Method for preparing lactones by biomimetic catalytic oxidation of ketone compounds
A technology for the biomimetic catalytic oxidation of ketones and ketones, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problems of complex catalyst preparation, environmental pollution, etc., and achieve easy separation and no pollution Cost, effectiveness of process cleaning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
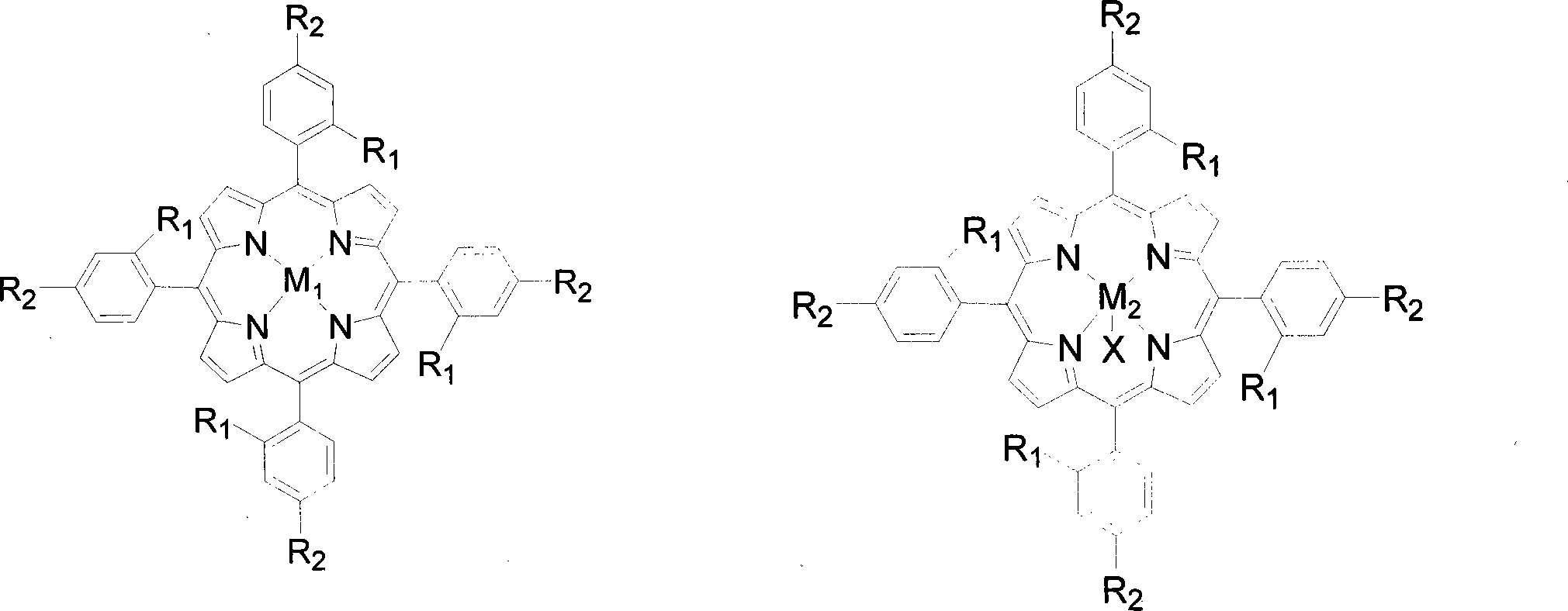
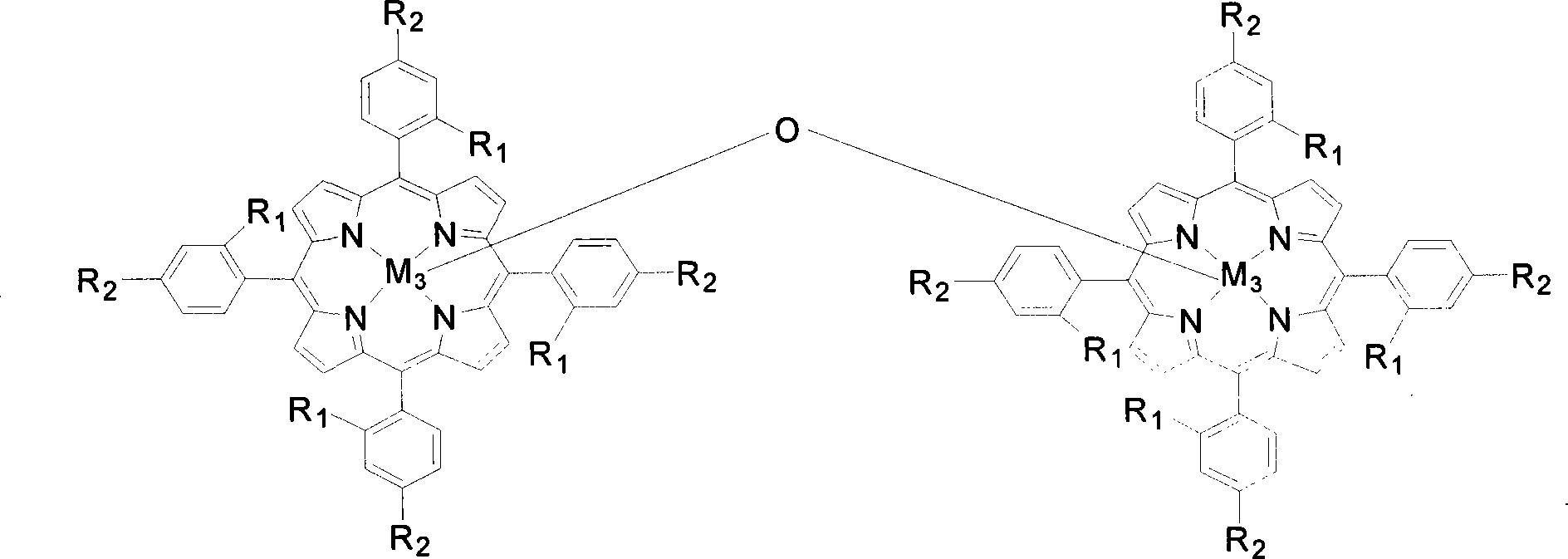
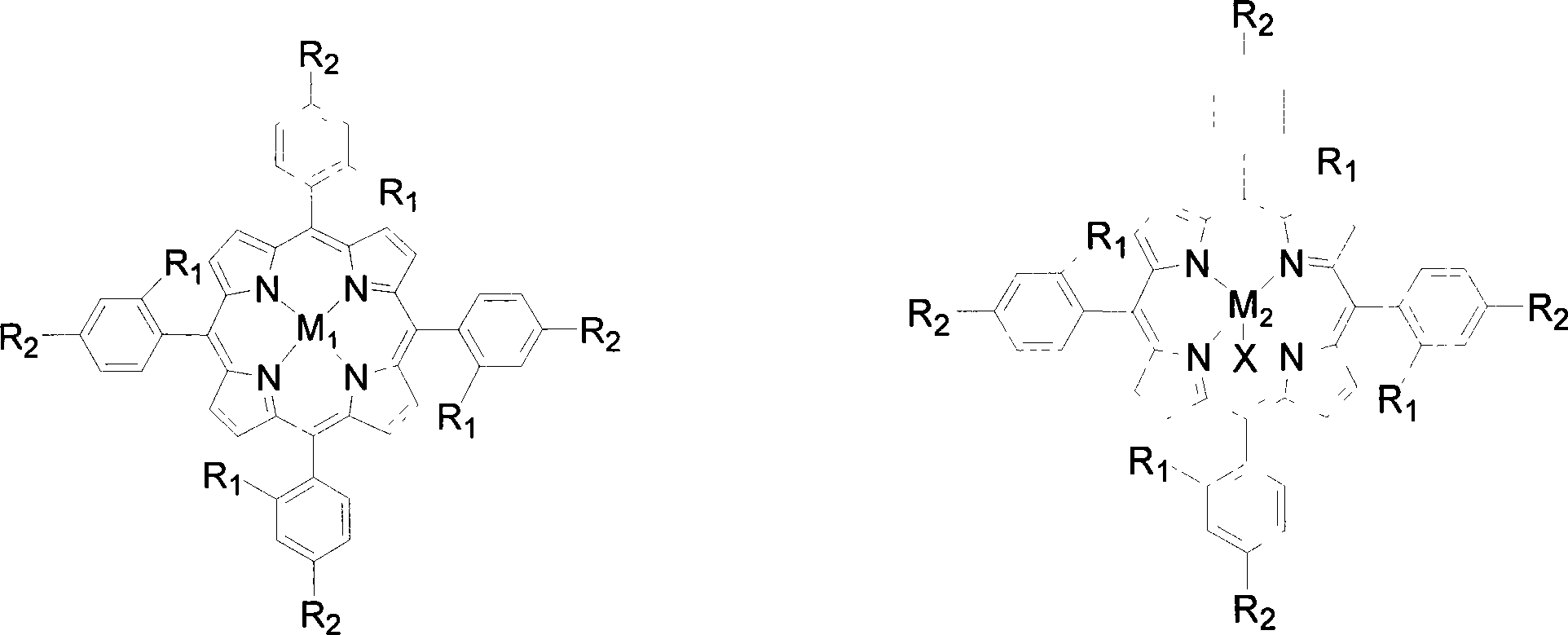
[0020] Contains 2 x 10 in 5 mL -5 Four-(o-nitrophenyl) manganese porphyrin of mol / L (being R in the general formula (I) 1 = NO 2 , R 2 = H, M 1 =Mn) in the toluene solution, add 0.02mol / L cyclohexanone and 0.05mmol benzaldehyde, feed the oxygen of 0.1MPa, stir reactant 6 hours at 40 ℃, the transformation rate of cyclohexanone is 96%, The yield of cyclocaprolactone was 96%.
[0021]
Embodiment 2
[0023] Contains 2 x 10 in 5 mL -4 The tetra-(ortho-chlorophenyl) iron porphyrin of mol / L (being R in the general formula (I) 1 = Cl, R 2 = H, M 1 =Fe) in the toluene solution, add 0.1mol / L 4-methylcyclohexanone and 0.5mmol benzaldehyde, feed 0.5MPa oxygen, stir the reactant at 60°C for 6 hours, 4-methylcyclohexanone The conversion of ketone was 90%, and the yield of 4-methylcyclocaprolactone was 90%.
[0024]
Embodiment 3
[0026] Contains 2 x 10 in 5 mL -3 The four-(o-methoxyphenyl) cobalt porphyrin of mol / L (being R in the general formula (I) 1 =OCH 3 , R 2 = H, M 1 =Co) in the toluene solution, add 2-methylcyclohexanone and 5mmol benzaldehyde of 0.5mol / L, pass into the oxygen of 0.8MPa, stir reactant 2 hours at 80 ℃, 2-methylcyclohexanone The conversion rate of is 85%, and the yield of 2-methylcyclocaprolactone is 85%.
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com