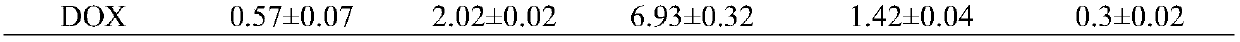Piperidone derivatives with antitumor activity and preparation method thereof
A technology of anti-tumor activity and piperidone, applied in the field of N-methyl-3,5-dibenzylidene)-4-piperidone derivatives and their preparation, anti-tumor drugs and their preparation, can Solve the problems of unstable chemical structure, low bioavailability, and limited application, and achieve the effects of avoiding genotoxicity, simple preparation method, and clear targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of N-methyl-3,5-bis(3-aminobenzylidene)-4-piperidone
[0021] Mix 0.01mol of N-methyl-4-piperidone and 0.022mol of m-nitrobenzaldehyde in a solution of 20mL of methanol and water, add 20mL of 20% sodium hydroxide solution at room temperature, stir and react at room temperature for 12h, pass Thin layer chromatography (TLC) TLC analysis determined the reaction endpoint. The precipitate was suction filtered to obtain the intermediate as light yellow powder. Add the intermediate and 0.065mol of stannous chloride to 25mL of concentrated hydrochloric acid and stir for 6-8h, and determine the end point of the reaction through thin-layer chromatography (TLC) analysis, precipitate and suction filter, 10% sodium carbonate solution lotion, 15mL ethanol / Water (volume ratio 1:1) was recrystallized to obtain a yellow powder, namely N-methyl-3,5-bis(3-aminobenzylidene)-4-piperidone.
Embodiment 2
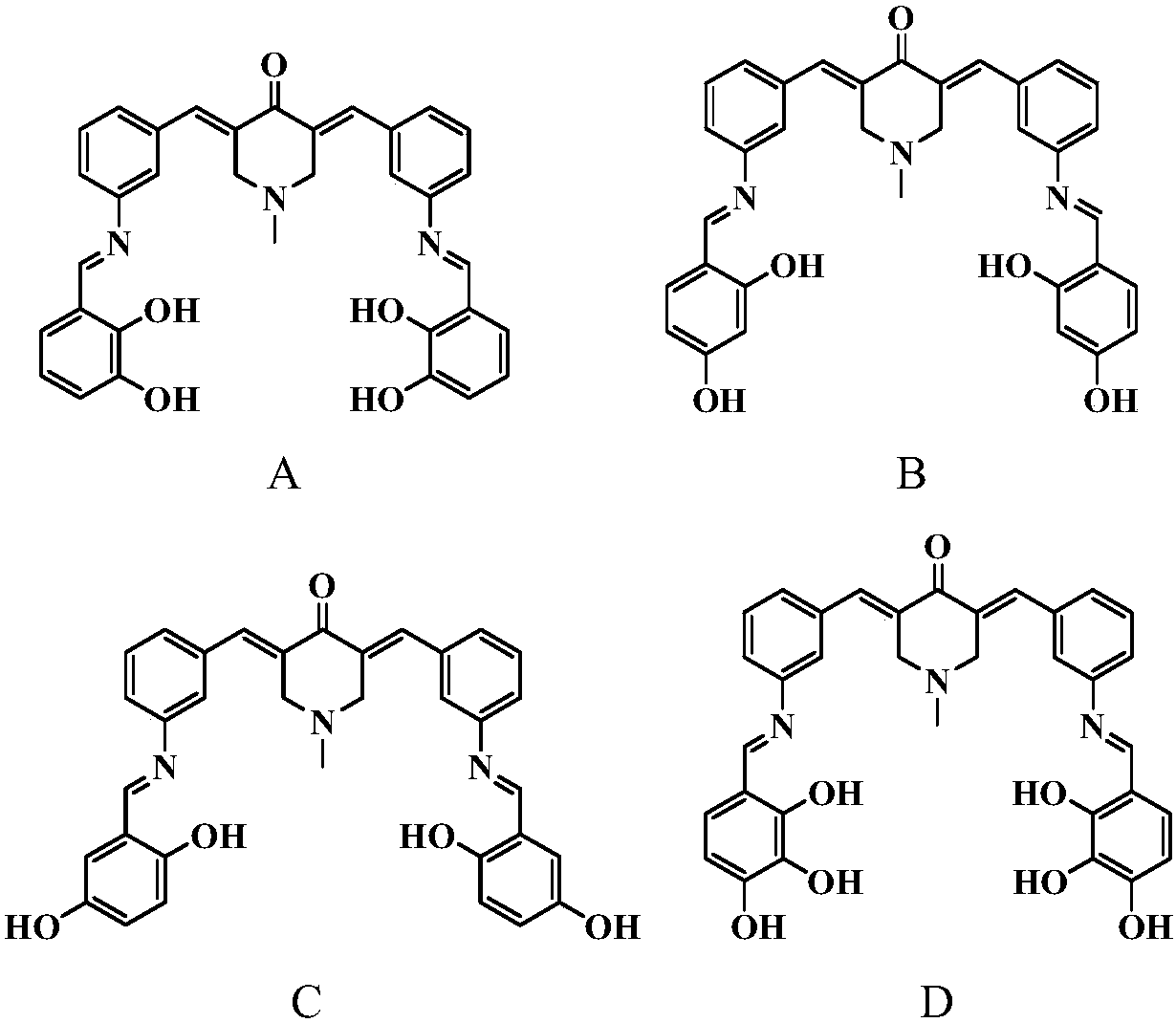
[0023] N-methyl-3,5-bis(3-(2,3-dihydroxybenzylideneamino)benzylidene)-4-piperidone (A)
