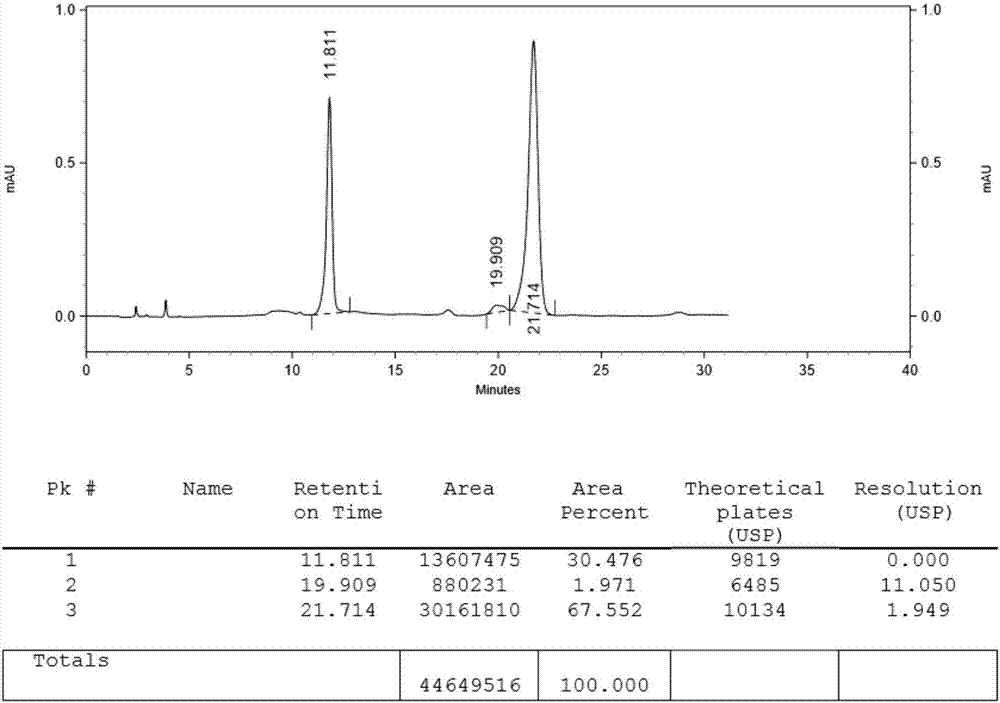
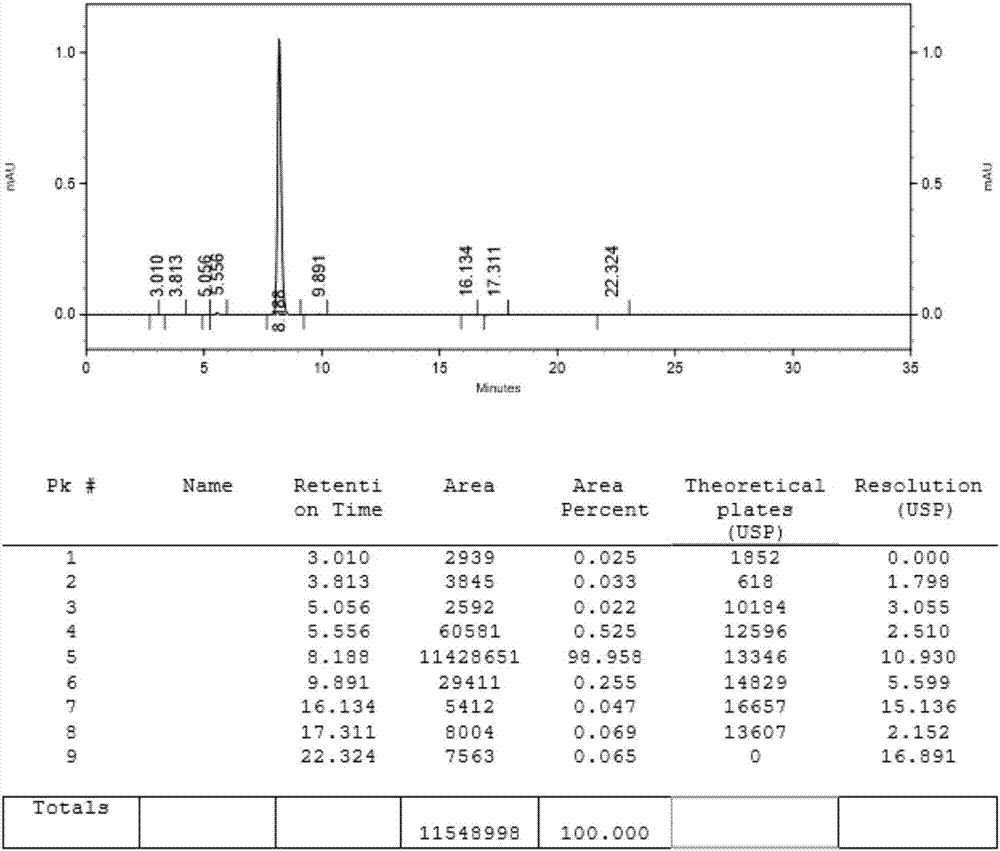
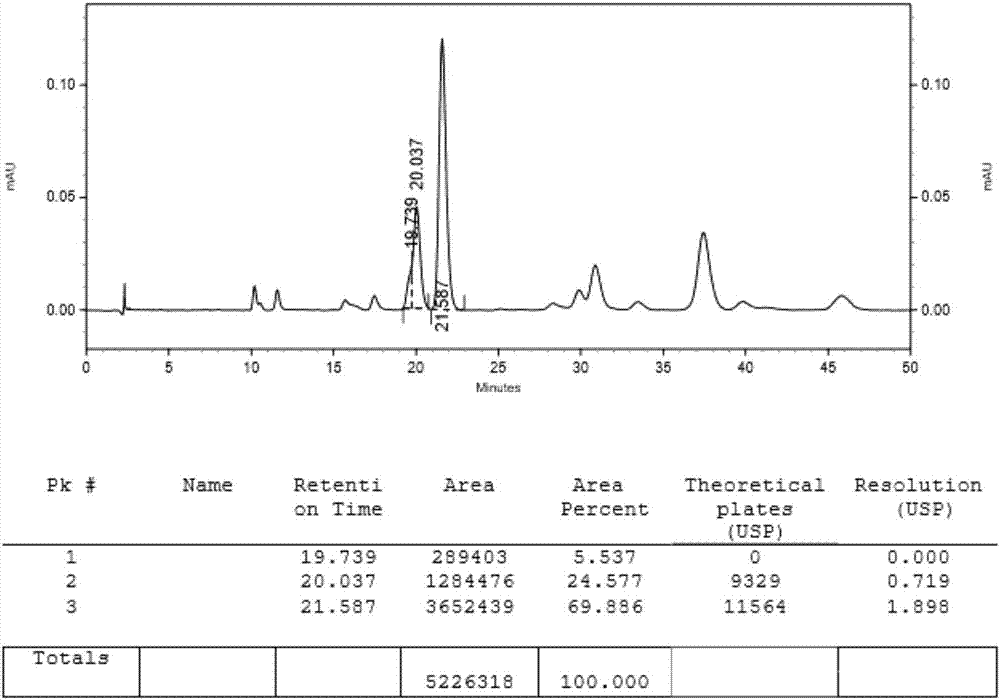
Preparing methods of ipragliflozin intermediates
A technology of methyl group and thiophene, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as affecting the field of medicine, being unsuitable for the field of medicine, affecting the quality of raw materials of final products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1S)-2,3,4,6-tetraacetoxy-1,5-anhydro-1-[3-(1-benzothiophen-2-ylmethyl)-4-fluorophenyl]-D -The preparation method of sorbitol, comprising steps:
[0051](1) Add 22 mL of tetrahydrofuran and 220 mL of toluene to the reaction flask, and add 22.0 g of 2-(5-bromo-2-fluorobenzyl)benzothiophene (compound 1) under stirring. Cool the reaction solution to -70°C, add 31.5mL of n-butyl lithium in n-hexane solution (2.5mol / L) dropwise under the protection of nitrogen, control the temperature of the reaction solution not higher than -60°C, and the dropping rate is 3 drops / second, After about 5 minutes of dripping, keep at -70°C for 1 hour.
[0052] Dissolve 36.8 g of 2,3,4,6-tetra-O-trimethylsilyl-D-gluconolactone (compound 2) in 20 mL of toluene, and add it dropwise to the above system, controlling the temperature of the reaction solution not to exceed -60°C, after about 10 minutes, the dripping was completed, and the reaction was continued at -70°C for 6 hours to obtain compound...
Embodiment 2
[0062] (1S)-2,3,4,6-tetraacetoxy-1,5-anhydro-1-[3-(1-benzothiophen-2-ylmethyl)-4-fluorophenyl]-D -The preparation method of sorbitol, comprising steps:
