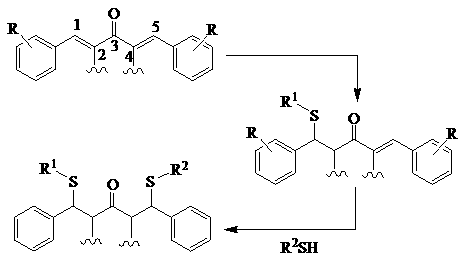
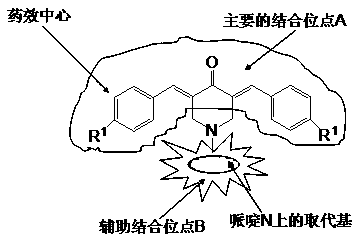
3,5-Dibenzylidene-4-Piperidinone Derivative for Antitumor and Preparation Method thereof
A technology of benzylidene and trifluoromethylbenzylidene is applied in the field of antitumor drugs and preparation thereof, and achieves the effects of novel structure, great application prospect and avoiding genotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
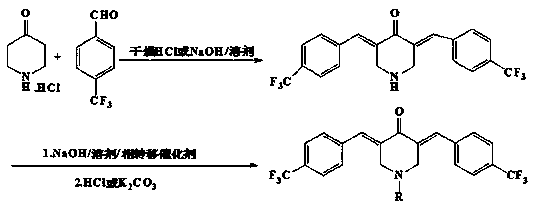
Embodiment 1
[0029] Synthesis of 3,5-bis(4-trifluoromethylbenzylidene)-4-piperidinone a
[0030] Mix 0.005mol of 4-piperidone hydrochloride and 0.01mol of 4-trifluoromethylbenzaldehyde in 12.5mL of glacial acetic acid, pass through dry HCl gas at room temperature for 60min, stir for 8h, and pass thin-layer chromatography Thin layer analysis (TLC) was used to determine the end point of the reaction. After completion of the reaction, suction filtration, the resulting precipitate was added to a mixture of 12.5 mL of potassium carbonate solution with a mass fraction of 25% and 12.5 mL of acetone solution, stirred at room temperature for 0.5 h, suction filtration, and recrystallized with a mixed solvent of methanol and chloroform to obtain 3, 5-bis(4-trifluoromethylbenzylidene)-4-piperidone 1.21g, yield 59%.
[0031]
Embodiment 2
[0033] Synthesis of N-(4-nitrobenzoyl)-3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone b
[0034] Mix 2 mL of 25% NaOH solution with 1 mmol of 3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone in 5 mL of tetrahydrofuran solution, add 0.02 g of 5 % tetrabutylammonium bromide, stirred at 0-5°C for 15 minutes, added dropwise 1.5mmol of 4-nitrobenzoyl chloride solution, continued to stir for 2 hours, and determined the reaction end point by thin layer chromatography (TLC) analysis. Suction filtration, add 10 mL of potassium carbonate solution with a mass fraction of 10% to the residue, stir at room temperature for 2 h, collect the precipitate, and recrystallize with methanol-chloroform mixed solvent to obtain N-(4-nitrobenzoyl)-3,5- Bis(4-trifluoromethylbenzylidene)-4-piperidone 0.44g, yield 78%.
[0035]
Embodiment 3
[0037] Synthesis of N-(4-methylbenzenesulfonyl)-3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone c
[0038] Mix 2 mL of 25% NaOH solution with 1 mmol of 3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone in 5 mL of 1,2-dichloroethane solution, add 0.01g of tetrabutylammonium chloride with a mass fraction of 5%, stirred at 0-5°C for 15min, added dropwise 1.5mmol of 4-methylbenzenesulfonyl chloride solution, continued to stir for 5h, and passed thin layer chromatography (TLC). Layer analysis to determine the reaction end point, with 2mol L -1 hydrochloric acid to adjust the pH to 1-4, collect the precipitate, and recrystallize with chloroform solvent to obtain N-(4-methylbenzenesulfonyl)-3,5-bis(4-trifluoromethylbenzylidene)-4 - piperidone 0.45g, yield is 79%.
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com