[0024] Dissolve 0.001mol N-methyl-3,5-bis(3-aminobenzylidene)-4-piperidone and 0.003mol 2,3-dihydroxybenzaldehyde in 40mL of methanol, add 3 drops of formic acid , stirred at room temperature for 8 h, and determined the end point of the reaction by thin layer chromatography (TLC) analysis. The precipitate was suction filtered, washed with methanol, and dried under vacuum to obtain red powder N-methyl-3,5-bis(3-(2,3-dihydroxybenzylideneamino)benzylidene)-4-piperidone (A) 0.3133 g, yield 56%.
[0025] IR (cm -1 ):3253(br),1614(s),1573(s),1460(m),1363(m),1273(s),1209(s),1183(m),1028(w),939(w ),850(w),789(m),734(s),684(s),540(w). 1 H NMR(400MHz,DMSO,25℃,TMS,ppm):δ13.03(s,2H),9.26(s,2H),8.97(s,2H),7.69(s,2H),7.62-7.50(m ,4H),7.50-7.40(m,4H),7.13(d,J=8Hz,2H),6.98(d,J=8Hz,2H),6.81(t,J=8Hz,2H),3.82(s, 4H), 2.42(s,3H).13C NMR (100MHz, CDCl 3 ):186.27,164.75,149.25,148.42,145.63,135.85,134.47...
Embodiment 3
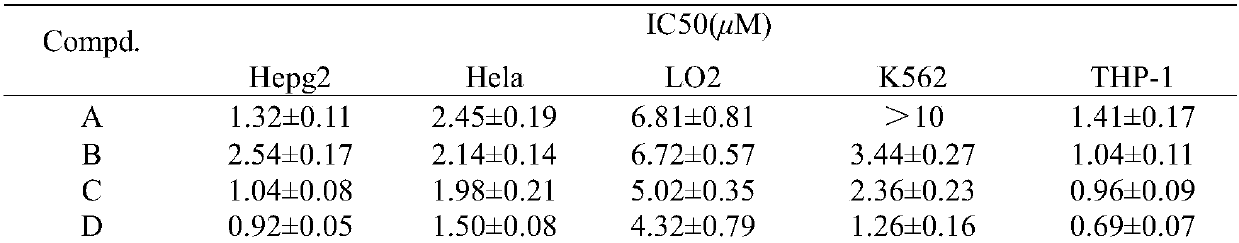
[0027] N-methyl-3,5-bis(3-(2,4-dihydroxybenzylideneamino)benzylidene)-4-piperidone (B)
[0028] Dissolve 0.001mol N-methyl-3,5-bis(3-aminobenzylidene)-4-piperidone and 0.0035mol 2,4-dihydroxybenzaldehyde in 45mL methanol, add 6 drops of formic acid , stirred at room temperature for 9 h, and determined the end point of the reaction by thin layer chromatography (TLC) analysis. The precipitate was suction filtered, washed with methanol, and dried in vacuo to obtain yellow powder N-methyl-3,5-bis(3-(2,4-dihydroxybenzylideneamino)benzylidene)-4-piperidone (B) 0.3025 g, yield 54%.
[0029] IR (cm -1 ):3144(br),1672(w),1607(s),1568(s),1509(w),1455(w),1329(w),1281(w),1208(s),1176(m ),1127(m),977(m),850(m),788(s),691(s),651(w),538(w). 1 H NMR(400MHz,DMSO,25℃,TMS,ppm):δ13.38(s,2H),10.50(s,2H),8.84(s,2H),7.66(s,2H),7.53(t,J =8Hz,2H),7.46(d,J=8Hz,4H),7.38(t,J=8Hz,4H),6.43(d,J=8Hz,2H),6.31(s,2H),3.78(s, 4H),2.40(s,3H). 13C NMR (100MHz, CDCl 3 ): δ186.38, 163.44, 162.95, 162.66, 148...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com