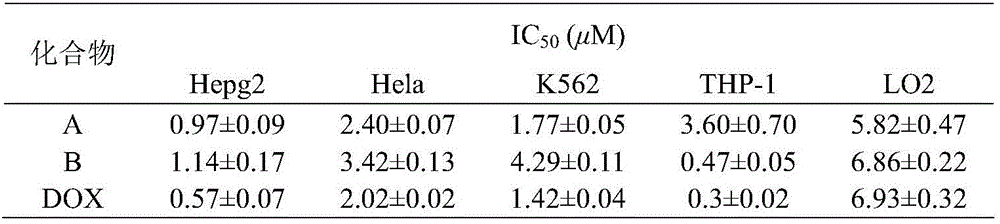
3,5-bis(3-aminobenzylidene)-4-piperidone derivatives with antitumor activity and preparation method thereof
An aminobenzylidene, antitumor activity technology, applied in antitumor drugs, organic chemistry, drug combination and other directions, to achieve the effects of high synthesis yield, low toxicity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
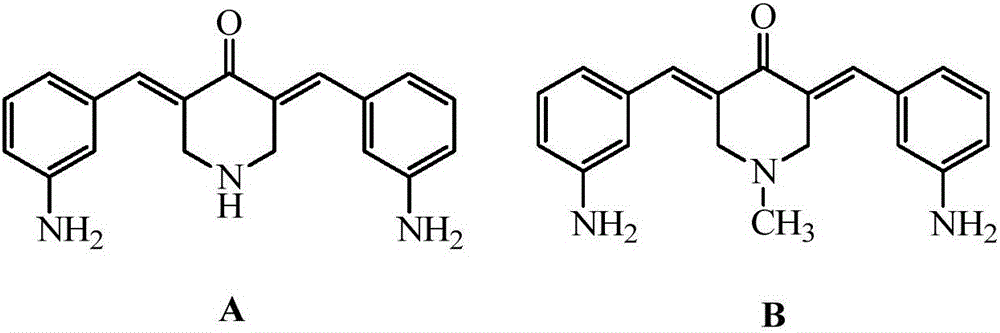
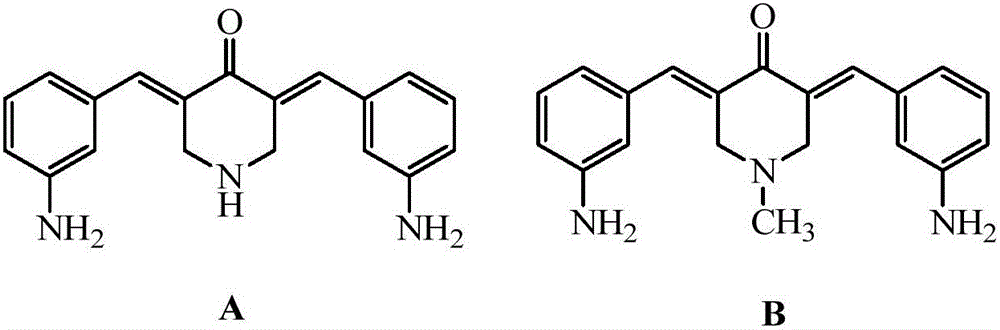
[0027] Synthesis of 3,5-bis(3-aminobenzylidene)-4-piperidinone (A)
[0028] Mix 0.01mol of 4-piperidone hydrochloride and 0.022mol of m-nitrobenzaldehyde in a solution of 25mL of ethanol and water, add dropwise 20mL of 20% sodium hydroxide solution at room temperature, stir and react at room temperature for 8 hours, pass through a thin layer Chromatography (TLC) thin layer analysis to determine the reaction endpoint. After the reaction was completed, it was suction filtered, and the obtained precipitate was washed with an appropriate amount of ether, and dried to obtain the intermediate as a light yellow powder. The intermediate and 0.06mol stannous chloride were added to 20mL of concentrated hydrochloric acid and stirred for 15h, the end point of the reaction was determined by thin-layer chromatography (TLC) analysis, the precipitate was suction filtered, 10% sodium carbonate solution lotion, 20mL ethanol / water ( Volume ratio 1:1) recrystallization to obtain a yellow powder,...
Embodiment 2
[0031] Synthesis of N-methyl-3,5-bis(3-aminobenzylidene)-4-piperidone (B)
[0032] Mix 0.01mol of N-methylpiperidone and 0.024mol of m-nitrobenzaldehyde in a solution of 20mL of methanol and water, add 25mL of 10% sodium hydroxide solution at room temperature, stir and react at room temperature for 12h, and pass thin-layer chromatography Thin layer analysis (TLC) was used to determine the end point of the reaction. The precipitate was suction filtered to obtain light yellow powder. Add the light yellow powder and 0.066mol tin protochloride to 25mL concentrated hydrochloric acid and stir for 6h, determine the end point of the reaction by thin layer chromatography (TLC) analysis, precipitate and suction filter, 10% sodium carbonate solution lotion, 25mL ethanol / water (volume ratio 1.5:1) recrystallization to obtain a yellow powder, namely N-methyl-3,5-bis(3-aminobenzylidene)-4-piperidone (B), 2.17g, yield 68 %.
[0033] IR (cm -1 ):3355(w),3347(w),3222(w),1600(s),1583(s),145...
Embodiment 3
[0035] Preparation method of 3,5-bis(3-aminobenzylidene)-4-piperidone with antitumor activity
[0036] Step 1: Preparation of 3,5-bis(3-nitrobenzylidene)-4-piperidone intermediate
[0037] Use m-nitrobenzaldehyde and 4-piperidone hydrochloride as raw materials, mix them in an alcoholic aqueous solution, add a catalyst, stir and react at room temperature for 4 hours, precipitate and filter to obtain an intermediate, which is directly used in the next reaction;
[0038] The molar ratio of m-nitrobenzaldehyde and 4-piperidone hydrochloride is greater than or equal to 2:1; the alcohol in the alcohol aqueous solution is isopropanol; the catalyst is potassium hydroxide solution with a mass concentration of 8% ;
[0039] Step 2: Preparation of 3,5-bis(3-aminobenzylidene)-4-piperidone derivative A
[0040] Perform reduction reaction with stannous chloride, concentrated hydrochloric acid and the intermediate obtained in step 1 for 18 hours, adjust the pH value to be greater than 12, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com