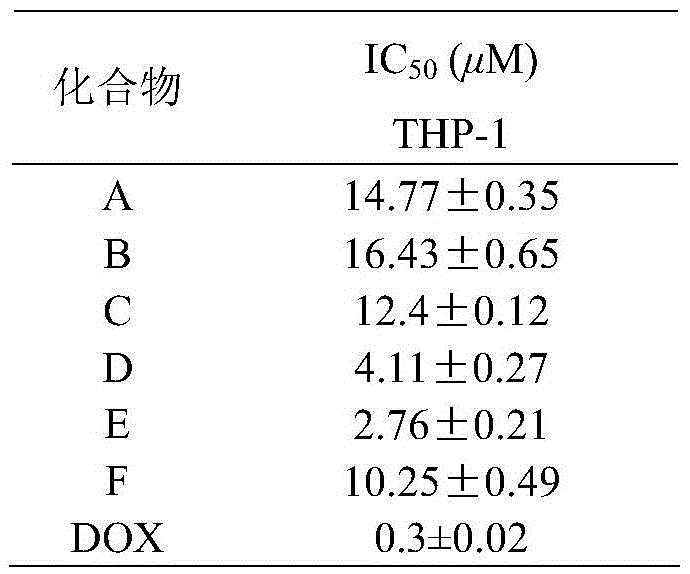
Tert-butyl substituted asymmetrical piperidone compounds with anti-tumor activities and preparation method thereof
A technology of anti-tumor activity and piperidone, which is applied in anti-tumor drugs, organic chemistry, drug combination, etc., can solve the problems of few reports on asymmetric derivatives, and achieve genotoxicity avoidance, low toxicity, and high synthesis yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
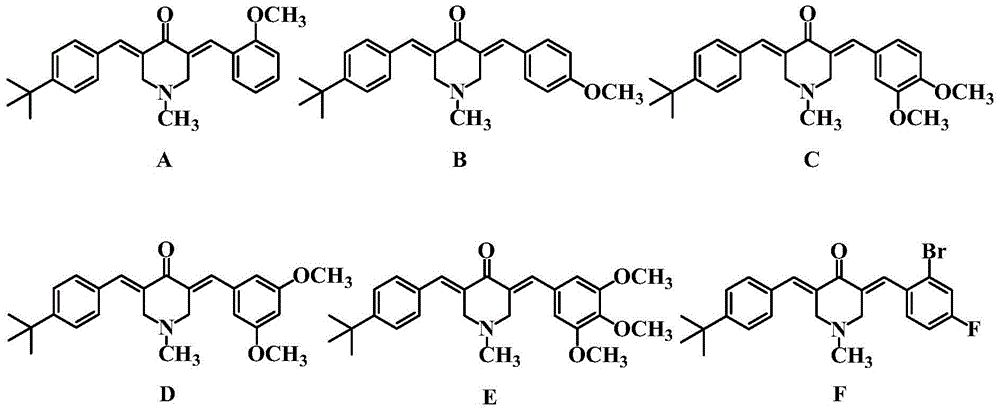
[0024] Synthesis of 3-(4-tert-butylbenzylidene)-5-(2-methoxybenzylidene)-4-piperidone (A)
[0025] Mix 0.01mol of N-methyl-4-piperidone with 0.01mol of 4-tert-butylbenzaldehyde and 0.01mol of 2-methoxybenzaldehyde in a solution of 25mL of ethanol and water, and add dropwise at room temperature 15 mL of 20% sodium hydroxide solution was stirred at room temperature for 4 h, and the end point of the reaction was determined by thin-layer chromatography (TLC) analysis. After the reaction, the obtained oil was chromatographed on a 200-300 mesh silica gel column (eluent: petroleum ether / ethyl acetate = 3:1) to obtain a yellow oil, which was the inventive product 3-(4-tert-butylbenzylidene yl)-5-(2-methoxybenzylidene)-4-piperidone (A), 2.23 g, the yield is 61%.
[0026] 1 HNMR (400MHz, CDCl 3 )δ8.06(s, 1H, -C=CH), 7.80(s, 1H, -C=CH), 7.44(d, J=7.5Hz, 2H, -C 6 h 4 ),7.36(m,3H,-C 6 h 4 ),7.20(d,J=7.4Hz,1H,-C 6 h 4 ),6.97(t,J=7.6Hz,1H,-C 6 h 4 ),6.93(d,J=8.4Hz,1H,-C 6 h 4 ),...
Embodiment 2
[0028] Synthesis of 3-(4-tert-butylbenzylidene)-5-(4-methoxybenzylidene)-4-piperidone (B)
[0029] Mix 0.01mol of N-methyl-4-piperidone with 0.011mol of 4-tert-butylbenzaldehyde and 0.010mol of 4-methoxybenzaldehyde in a solution of 16mL of methanol and water, add dropwise at room temperature 15 mL of 10% potassium hydroxide solution was stirred at 40° C. for 3 h, and the end point of the reaction was determined by thin layer chromatography (TLC) analysis. After the reaction, the precipitate was filtered with suction, and the resulting precipitate was chromatographed on a 200-300 mesh silica gel column (eluent: petroleum ether / ethyl acetate = 4:1) to obtain a bright yellow powder, which is the inventive product 3-(4-tert-butyl Benzylidene)-5-(4-methoxybenzylidene)-4-piperidone (B), 2.32 g, yield 62%.
[0030] Mp:154~156℃.IR(cm -1 ):2954(s), 2783(m), 1675(m), 1604(s), 1505(s), 1456(s), 1253(s), 1166(s), 1031(s), 918(s) ),830(s). 1 HNMR (400MHz, CDCl 3 )δ7.78(d,2H,-C=CH),7....
Embodiment 3
[0032] Synthesis of 3-(4-tert-butylbenzylidene)-5-(3,4-dimethoxybenzylidene)-4-piperidone (C)
[0033] Mix 0.01mol of N-methyl-4-piperidone with 0.009mol of 4-tert-butylbenzaldehyde and 0.011mol of 3,4-dimethoxybenzaldehyde in 20mL of propanol and water solution, room temperature 15 mL of 10% sodium hydroxide solution was added dropwise, stirred at 50° C. for 3.5 h, and the end point of the reaction was determined by thin layer chromatography (TLC) analysis. After the reaction was completed, the precipitate was filtered by suction, and the resulting precipitate was chromatographed on a 300-400 mesh silica gel column (eluent: petroleum ether / ethyl acetate = 4:1) to obtain a yellow powder, which is the inventive product 3-(4-tert-butylbenzene Methylene)-5-(3,4-dimethoxybenzylidene)-4-piperidone (C), 2.3 g, yield 57%.
[0034] Mp:155~157℃.IR(cm -1 ):2956(s), 2837(m), 1670(m), 1609(s), 1515(s), 1460(s), 1325(s), 1253(s), 1142(s), 1024(s) ),990(m),927(s),855(s),766(m). 1 HNMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com