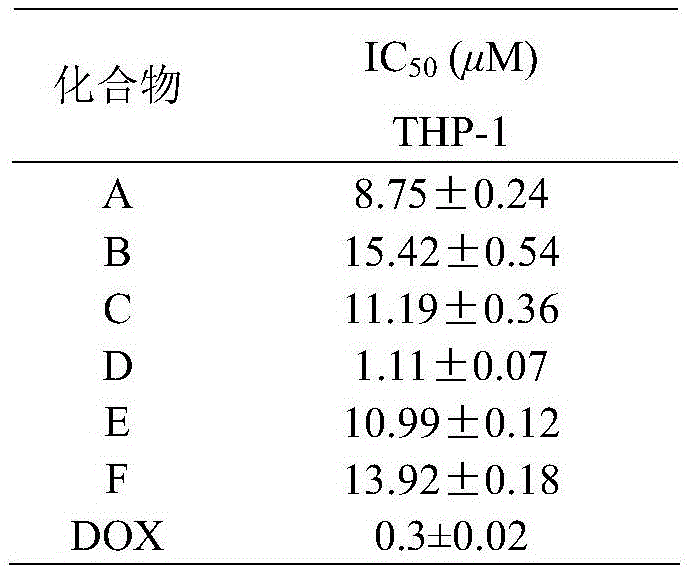
Fluorophenyl-substituted asymmetric piperidone compounds with antitumor activity and preparation method thereof
A technology of anti-tumor activity and piperidone, applied in anti-tumor drugs, active ingredients of heterocyclic compounds, organic chemistry, etc., can solve the problem of asymmetric molecular polarity, unclear influence of solubility and activity, and lack of system of structure-activity relationship Sexuality and other issues, to achieve the effect of avoiding genotoxicity, low toxicity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
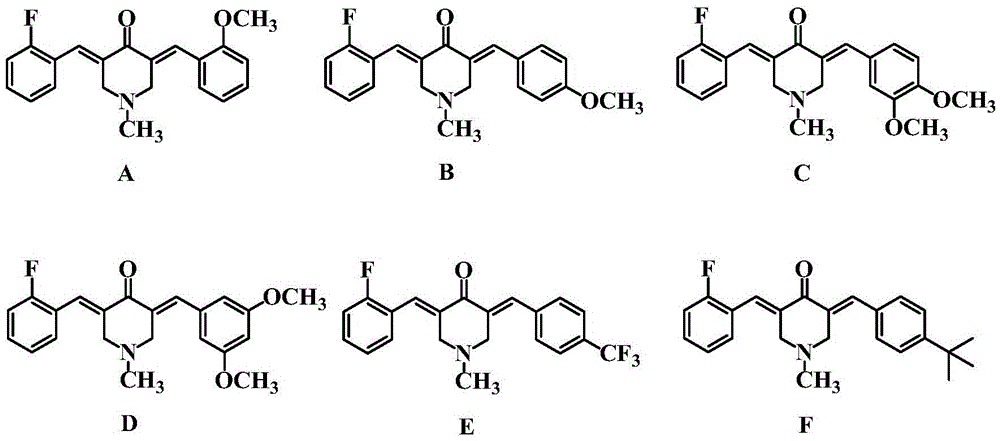
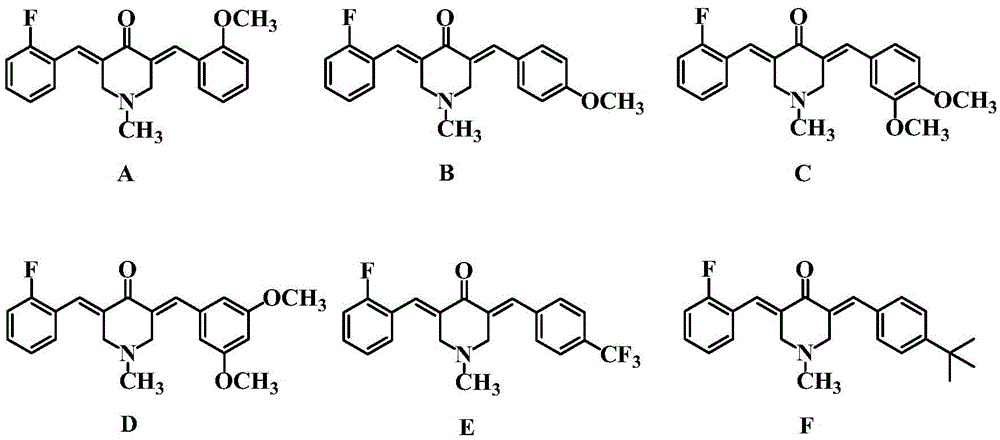
[0023] Synthesis of 3-(2-fluorobenzylidene)-5-(2-methoxybenzylidene)-4-piperidone (A)
[0024] Mix 0.01 mol of N-methyl-4-piperidone with 0.01 mol of 2-fluorobenzaldehyde and 0.01 mol of 2-methoxybenzaldehyde in a solution of 25 mL of ethanol and water, add 10 mL of 20% hydroxide dropwise at room temperature Sodium solution, stirred at room temperature for 4 h, and the end point of the reaction was determined by thin-layer chromatography (TLC) analysis. After the reaction was completed, the precipitate was suction filtered, and the obtained precipitate was chromatographed on a 200-300 mesh silica gel column (eluent: petroleum ether / ethyl acetate = 3:1) to obtain a yellow powder, which is the inventive product 3-(2-fluorobenzylidene yl)-5-(2-methoxybenzylidene)-4-piperidone (A), 1.81 g, yield 54%.
[0025] Mp: 89~91℃. IR(cm-1): ν2943, 2773, 1742, 1675, 1617, 1459, 1243, 1174, 1108, 1028, 922, 751. 1 HNMR (300MHz, CDCl 3 )δ8.11(s,1H),7.90(s,1H),7.40-6.92(m,8H),3.88(s,3H),3.70...
Embodiment 2
[0027] Synthesis of 3-(2-fluorobenzylidene)-5-(4-methoxybenzylidene)-4-piperidone (B)
