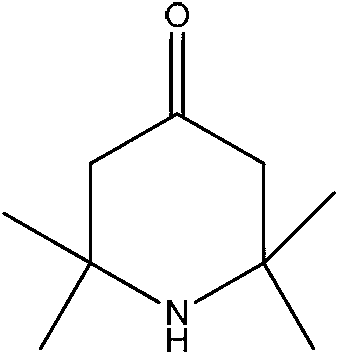
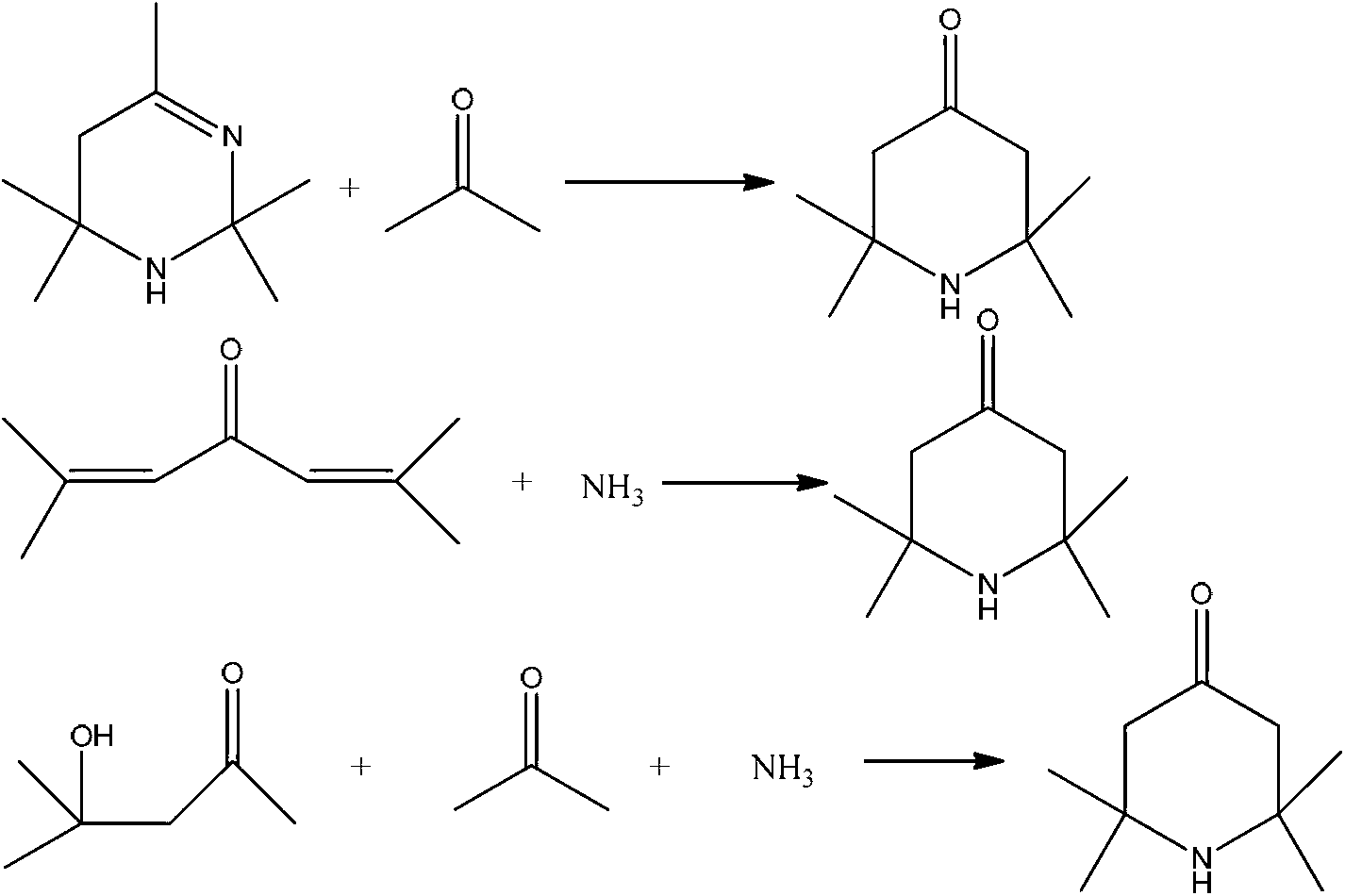
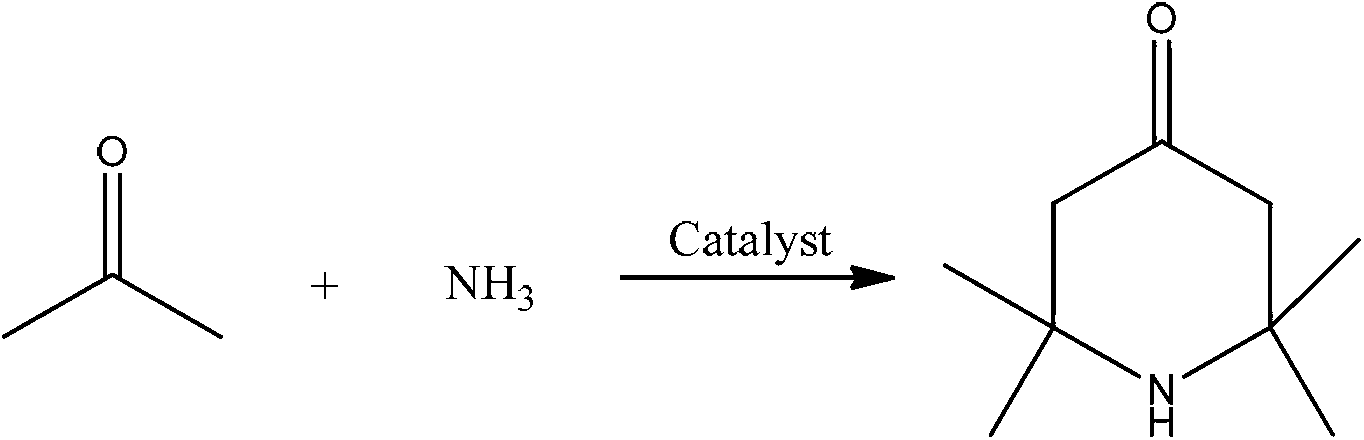
2,2,6,6,-tetramethyl-4-piperidone continuous synthesis method
A synthesis method and piperidone technology are applied in the field of synthesis of 2,2,6,6-tetramethyl-4-piperidone, which can solve the problems of increasing production cost, reducing selectivity and high energy consumption, and achieving The effect of avoiding pollution, low cost and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A kind of 2,2,6,6-tetramethyl-4-piperidone continuous synthesis method, comprises the steps:
[0025] (1) The reactor is a fixed-bed single-tubular reactor with an inner diameter of 14mm and a tube length of 650mm. It is filled with 40mL of sulfonic acid resin D061, and heated to keep the temperature of the fixed-bed single-tubular reactor at 60°C;
[0026] (2) Acetone and ammonia gas are passed into the reactor from the upper end of the fixed-bed single-tubular reactor through the preheater according to the molar ratio of 6:1, and the volume space velocity of acetone is 0.39h -1 , the volumetric space velocity of ammonia gas is 20.7h -1 ;
[0027] (3) The reaction product flows out from the lower end of the fixed-bed single-tubular reactor, and the effluent is cooled and gas-liquid separated to obtain the crude product of 2,2,6,6-tetramethyl-4-piperidone. After gas chromatography analysis, the selectivity was 65%.
Embodiment 2
[0029] A kind of 2,2,6,6-tetramethyl-4-piperidone continuous synthesis method, comprises the steps:
[0030] (1) The reactor is a fixed-bed single-tubular reactor with an inner diameter of 14mm and a tube length of 650mm. It is filled with 40mL of sulfonic acid resin D072 and heated to keep the temperature of the fixed-bed single-tubular reactor at 50°C;
