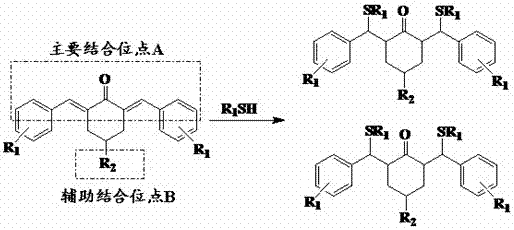
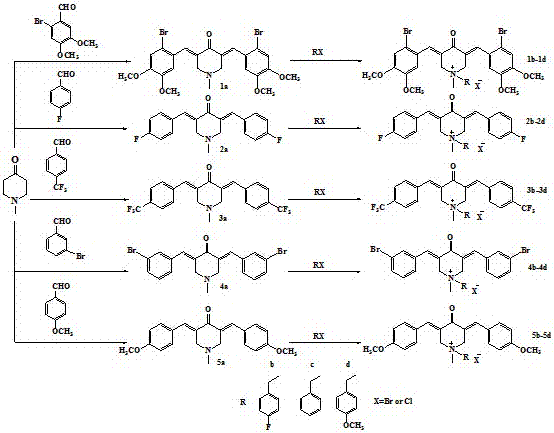
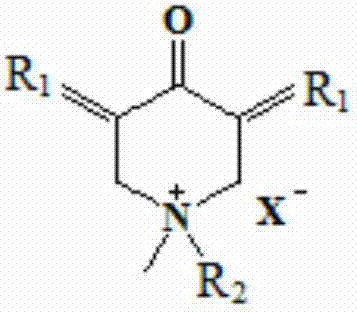
N-methyl-3,5-diaryl methylene-4-piperidone and its quaternary ammonium salt derivatives for antitumor
A technology of diarymethylene and methoxybenzylidene, applied in the field of N-methyl-3,5-diarymethylene-4-piperidone and its quaternary ammonium salt derivatives, reaching Novel structure, great application prospects, and the effect of avoiding genotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of N-methyl-3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone intermediate 3a
[0035] Mix 0.005 mol of N-methyl-4-piperidone and 0.01 mol of 4-trifluoromethylbenzaldehyde in 12.5 mL of glacial acetic acid, feed dry HCl gas for 45 min at room temperature, and stir for 7-24 h. The reaction endpoint was determined by TLC thin layer chromatography thin layer analysis. After the reaction was completed, suction filtered, and the resulting precipitate was added to 12.5 mL of 10% sodium hydroxide solution, stirred at room temperature for 0.5 h, suction filtered, and recrystallized with methanol to obtain N-methyl-3,5-bis(4- Trifluoromethylbenzylidene)-4-piperidone 2.82g, yield 48%, melting point 122-126°C.
[0036] UVλmax(logε)316(0.848),214(2.102)nm.IR(KBr Pellet cm-1):2785(w),1415(m),3347(m),696(m),1614(s),1583 (m), 2360(m), 2332(m), 1325(s), 1277(s), 1171(s), 825(m), 924(m), 1117(s), 1070(s).1H NMR(400MHz,DMSO,25℃,TMS,ppm):δ=3.01(s,3H,NCH3),3.69(s,3H,aryl OCH3...
Embodiment 2
[0038] Synthesis of N-methyl-N-(4-fluorobenzyl)-3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone bromide salt 3b
[0039] Mix 0.001mol of intermediate 3a and 1.5mL of N,N-dimethylformamide solution in a 25ml two-neck flask, stir and heat at 80°C until intermediate 3a dissolves, then add 0.0015mol of p-fluorobenzyl bromide solution dropwise, and use TLC Detect the progress of the reaction. In the TLC detection, the developing agent dichloromethane:methanol is 15:1. After the reaction is completed, wash with ether 2-3 times until the precipitation is complete. The precipitate is collected by suction filtration, and then recrystallized with methanol. 0.315 g of N-methyl-N-(4-fluorobenzyl)-3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone bromide was obtained, with a yield of 59.0% , melting point 184-189°C.
[0040] UVλmax(logε)308(0.436),220(0.360)nm.IR(KBr Pellet cm-1):3400(w),1415(m),1514(m),600.99(m),696(m),854 (m), 1612(s), 1327(s), 1174(s), 1070(s), 1119(s).1H NMR (400...
Embodiment 3
[0042] Synthesis of N-methyl-N-benzyl-3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone bromide salt 3c
[0043] Add 1.5 mL of N,N-dimethylformamide solution to 0.001 mol of intermediate 3a, heat and stir in an oil bath at 80°C until intermediate 3a dissolves, then add 0.0015 mol of benzyl bromide dropwise, and use TLC to detect the progress of the reaction. In the TLC detection, the developer dichloromethane:methanol is 20:1. After the reaction is completed, wash with ether 2-3 times until the precipitation is complete. The precipitate is collected by suction filtration, and then recrystallized with acetone, and vacuum-dried at 30°C for two hours, N-methyl-N-benzyl-3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone bromide salt 0.39g, yield 75.6%, melting point 194 -196°C.
[0044] UVλmax(logε)423(0.001),306(0.278),220(0.251),208(0.183)nm.IR(KBrPellet cm-1):2995(w),1415(m),1475(m),3429( m), 611(m), 708(m), 854(m), 1614(s), 1329(s), 1167(s), 1070(s).1HNMR(400MHz,DMSO,25℃,TM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com