C, slowly add 36g of ethylamine dropwise under stirring, and the dropwise addition is completed Then keep stirring for 1 h to end the reaction. Low boilers were recovered by distillation under reduced pressure, and steamed to an internal temperature of 120°C / 3mmHg. There was no distillate, cooled to room temperature, sampled for GC analysis, the diester content was 90.6%, and 173.4 g of orange liquid N,N-bis(β-ethyl acrylate)ethylamine was obtained, with a yield of 80.3%.
[0031] (2) Add 5ml of methanol and 3.5g of sodium into the reaction flask, add 70ml of toluene after 10 minutes of reaction, heat to reflux, and slowly drop the mixture of 27g of diester and 30ml of toluene prepared above into the reaction system under stirring In the process, after the dropwise addition of the mixed solution is completed, keep warm and reflux. During ...
Embodiment 3
[0034] (1) Add 200g of ethyl acrylate (containing a polymerization inhibitor) and 100ml of tetrahydrofuran into the reaction bottle and heat up to 60°C. Slowly add 85.6g of benzylamine dropwise while stirring. After the dropwise addition, keep stirring for 1 hour to end the reaction . Recover low boilers by distillation under reduced pressure, and steam to an internal temperature of 130°C / 3mmHg. There was no distillate, cooled to room temperature, sampled for GC analysis, the diester content was 90.8%, and 238.0 g of orange liquid N,N-bis(β-ethyl acrylate) benzylamine was obtained, with a yield of 88.2%.
[0035] (2) Add 5ml of ethanol and 3.5g of sodium into the reaction flask, add 70ml of benzene after reacting for 10min, heat to reflux, slowly add the mixed solution of 34g of diester and 30ml of benzene into the reaction system dropwise under stirring, when After the dropwise addition of the mixed solution is completed, heat-preserve and reflux. During the reaction process...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com