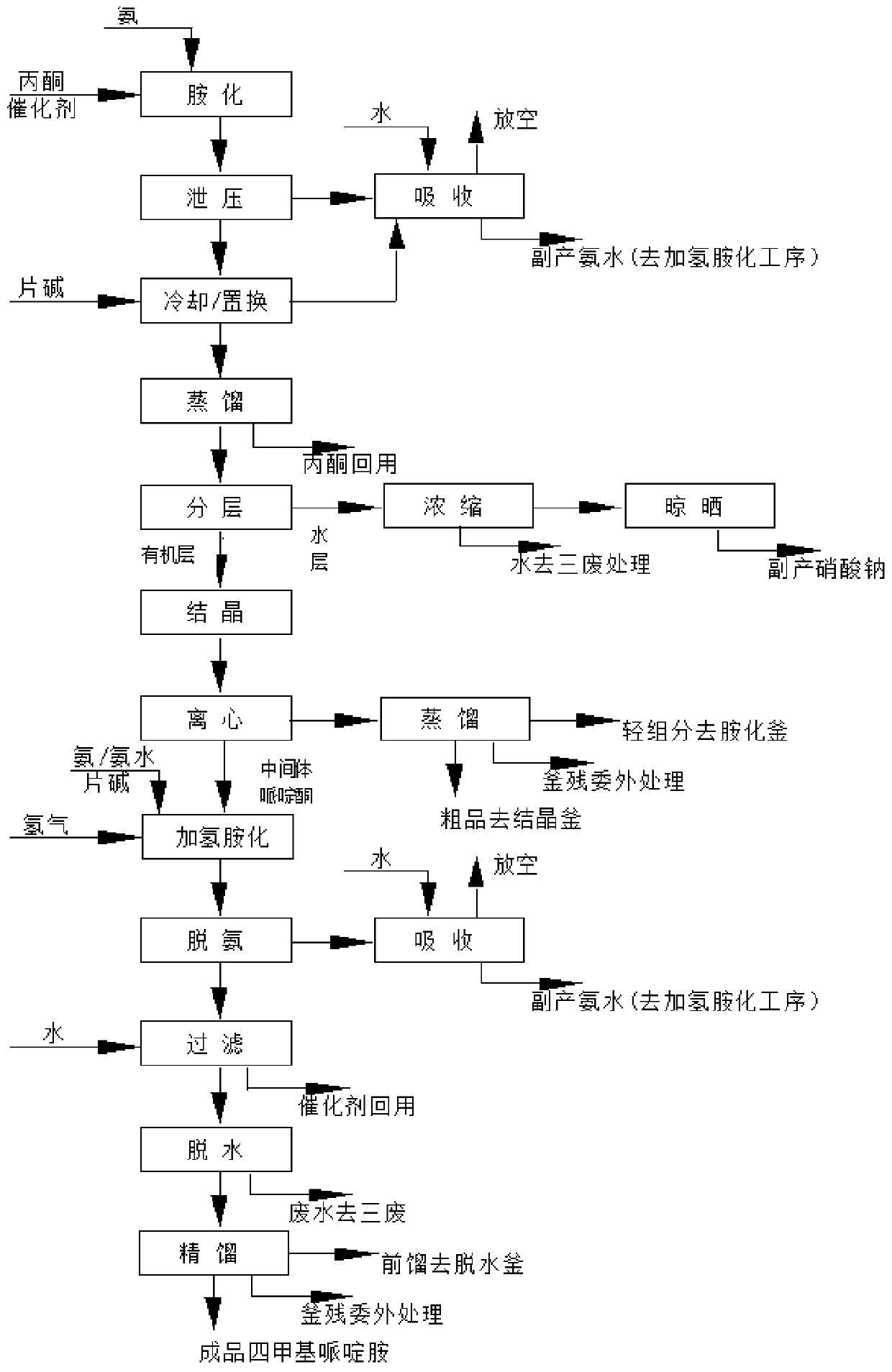
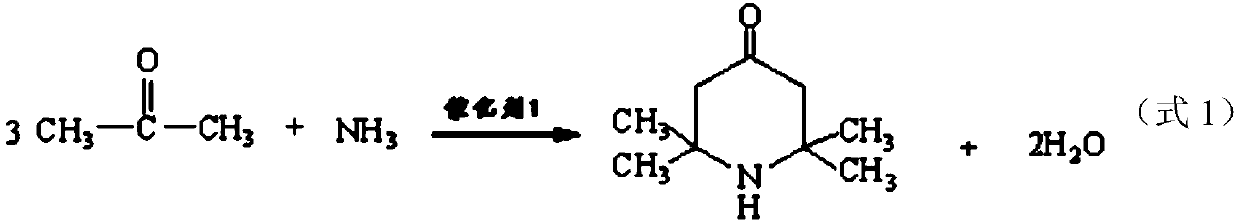
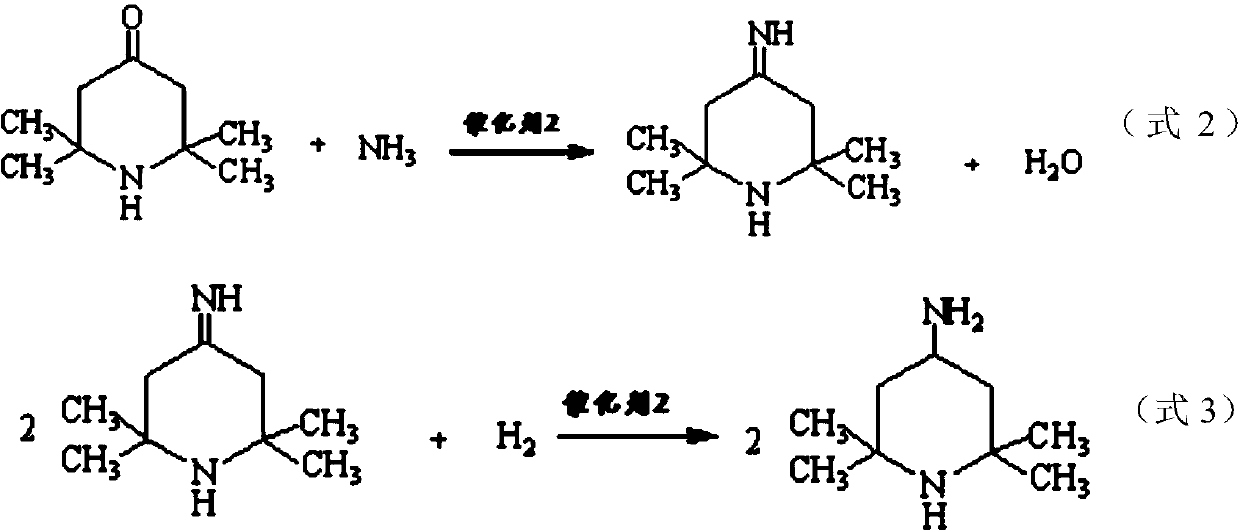
Method for preparing intermediate 2,2,6,6-tetramethyl-4-piperidylamine
A technology of tetramethylpiperidine amine and tetramethylpiperidine imine is applied in the field of preparation of pharmaceutical and plastic additive intermediates, and can solve the problem of low raw material reuse rate, low acetone conversion rate, easy deactivation of catalysts, etc. problems, to reduce the risk of inactivation or poisoning, prolong the service life and the number of cycles, and shorten the response time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: Preparation of nickel-skeleton catalyst modified by sodium hydroxyphenyl phosphate
[0049] 1) In a 1L three-necked flask equipped with a stirring paddle, add 600ml of 10% sodium hydroxide aqueous solution, heat to 90°C, and under stirring, add 40g of nickel-aluminum-zinc (Ni3Al2Zn1) alloy in batches. The speed of addition should keep the temperature of the solution between 90 and 95°C, and the addition should be completed within about 20 to 30 minutes. Stirring was continued for another 1 hour. Let it stand, let the nickel sink, discard the supernatant, and then wash five times by decantation, each time using 200ml. Then wash five times with ethanol, 50ml each time. During the washing process, the nickel powder should always be covered by the liquid, and the catalyst should not be in contact with the air. The prepared catalyst should be stored in absolute ethanol for subsequent use;
[0050] 2) Take 50g of the skeleton nickel catalyst prepared above, ...
Embodiment 2
[0051] Example 2: Preparation of 2,2,6,6-tetramethylpiperidinium amine using a modified nickel skeleton catalyst. Add 100 L of acetone and 500 g of ammonium nitrate into a 300 L amination kettle, then start stirring and raise the temperature to 50 ° C, and start to add amine Ammonia gas is fed into the kettle at a rate of about 1kg / min. The reaction temperature of the amination process is controlled at 50-70° C., and the pressure is 0.2 MPa. After the completion of the ammonia flow, keep the reaction for 2 hours to complete the reaction, and then cool down to 30°C to release the pressure, and at the same time absorb ammonia with water to produce by-product ammonia water. Add 5g caustic soda in still, to destroy catalyst ammonium nitrate. After stirring for 30 minutes, the temperature was lowered to normal temperature and transferred to a still, and the reaction liquid was absorbed and treated for the ammonia in the system before being transferred into the still.
[0052] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com