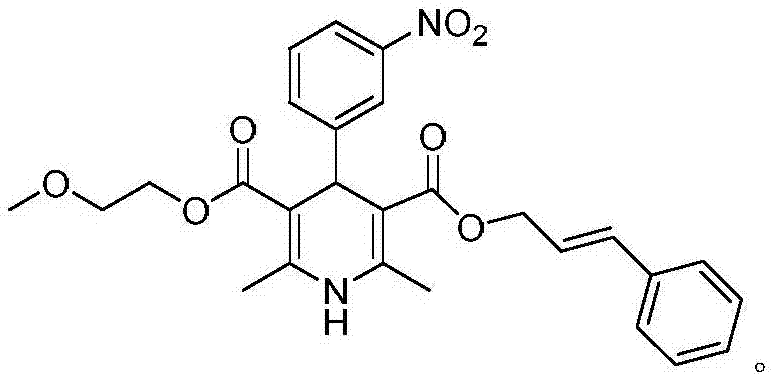
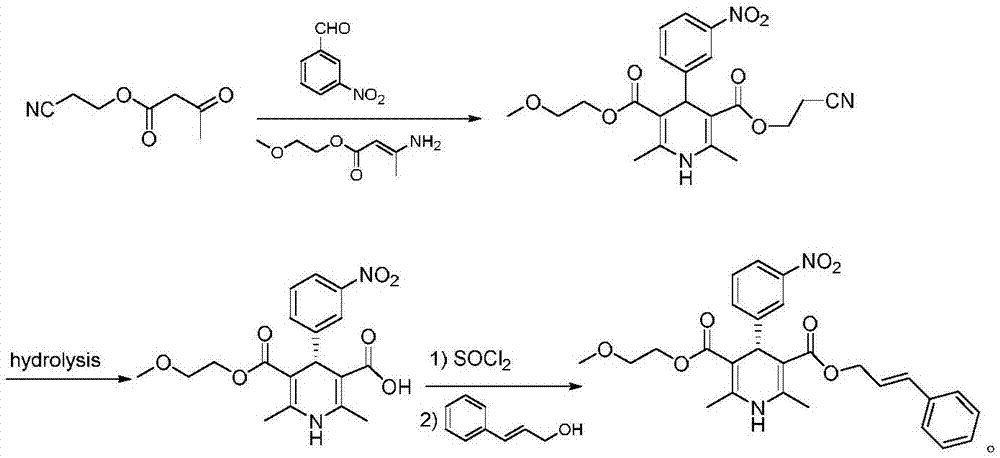
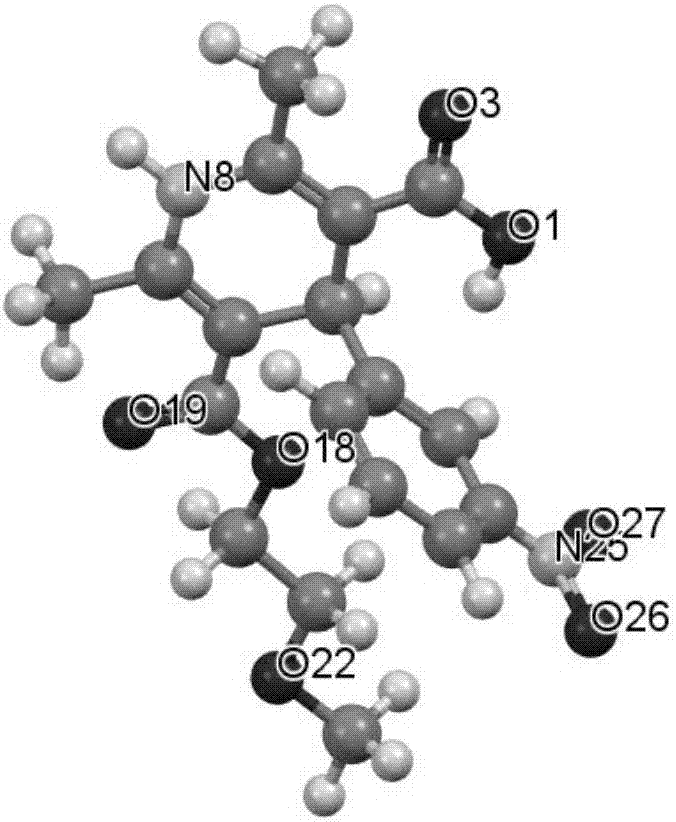
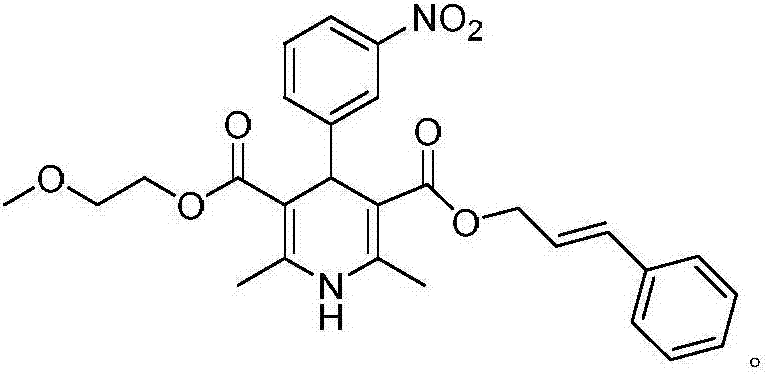
Patents
Literature
44 results about "Meta-nitrobenzaldehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
3-nitrobenzaldehyde; meta-nitrobenzaldehyde; from MeSH. Depositor-Supplied Synonyms. Chemical names and identifiers provided by individual data contributors and associated to PubChem Substance records. Synonyms of Substances corresponding to a PubChem Compound record are combined.
