Micro-pore zeolite, preparation method and application thereof
A technology of microporous zeolite and zeolite, applied in chemical instruments and methods, other chemical processes, inorganic chemistry, etc., to achieve good technical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Sodium aluminate (containing 42.0% by weight Al 2 o 3 ) 6.1 grams and 2.0 grams of sodium hydroxide are dissolved in 270 grams of water, then add 34.7 grams of hexamethyleneimine under the situation of stirring, then add 150 grams of silica sol (containing 40% by weight SiO 2 ), dimethyldichlorosilane 6.5 grams, the material proportion (mol ratio) of reactant is:
[0024] SiO 2 / Al 2 o 3 =40
[0025] NaOH / SiO 2 =0.05
[0026] Dimethyldichlorosilane / SiO 2 =0.05
[0027] Hexamethyleneimine / SiO 2 =0.35
[0028] h 2 O / SiO 2 =20
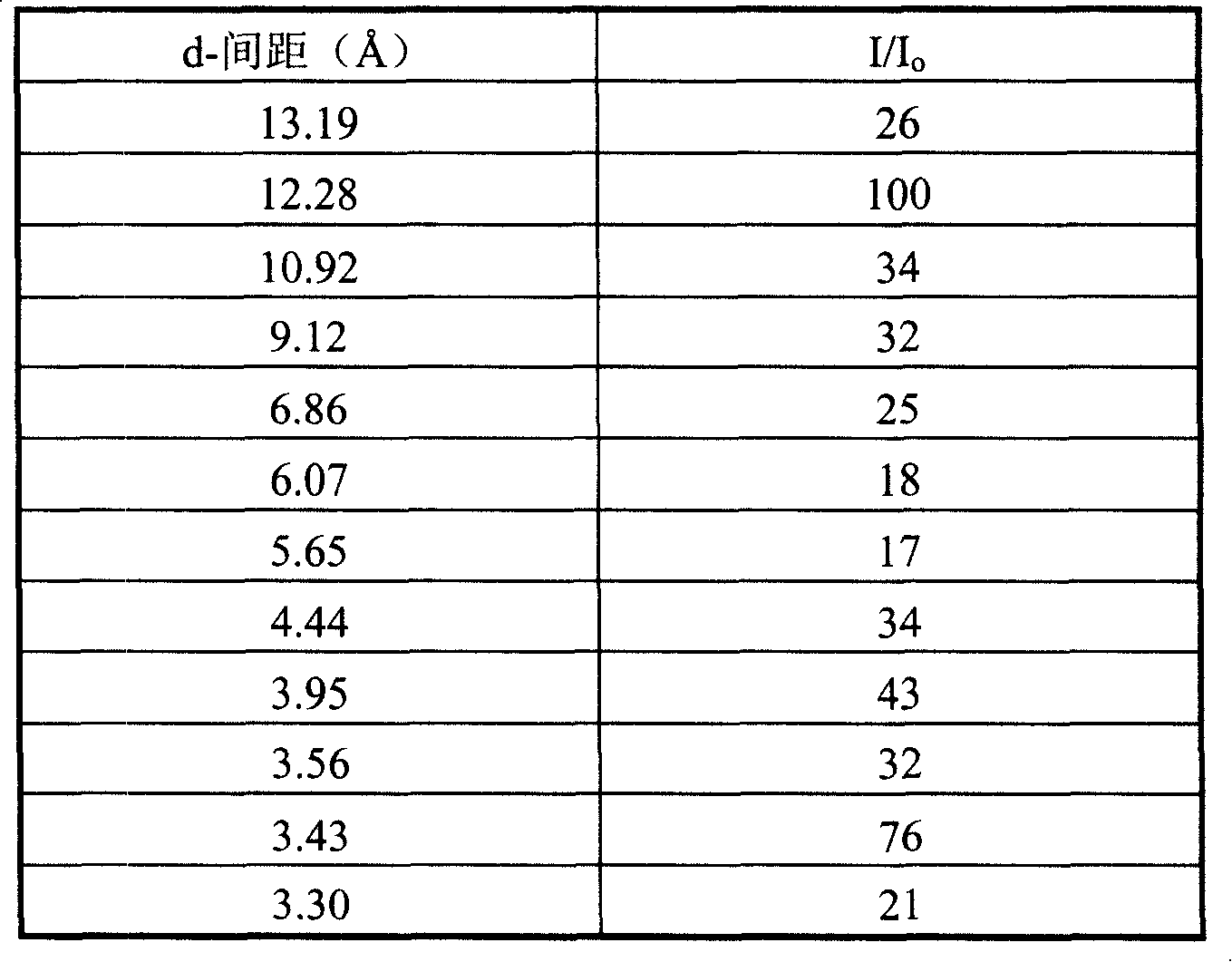
[0029] After the reaction mixture was stirred evenly, it was put into a stainless steel reaction kettle, and crystallized at 150° C. for 55 hours while stirring. After taking out, it is filtered, washed and dried. SiO was obtained by chemical analysis 2 / Al 2 o 3 The molar ratio was 42.3.