[0063] (1) Add 22 mL of tetrahydrofuran and 220 mL of toluene to the reaction flask, and add 22.0 g of 2-(5-bromo-2-fluorobenzyl)benzothiophene (compound 1) under stirring. Cool the reaction solution to -60°C, add 31.5mL of n-butyl lithium in n-hexane solution (2.5mol / L) dropwise under the protection of nitrogen, control the temperature of the reaction solution not higher than -60°C, and the dropping rate is 3 drops / second, After about 5 minutes of dripping, keep at -60°C for 1 hour.
[0064] Dissolve 36.8 g of 2,3,4,6-tetra-O-trimethylsilyl-D-gluconolactone (compound 2) in 20 mL of toluene, and add it dropwise to the above system, controlling the temperature of the reaction solution not to exceed -60°C, the dripping was completed in about 10 minutes, and the reaction was continued at -60°C for 5 hours to obtain compound 3....
Embodiment 3
[0074] (1S)-2,3,4,6-tetraacetoxy-1,5-anhydro-1-[3-(1-benzothiophen-2-ylmethyl)-4-fluorophenyl]-D -The preparation method of sorbitol, comprising steps:
[0075] (1) Add 2.2 L of tetrahydrofuran and 22 L of toluene to the reaction flask, and add 2,200 g of 2-(5-bromo-2-fluorobenzyl)benzothiophene (compound 1) under stirring. Cool the reaction solution to -70°C, add 3150mL of n-butyllithium n-hexane solution (2.5mol / L) dropwise under the protection of nitrogen, control the temperature of the reaction solution not higher than -60°C, the rate of addition is 3 drops / second, about After 1 hour of dripping, keep warm at -60°C for 1 hour.
[0076] Dissolve 3680g of 2,3,4,6-tetra-O-trimethylsilyl-D-gluconolactone (compound 2) in 2L of toluene and add it dropwise to the above system, controlling the temperature of the reaction solution not to exceed - 60°C, about 1 hour to complete the dripping, keep warm at -70°C and continue to react for 6 hours to obtain compound 3.
[0077] (2) A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com