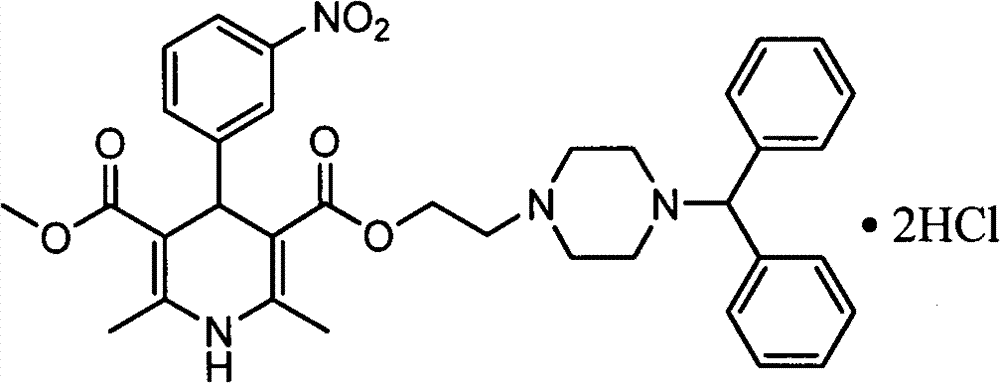
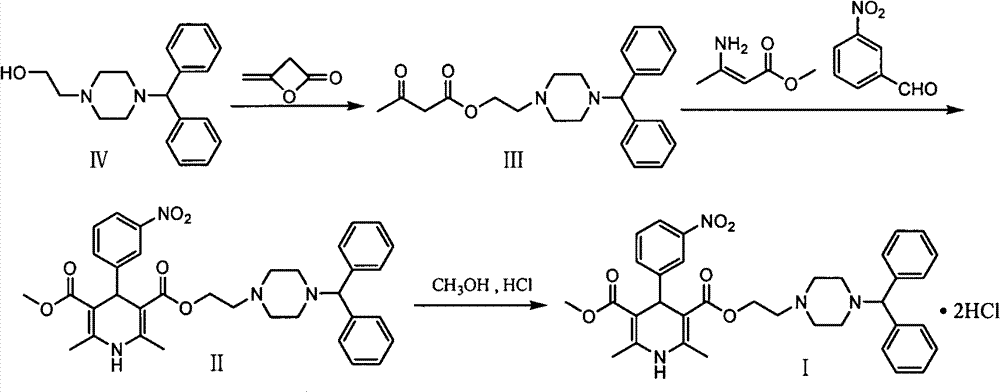
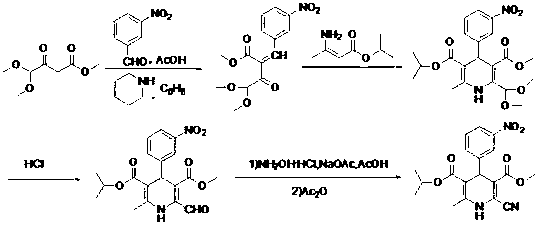
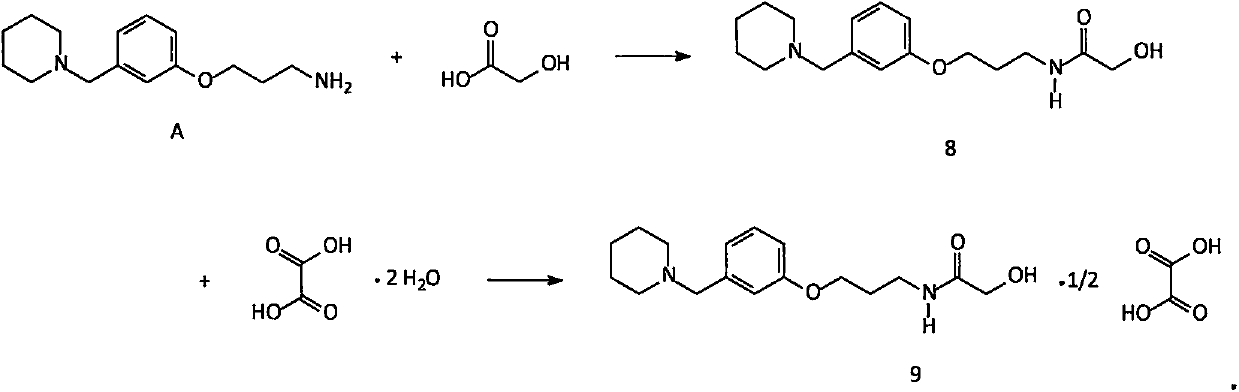
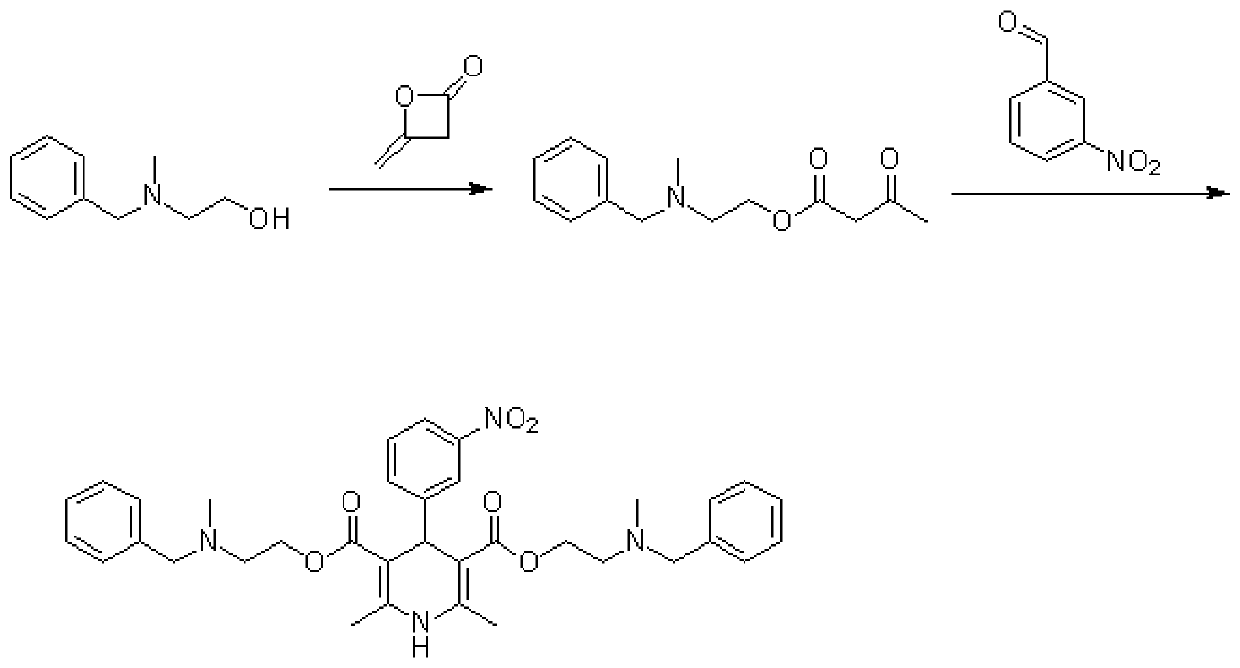
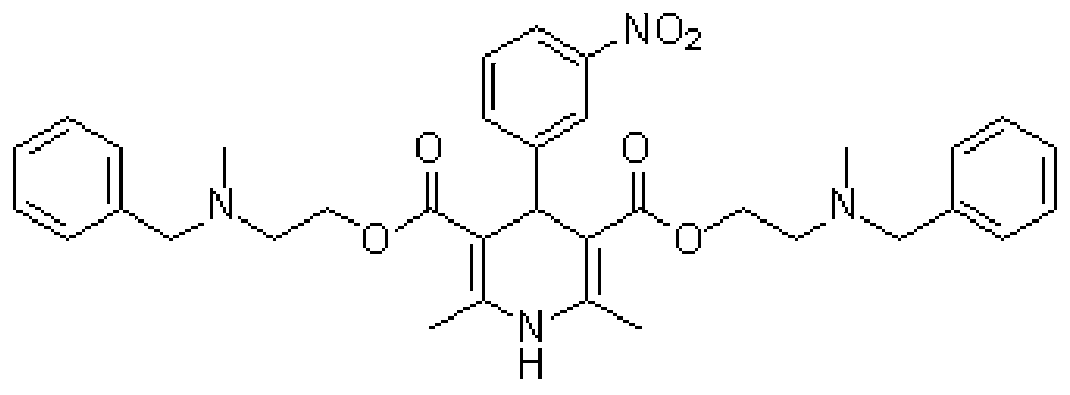
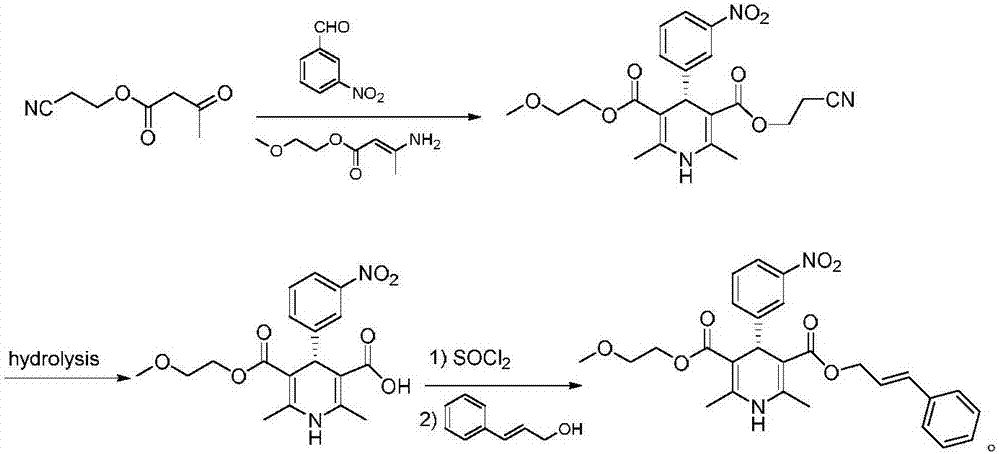
Improved method for synthesis process of manidipine hydrochloride
ActiveCN102875451ASuitable for industrial productionEasy to operateOrganic chemistryManidipine hydrochlorideImproved method
The invention discloses an improved method for a synthesis process of manidipine hydrochloride. The method comprises the following steps: 1-benzhydryl-4-(2-hydroxyethyl)piperazine is taken as a raw material to be acylated with diketene, cyclized with m-nitrobenzaldehyde