[0030]After drying the sample was determined, its Si 29 The NMR solid-state nuclear magnetic spectrum peak appears at -18.4ppm. Its X-ray d...
Embodiment 2
[0034] Sodium aluminate (containing 42.0% by weight Al 2 o 3 ) 6.1 grams and 2.0 grams of sodium hydroxide are dissolved in 180 grams of water, then add 6.6 grams of trimethylchlorosilane under the situation of stirring, hexahydropyridine 43.0 grams, then add 150 grams of silica sol (containing 40% by weight SiO 2 ), the material ratio (molar ratio) of reactant is:
[0035] SiO 2 / Al 2 o 3 =40
[0036] NaOH / SiO 2 =0.05
[0037] Chlorotrimethylsilane / SiO 2 =0.06
[0038] Hexahydropyridine / SiO 2 =0.50
[0039] h 2 O / SiO 2 =15
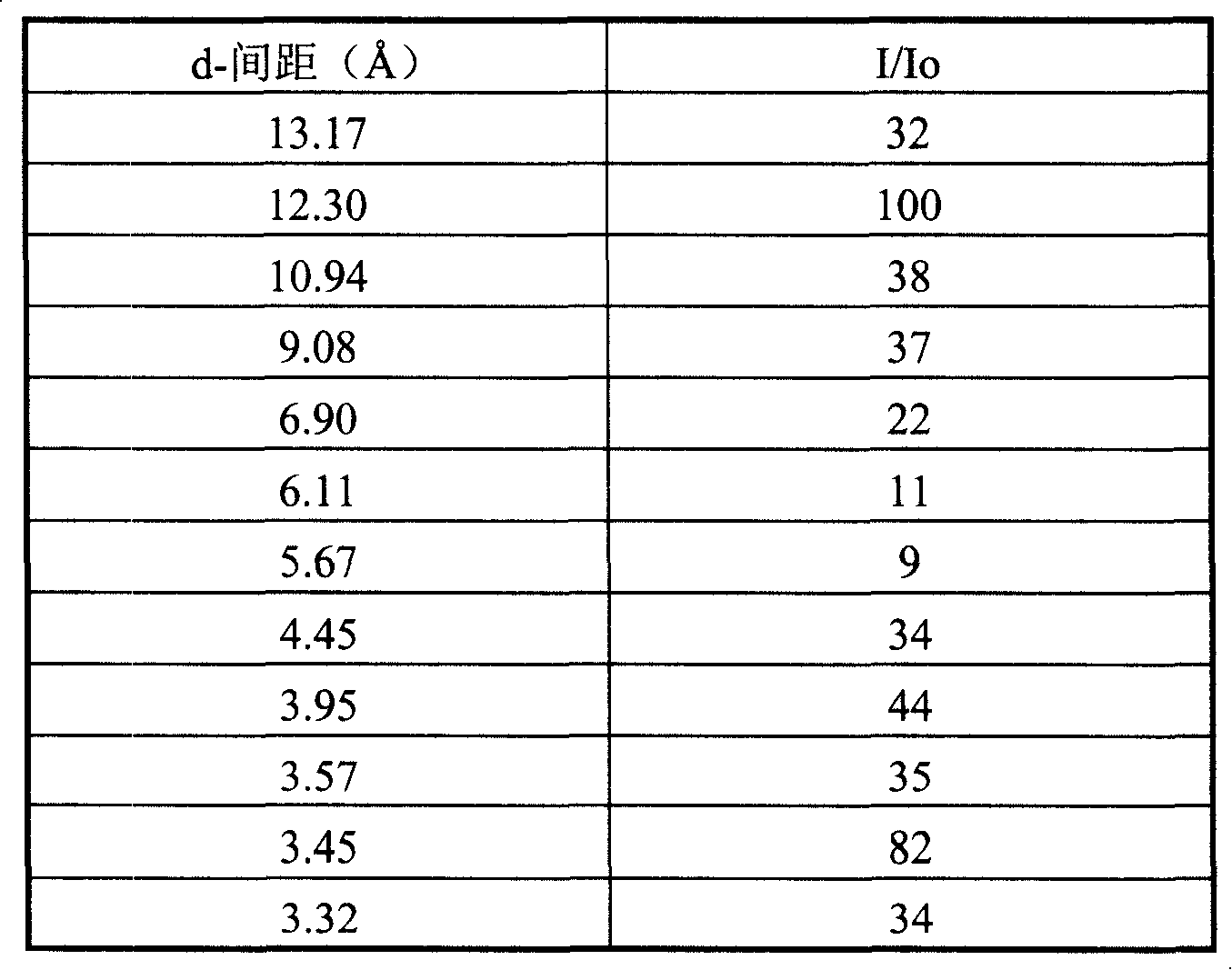
[0040] After the reaction mixture was stirred evenly, it was put into a stainless steel reaction kettle, and crystallized at 152° C. for 50 hours while stirring. After taking out, it is filtered, washed and dried. SiO was obtained by chemical analysis 2 / Al 2 o 3 The molar ratio was 42.1.
