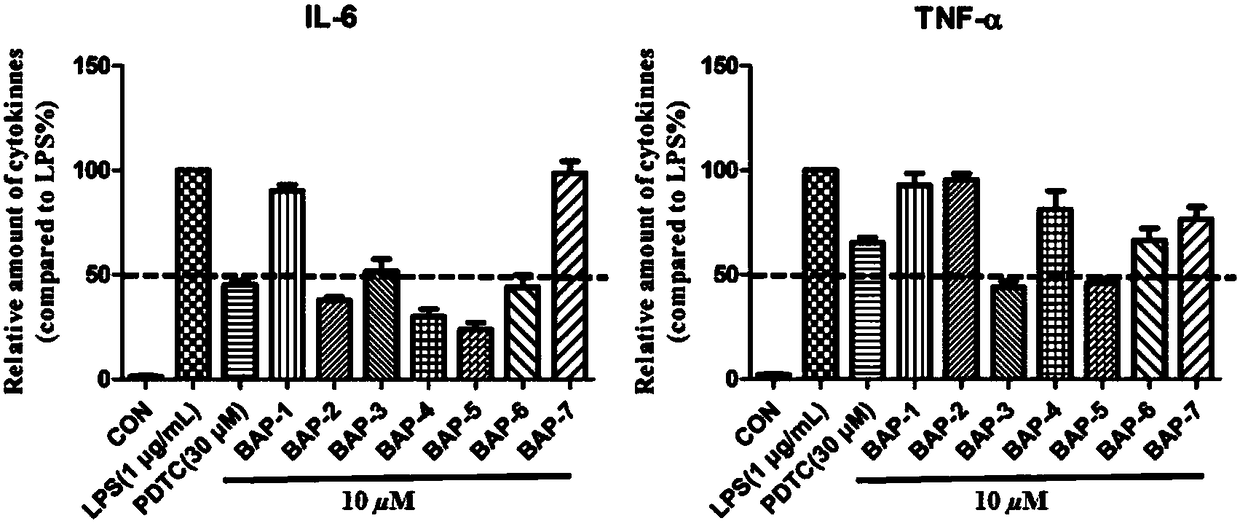
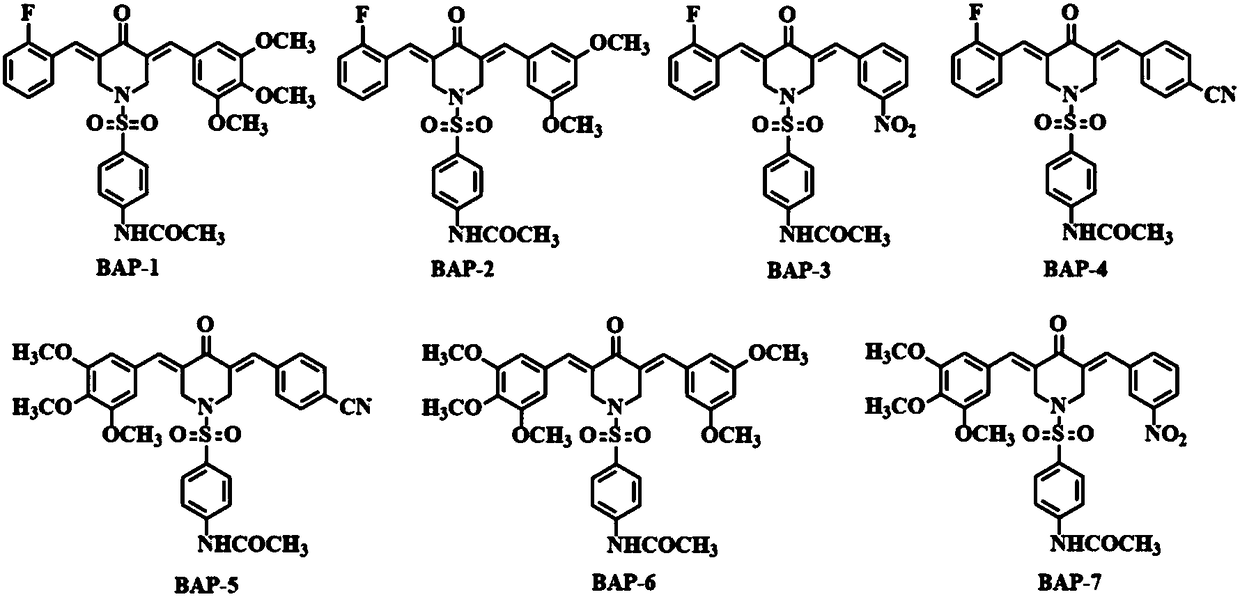
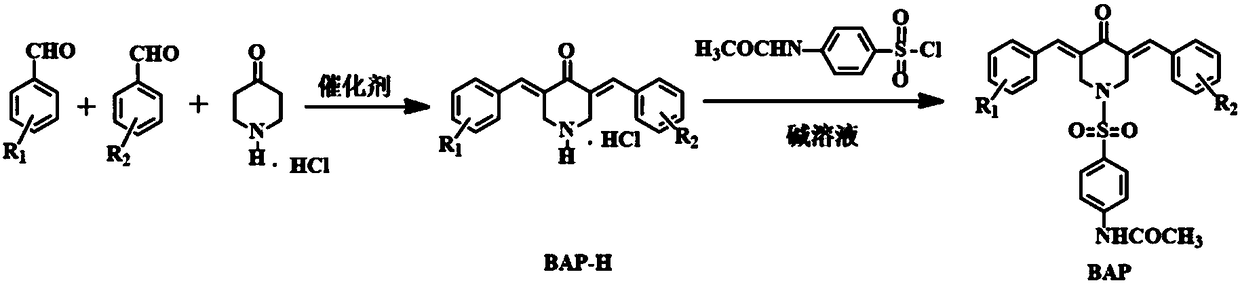
4-acetylamino benzene sulfonyl substituted 3,5-bis(arylidene)-4-piperidinone compounds and preparation method thereof
An acetamidobenzenesulfonyl and diarylmethylene technology is applied in the field of anti-tumor and anti-inflammatory drugs and their preparation, and can solve the problem of less anti-inflammatory activity, less compound reports, structure-activity relationship, and anti-tumor and anti-inflammatory activities Lack of systemicity and other problems, to achieve the effects of mild reaction conditions, high synthesis yield, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 3-(3,4,5-trimethoxybenzylidene)-5-(2-fluorobenzylidene)-N-(4-acetylaminobenzenesulfonyl)-4-piperidone (BAP- 1) Synthesis of
[0031] Mix 0.01mol of 4-piperidone hydrochloride with 0.01mol of 2-fluorobenzaldehyde and 0.01mol of 3,4,5-trimethoxybenzaldehyde in 10mL of acetic acid, and continuously feed dry hydrogen chloride gas for 45min , stirred at room temperature for 15 hours, and determined the end point of the reaction by thin layer chromatography (TLC). After the reaction, the precipitate was suction filtered, the precipitate was dissolved in water and the pH value was adjusted to neutrality with sodium hydroxide solution, and the resulting precipitate was chromatographed on a 200-300 mesh silica gel column (eluent: petroleum ether / ethyl acetate / methanol=10: 10:1) to obtain a yellow solid, namely the intermediate BAP-H (1), then, the intermediate BAP-H (1) and 4-acetylaminobenzenesulfonyl chloride were dissolved in 10mL of dichloromethane, and 3-5 drops of Pyridi...
Embodiment 2
[0034] 3-(3,5-dimethoxybenzylidene)-5-(2-fluorobenzylidene)-N-(4-acetylaminobenzenesulfonyl)-4-piperidone (BAP-2 ),Synthesis