and methyl 3-aminocrotonate and finally acidized by hydrochloric acid to obtain the manidipine hydrochloride. Due to the optimization of an aftertreatment method, high-vacuum rectification, column chromatography and other purification operation are removed; the maximum single impurity of the product is controlled within 0.1%, and the method has the advantages of simplicity in operation, suitability for industrial production, high product purity and the like.
Owner:CHANGZHOU PHARMA FACTORY
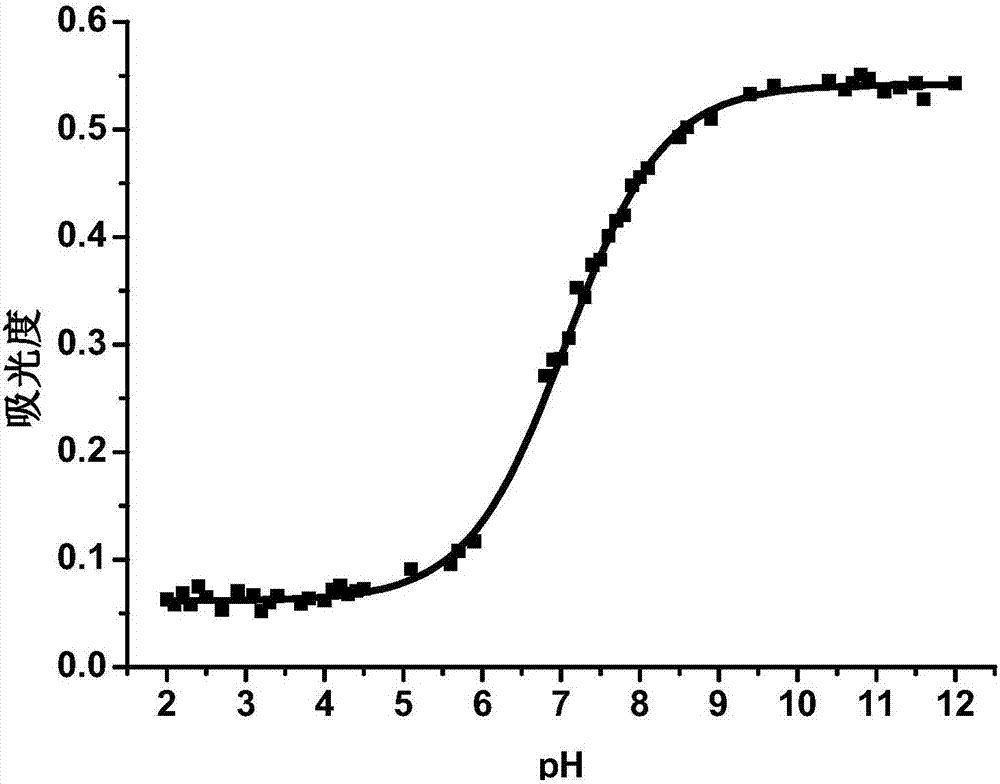
Phenanthroimidazole-fluorescein pH fluorescent probe containing two hydroxyl groups and preparation method of phenanthroimidazole-fluorescein pH fluorescent probe
