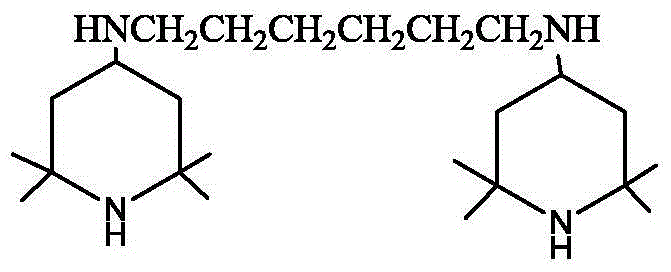Preparation method of hexamethylenediamine piperidine
A technology of hexamethylenediamine piperidine and sevbase, which is applied in the field of compound preparation, can solve problems such as difficulty in realizing industrialized production, difficulty in synthesis and separation, and increase in production cost, and achieves simple and convenient post-processing method, cost reduction, and equipment reduction. The effect of stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
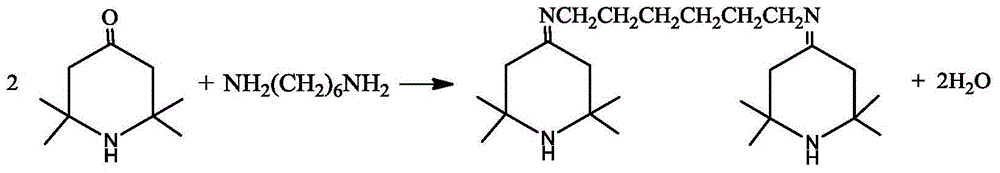
[0025] Add 5.7kg (49mol) of 1,6-hexanediamine and 16kg (103mol) of tetramethylpiperidone into a 100L glass reactor with 24kg of toluene, stir, vacuumize -0.070~-0.095MPa, heat up, and reflux for dehydration reaction Until no water comes out, the amount of dehydration water is measured during the reaction process.
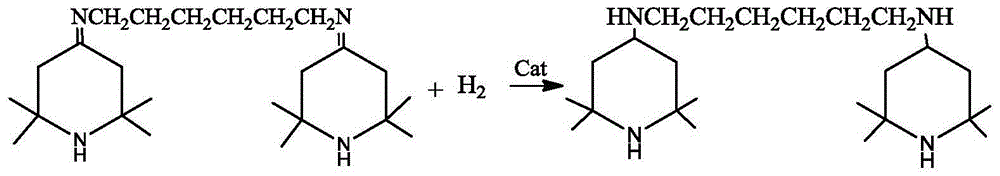
[0026] Add the above-mentioned Schiff base solution and the skeleton nickel catalyst into the hydrogenation reactor, replace with nitrogen three times, replace with hydrogen three times, and replenish hydrogen to 1.0 MPa. After the replacement is completed, stir and heat up. When the temperature rises to 70-75°C, the reaction starts, and the hydrogen pressure is maintained at 1.0-1.5 MPa. After 5 hours, the hydrogen absorption rate becomes very slow. Turn on the heating device and control the temperature in the kettle to maintain at 85-1.5 MPa. React at 90°C for 1 hour, take samples midway, and control in GC analysis, the GC of the raw material is 0.8%, and the prod...
Embodiment 2
[0028] Dissolve 9.5kg (81.9mol) of 1,6-hexanediamine and 26.7kg (172mol) of tetramethylpiperidone in 40kg of cyclohexane to make a solution, add it to a 200L reactor, stir, and vacuum -0.070 ~-0.095MPa, heat up, reflux dehydration reaction until no water comes out, measure the amount of dehydration water during the reaction process.
[0029] Add the above-mentioned Schiff base solution and the skeleton nickel catalyst into the hydrogenation reactor, replace with nitrogen three times, replace with hydrogen three times, and replenish hydrogen to 1.0 MPa. After the replacement is completed, stir and heat up. When the temperature rises to 70-75°C, the reaction starts, and the hydrogen pressure is maintained at 1.0-1.5 MPa. After 5 hours, the hydrogen absorption rate becomes very slow. Turn on the heating device and control the temperature in the kettle to maintain at 70-1.5 MPa. React at 80°C for 1 hour, take samples midway, and control in GC analysis. The GC of the raw material i...
Embodiment 3
[0031] Add 28.50kg (245mol) of 1,6-hexanediamine and 81.83kg (526.73mol) of tetramethylpiperidone into a 500L reactor with 120kg of xylene, stir, vacuumize -0.070~-0.095MPa, heat up, and reflux The dehydration reaction is performed until no water comes out, and the amount of dehydration water is measured during the reaction process.
[0032] Add the above-mentioned Schiff base solution and the skeleton nickel catalyst into the hydrogenation reactor, replace with nitrogen three times, replace with hydrogen three times, and replenish hydrogen to 2.0 MPa. After the replacement is completed, stir and heat up. When the temperature rises to 70-75°C, the reaction starts, and the hydrogen pressure is maintained at 2.0-3.0 MPa. After 7 hours, the hydrogen absorption rate becomes very slow. Turn on the heating device and control the temperature in the kettle to maintain at 85-3.0 MPa. React at 90°C for 1 hour, take samples midway, and control in GC analysis. The GC of the raw material i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com