[0035] Mix 0.01mol of 4-piperidone hydrochloride with 0.01mol of 2-fluorobenzaldehyde and 0.01mol of 3,5-dimethoxybenzaldehyde in 10mL of acetic acid, and continuously feed dry hydrogen chloride gas for 45min. The reaction was stirred at room temperature for 15 hours, and the end point of the reaction was determined by thin layer chromatography (TLC). After the reaction, the precipitate was suction filtered, the precipitate was dissolved in water and the pH value was adjusted to neutral with sodium hydroxide solution, and the obtained precipitate was chromatographed on a 200-300 mesh silica gel column (eluent: petroleum ether / ethyl acetate = 2:1) Obtain a yellow solid, namely the intermediate BAP-H (2), then, dissolve the intermediate BAP-H (2) and 4-acetylaminobenzenesulfonyl chloride in 10 mL of dichloromethane, add 3-5 drops of pyridine, and stir a...
Embodiment 3
[0038] Synthesis of 3-(3-nitrobenzylidene)-5-(2-fluorobenzylidene)-N-(4-acetylaminobenzenesulfonyl)-4-piperidinone (BAP-3)
[0039] Mix 0.01mol of 4-piperidone hydrochloride with 0.01mol of 2-fluorobenzaldehyde and 0.01mol of 3-nitrobenzaldehyde in a solution of 15mL of methanol and water, add dropwise 2-3mL of 20% sodium hydroxide The solution was stirred and reacted at 40° C. for 6 hours, and the end point of the reaction was determined by thin layer chromatography (TLC). After the reaction, the precipitate was suction filtered, and the resulting precipitate was chromatographed on a 200-300 mesh silica gel column (eluent: petroleum ether / ethyl acetate = 1:1) to obtain a yellow solid, which is the intermediate BAP-H (3). Then, Dissolve the intermediate BAP-H(3) and 4-acetylaminobenzenesulfonyl chloride in 10 mL of dichloromethane, add 3-5 drops of pyridine, stir overnight at room temperature, and determine the end point of the reaction by thin layer chromatography (TLC). The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com