InactiveCN106905343AGood linear relationshipClear pH ResponseOrganic chemistryFluorescence/phosphorescenceHydrazine compoundStructural formula
The invention provides a phenanthroimidazole-fluorescein pH fluorescent probe containing two hydroxyl groups and a preparation method of the phenanthroimidazole-fluorescein pH fluorescent probe, relates to fluorescein pH fluorescent probes and preparation methods thereof and aims to solve the technical problems that the conventional fluorescein pH fluorescent probes have fewer modification sites and single structures and have no clear response sites and pH values cannot be detected quantitatively. The structural formula of the phenanthroimidazole-fluorescein pH fluorescent probe containing the two hydroxyl groups is shown in the specification. The preparation method comprises the following steps: 1, an intermediate compound I is prepared from phenanthrenequinone, m-nitrobenzaldehyde, aniline and ammonium acetate as raw materials and glacial acetic acid as a solvent; 2, an intermediate compound II is synthesized from the intermediate compound I, Raney nickel and a hydrazine hydrate solution; 3, an intermediate compound III is synthesized from fluorescein, a methanol solution, a sodium hydroxide solution and chloroform; 4, the pH fluorescent probe is synthesized from the intermediate compound II and the intermediate compound III as raw materials and an acid medium as a solvent. The pH fluorescent probe can be used for detecting and monitoring the pH values.