[0031] (2) Pass acetone and ammonia gas into the reactor from the upper end of the fixed-bed single-tubular reactor through the preheater according to the molar ratio of 9:1, and the volumetric space velocity of acetone is 0.15h -1 , the volumetric space velocity of ammonia gas is 5.25h -1 ;
[0032] (3) The reaction product flows out from the lower end of the fixed-bed single-tubular reactor, and the effluent is cooled and gas-liquid separated to obtain the crude product of 2,2,6,6-tetramethyl-4-piperidone. After gas chromatography analysis, the selectivity was 60%.
Embodiment 3
[0034] A kind of 2,2,6,6-tetramethyl-4-piperidone continuous synthesis method, comprises the steps:
[0035] (1) The reactor is a fixed-bed tubular reactor with an inner diameter of 14 mm and a tube length of 650 mm. It is filled with 40 mL of sulfonic acid resin NKC-9, and heated to keep the temperature of the fixed-bed single-tubular reactor at 70 ° C;
[0036] (2) According to the molar ratio of 3:1, acetone and ammonia are passed into the reactor from the upper end of the fixed-bed single-tubular reactor through the preheater respectively, and the volumetric space velocity of acetone is 1.17h -1 , the volume space velocity of ammonia gas is 124.2h -1 ;
[0037] (3) The reaction product flows out from the lower end of the fixed-bed single-tubular reactor, and the effluent is cooled and gas-liquid separated to obtain the crude product of 2,2,6,6-tetramethyl-4-piperidone. After gas chromatography analysis, the selectivity was 54%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com