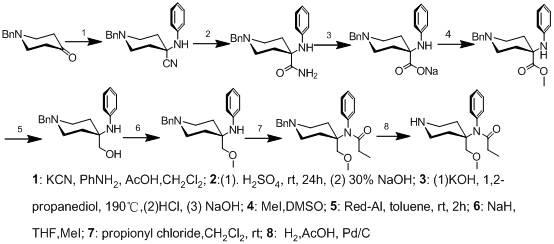
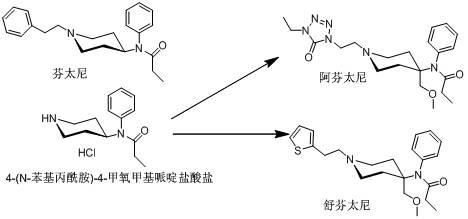
Method for preparing 4-(N-phenylpropionamide)-4-methoxymethyl-piperidine hydrochloride
A technology of methoxymethylpiperidine and phenylpropanamide, which is applied in the field of preparation of 4--4-methoxymethylpiperidine hydrochloride, can solve the problems of unfavorable long-term storage and transportation, large discharge of three wastes, Resolve problems such as low reaction purity, and achieve the effects of facilitating storage and transportation, reducing three waste pollution, and improving purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Acetic acid (1600g), 1-phenyl-4-piperidone (500g, 2.64mol) and aniline (270.6g, 2.91mol) were added to a 5L reaction flask, and the solution was an orange-yellow clear liquid. After cooling in an ice bath to below 30°C, trimethylcyanosilane (275 g, 2.78 mol) was added dropwise, and the dropping temperature was controlled below 40°C. After the dropwise reaction was stirred at room temperature for 2 hours, TLC (petroleum ether: ethyl acetate = 3: 1) monitored the complete reaction of the raw material 1-phenyl-4-piperidone. Pour ammonia water into the reaction bottle, adjust the pH to around 9, and a large amount of white solids are precipitated. After standing for half an hour, the solid was collected by filtration, rinsed with 300ml of water, and dried to obtain 720g of off-white solid (I), with a yield of 93.5% (GC purity 98.5%, largest single impurity 0.3%). The product was directly put into the next step reaction without further purification.
[0031] Pre...
Embodiment 2
[0032] Example 2: Compound (I) (500g, 1.72mol) was added to a 3L reaction flask, concentrated H 2 SO 4 (4kg), control the temperature between 20-30°C, react at room temperature for 20h, TLC (petroleum ether: ethyl acetate = 3: 1) to monitor the complete reaction of raw materials, add ice cubes (3kg), a large amount of heat release, stir for 30min Afterwards, a white solid precipitated out. After cooling to 10°C, filter, and dissolve the collected white solid in a mixed solution (3.5 L) of isopropanol: water = 1: 8, and pour ammonia water to adjust the pH to about 9. After standing for 1 hour to cool to room temperature, filter and rinse with 150ml of water, and dry to obtain 490g of off-white solid (II), with a yield of 92.5% (GC purity 99.4%, largest single impurity 0.1%). Compound (II) does not need to be processed again, and is directly dropped into the next step reaction.
[0033] The present embodiment replaces the concentrated sulfuric acid with hydrochloric acid or p...
Embodiment 3
[0035] Example 3: Add compound (II) (450g, 1.46mol), 1,2-propanediol (1.55kg), potassium hydroxide (326g, 5.82mol) into the reaction flask, heat and control the temperature at 170°C for 12h, TLC (Ethyl acetate) After monitoring the complete reaction of raw materials, add 2000ml of water. Remove insoluble inorganic salts by filtration, adjust the pH of the aqueous layer to 5-6 with about 700ml of concentrated HCl, cool to 10°C, and let it stand for about 2 hours, a large amount of off-white solids precipitated. The off-white solid was collected by filtration and dried to obtain 388 g of product (Ⅲ), with a yield of 86% (HPLC purity 98.6%, maximum single impurity 0.8%). The white solid was directly put into the next reaction without further purification.
[0036] Present embodiment replaces potassium hydroxide with sodium hydroxide, replaces propylene glycol with ethylene glycol, all can obtain same result.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com