Preparation method of homopiperazine and derivative thereof
A technology for homopiperazine and derivatives, which is applied in the field of compound preparation, can solve the problems of high consumption of raw materials, many by-products, serious equipment corrosion, etc., and achieves the effects of reducing production costs, mild reaction conditions and easy control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
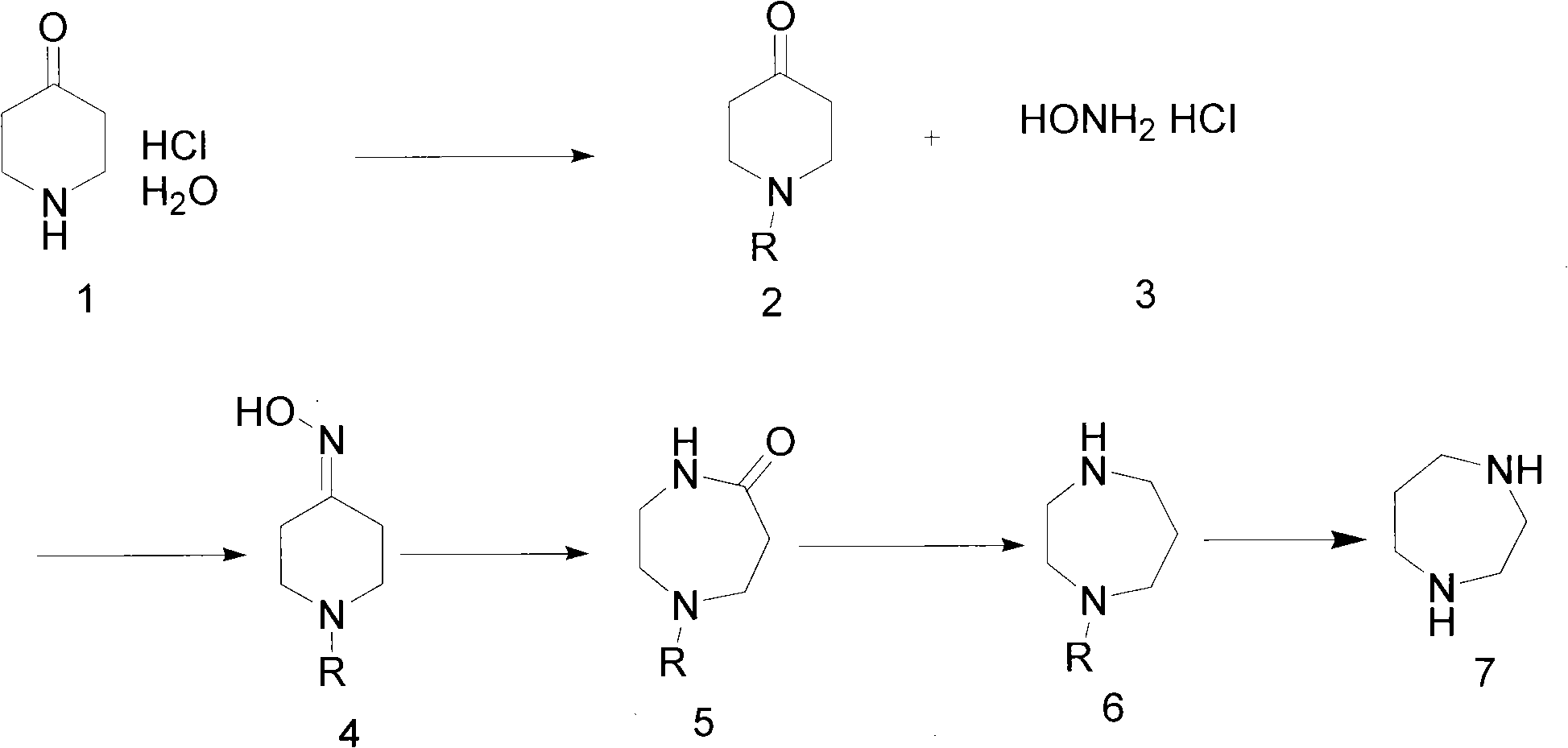
[0028] Example 1 Preparation of 1-benzyloxycarbonyl-4-piperidone, refer to literature ("PROTECTIVE GROUPS IN ORGANIC SYNTHESIS" written by GREENE T.W., translated by the Organic Teaching and Research Group of East China University of Science and Technology, first edition in October 2004 by East China University of Science and Technology Press Chapter 7 pp. 494-653)
[0029] Combine 4-piperidone hydrochloride hydrate (7.68 kg) and potassium carbonate (20.7 kg), at 0°C, add water (50 liters), stir well, add dichloromethane (40 liters), and then dropwise add benzene A solution of methoxycarbonylsuccinimide (12.4 kg) in dichloromethane (80 liters) was reacted at room temperature for two hours. The reaction solution was separated, and the aqueous layer was extracted with dichloromethane (2×15 L). The organic phases were combined, dried over anhydrous sodium sulfate, and concentrated to obtain 11.5 kg of 1-benzyloxycarbonyl-4-piperidone as a colorless oil.
Embodiment 2
[0030] Embodiment 2 prepares 1-benzyloxycarbonyl-4-piperidone oxime
[0031] Dissolve the 1-benzyloxycarbonyl-4-piperidone oxime (7.2 kg) prepared in Example 1 with about ethanol (40 liters), add potassium carbonate (6.4 kg) and water (20 liters) solution, drop at 0°C Add a solution of hydroxylamine hydrochloride (2.57 kg) in water (20 L), and stir at room temperature for 3 hours. Concentrate ethanol under reduced pressure, add ethyl acetate to extract (4×15 L), combine organic phases, dry and concentrate to obtain white solid 1-benzyloxycarbonyl-4-piperidone oxime.
Embodiment 3
[0032] Embodiment 3 prepares 1-benzyloxycarbonyl-5-carbonyl homopiperazine
[0033] Dissolve 1-benzyloxycarbonyl-4-piperidone oxime (4.2 kg) and p-toluenesulfonyl chloride (3.55 kg) in 50 liters of acetone prepared in Example 2, and slowly add aqueous sodium hydroxide solution (7.4 kg) dropwise at 0°C. liters, 2.5 equivalents), reacted at 65°C for 3 hours. Concentrate the acetone under reduced pressure, add 20 liters of water, extract with ethyl acetate (4×15 liters), combine the organic layers, dry and concentrate to obtain 1-benzyloxycarbonyl-5-carbonylhomopiperazine as a white solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com