[0028] Mix 0.01mol of N-methyl-4-piperidone with 0.011mol of 2-fluorobenzaldehyde and 0.009mol of 4-methoxybenzaldehyde in a solution of 18mL of methanol and water, add dropwise 15mL of 10% hydroxide at room temperature Potassium solution was stirred at room temperature for 3 h, and the end point of the reaction was determined by thin-layer chromatography (TLC) thin-layer analysis. After the reaction, the precipitate was filtered with suction, and the obtained precipitate was chromatographed on a 200-300 mesh silica gel column (eluent: petroleum ether / ethyl acetate = 5:1) to obtain a bright yellow powder, which is the inventive product 3-(2-fluorophenylene oxide) Methyl)-5-(4-methoxybenzylidene)-4-piperidone (B), 1.92 g, yield 57%.
[0029] Mp: 127~129℃.IR(cm-1):ν2940,2779,1669,1603,1573,1507,1454,1302,1259,1165,1023,922,826,759.Elementalanalysis(%)calcd.forC 21 h 20 FNO 2 (337.38):C74....
Embodiment 3
[0031] Synthesis of 3-(2-fluorobenzylidene)-5-(3,4-dimethoxybenzylidene)-4-piperidone (C)
[0032]Mix 0.01mol of N-methyl-4-piperidone with 0.009mol of 2-fluorobenzaldehyde and 0.011mol of 3,4-dimethoxybenzaldehyde in a solution of 20mL of ethanol and water, add dropwise 15mL of 20 % sodium hydroxide solution, stirred and reacted at 45°C for 3.5h, and the end point of the reaction was determined by thin-layer chromatography (TLC) thin-layer analysis. After the reaction was completed, the precipitate was suction-filtered, and the resulting precipitate was chromatographed on a 300-400 mesh silica gel column (eluent: petroleum ether / ethyl acetate = 4:1) to obtain a yellow powder, which was the inventive product 3-(2-fluorobenzylidene Dimethoxy)-5-(3,4-dimethoxybenzylidene)-4-piperidone (C), 1.90 g, yield 52%.
[0033] Mp: 116~118℃.IR(cm-1):ν2934,2782,1668,1608,1508,1446,1255,1146,1021,922,810,764.Elementalanalysis(%)calcd.forC 22 h 22 FNO 3 (367.41):C71.92,H6.04,N3.81; Found:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com