[0041] After drying the sample was determined, its Si 29 The NMR solid-state nuclear magnetic spectrum peak appears at 15.2ppm. Its X-ray diffract...
Embodiment 3
[0045] Dissolve 5.1 grams of aluminum oxide and 2.0 grams of sodium hydroxide in 540 grams of water, then add 42.5 grams of hexamethyleneimine while stirring, then add 60 grams of solid silicon oxide and 3.2 grams of hexamethyldisilazane , the material ratio (molar ratio) of reactant is:
[0046] SiO 2 / Al 2 o 3 =20
[0047] NaOH / SiO 2 =0.05
[0048] Hexamethyldisilazane / SiO 2 =0.04
[0049] Hexamethyleneimine / SiO 2 =0.5
[0050] h 2 O / SiO 2 =30
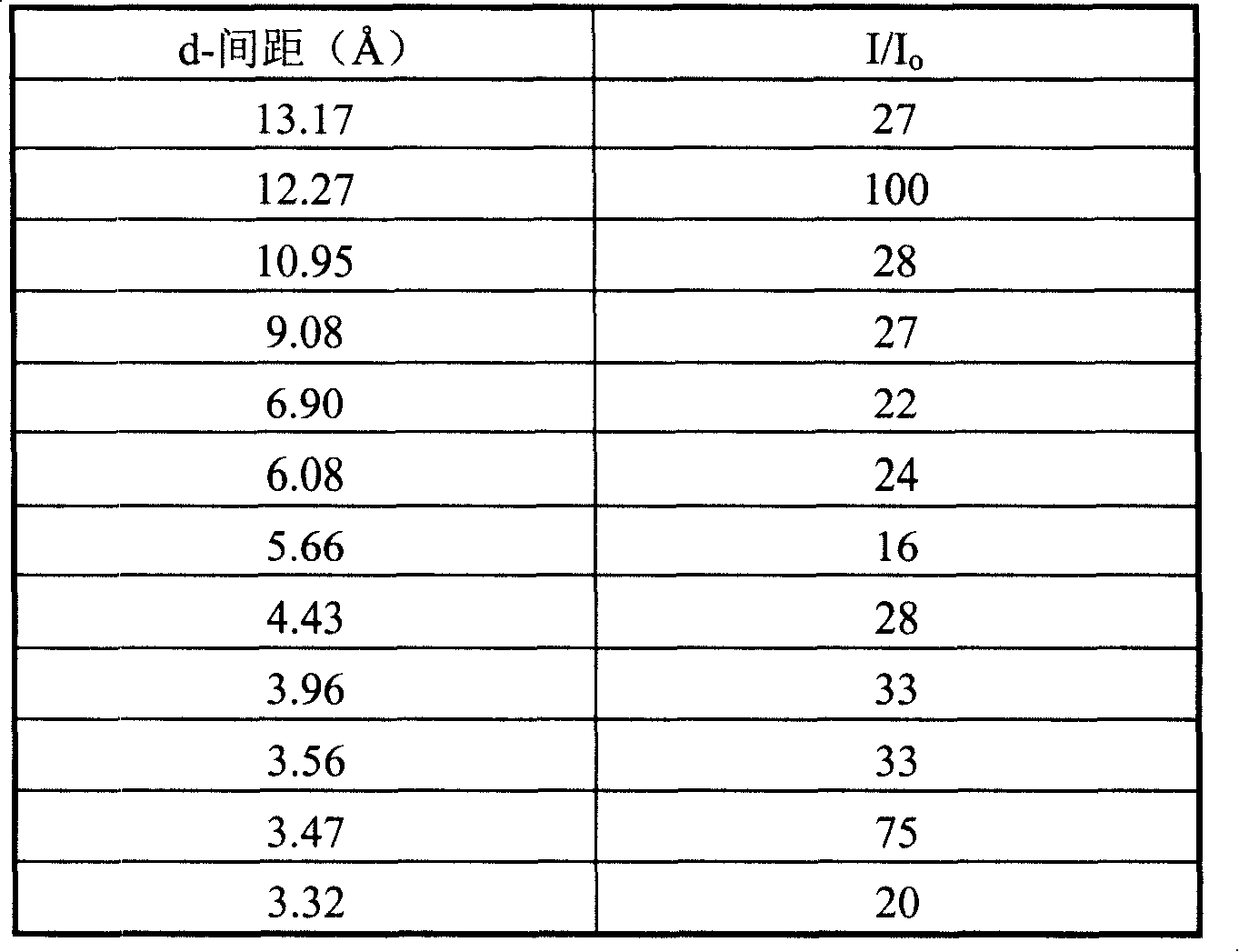
[0051] After the reaction mixture was stirred evenly, it was put into a stainless steel reaction kettle, and crystallized at 163° C. for 50 hours while stirring. After taking out, it is filtered, washed and dried. SiO was obtained by chemical analysis 2 / Al 2 o 3 The molar ratio was 17.7.
[0052] After drying the sample was determined, its Si 29 The NMR solid-state nuclear magnetic spectrum peak appears at 15.8ppm. Its X-ray diffraction data are shown in Table 3.
[0053] table 3
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com