Owner:QIQIHAR UNIVERSITY
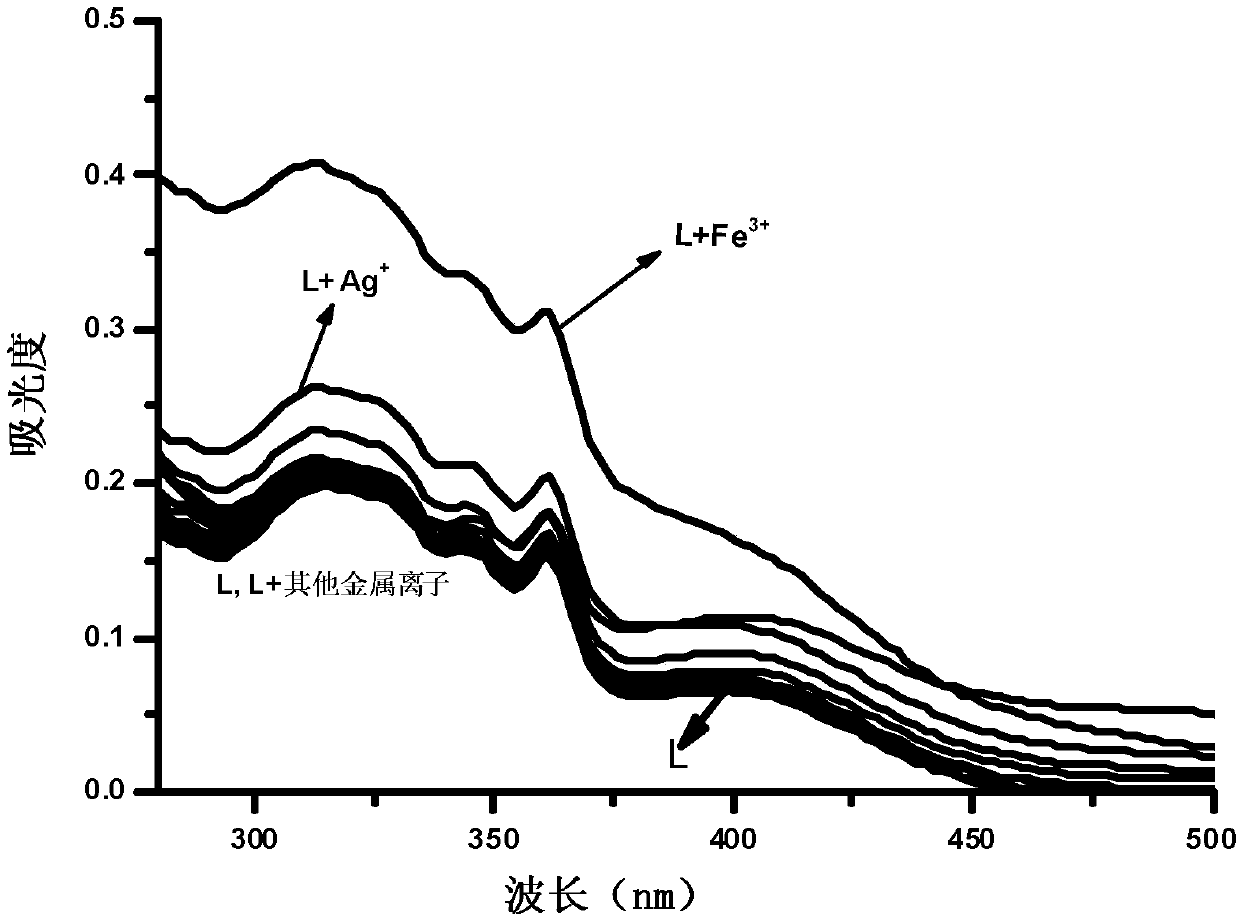
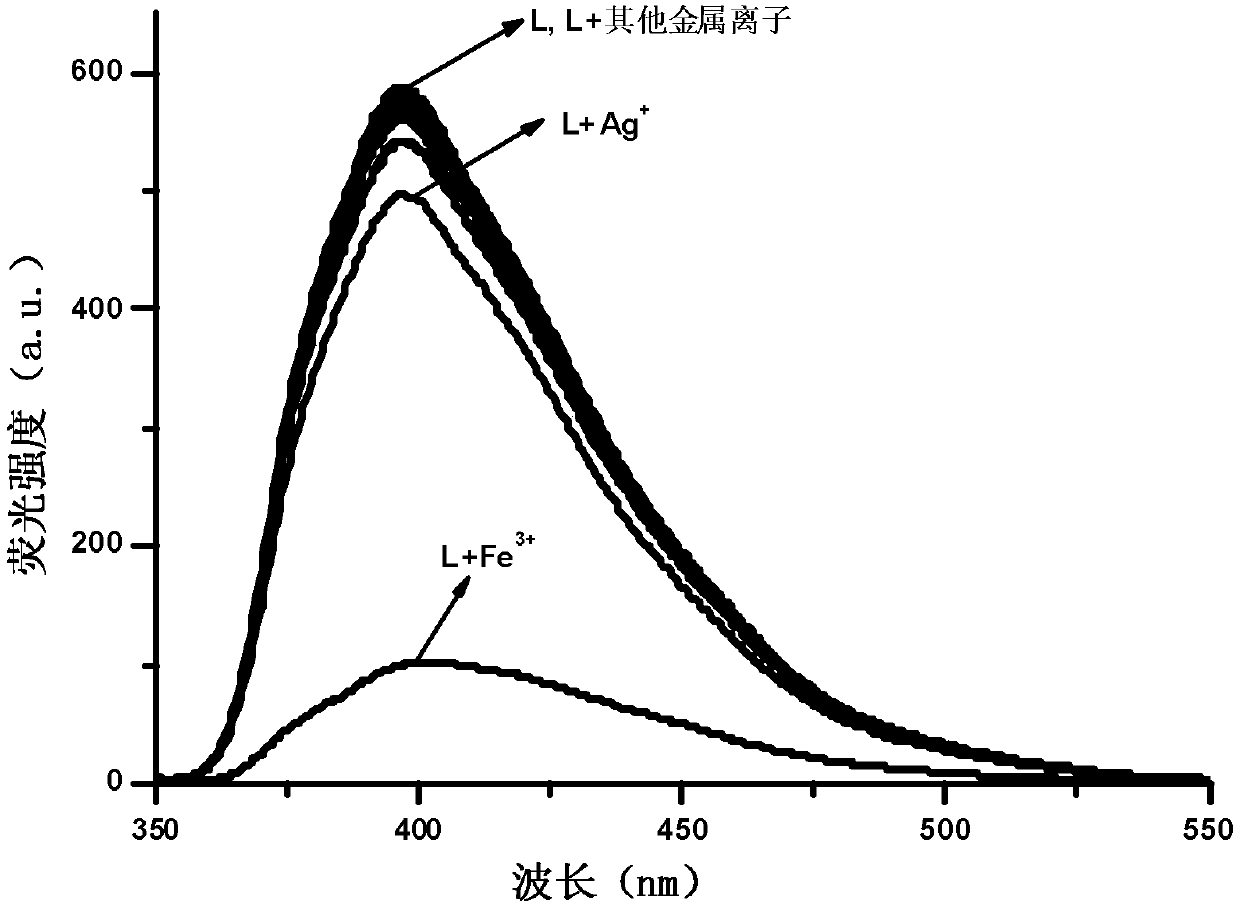
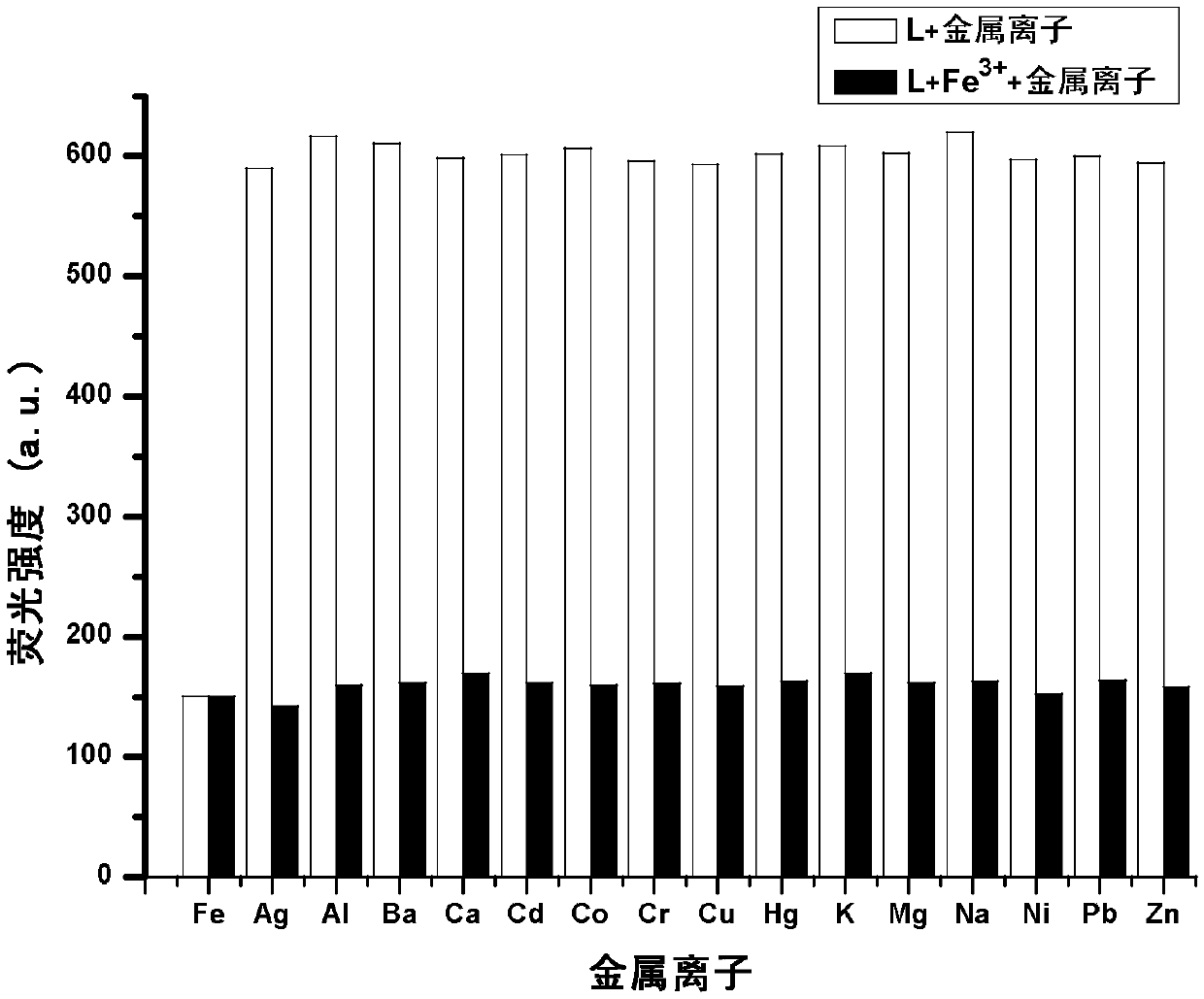
Phenanthrimidazole micromolecule Fe3+ fluorescent probe and synthesis method thereof
InactiveCN107629008AHigh selectivityHigh yieldOrganic chemistryFluorescence/phosphorescenceHydrazine compoundFluorescence
The invention provides a phenanthrimidazole micromolecule Fe3+ fluorescent probe and a synthesis method thereof and aims at solving the technical problems that an existing Fe3+ fluorescent probe is only applicable to acid and neutral identification environments and other metals interfere iron ion identification. The structural formula of the phenanthrimidazole micromolecule Fe3+ fluorescent probeis described in the specification. The synthesis method comprises the following steps: firstly, synthesizing an intermediate compound I with phenanthrenequinone, m-nitrobenzaldehyde and ammonium acetate; secondly, then synthesizing an intermediate compound II by utilizing the intermediate compound I, raney nickel and hydrazine hydrate solution; and thirdly, synthesizing the phenanthrimidazole micromolecule Fe3+ fluorescent probe with the intermediate compound II and 5-nitryl salicylaldehyde. The phenanthrimidazole micromolecule Fe3+ fluorescent probe can identify Fe3<+> in an aqueous phase system with the pH value of 3-12, is not interfered by other ions and can be used for detecting pollution of Fe<3+> in water.
Owner:QIQIHAR UNIVERSITY
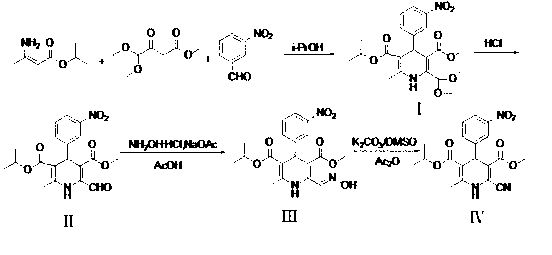
Preparation method of cardiovascular drug nilvadipine
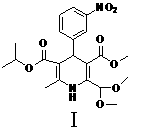
A preparation method of a cardiovascular drug nilvadipine comprises the following steps of (1) cyclizing isopropyl 3-aminocrotonate, methyl 4,4-dimethoxyacetylacetate and m-nitrobenzaldehyde without a solvent or in an organic solvent I to obtain an intermediate compound I, (2) dissolving the compound I in an organic solvent II, adding 2-6 equivalents of acid, stirring for hydrolysis reaction for 1-12h to generate an intermediate compound II, (3) dissolving the intermediate compound II, 1.1-1.3 equivalents of hydroxylamine hydrochloride and 1.4-1.6 equivalents of alkali in the organic solvent III for reaction for 2.5-4h, adding water, stirring and filtering to obtain an intermediate compound III, and (4) dehydrating the compound III in an alkaline environment to obtain nilvadipine IV. The preparation method is simple in process, low in cost, can greatly improve the purity of a nilvadipine intermediate, and is suitable for large-scale industrial production, and the yield of nilvadipine is higher.
Owner:HUNAN NORMAL UNIVERSITY
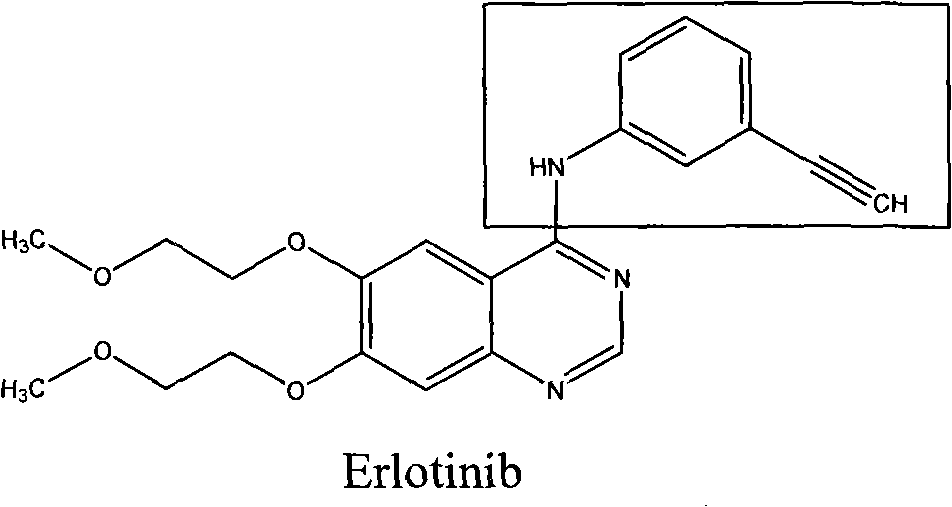
Synthesis method of erlotinib hydrochloride
InactiveCN102675225AEasy to separate and purifyMild reaction conditionsOrganic chemistryPropanoic acidSynthesis methods
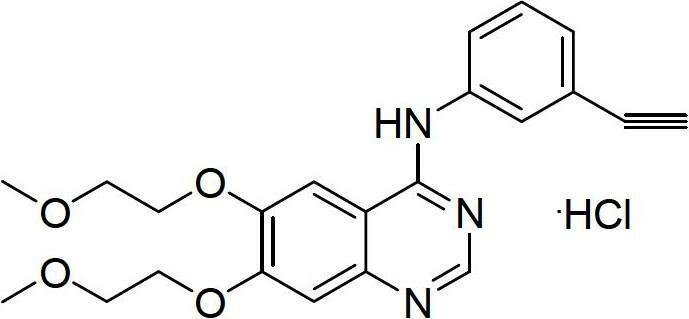
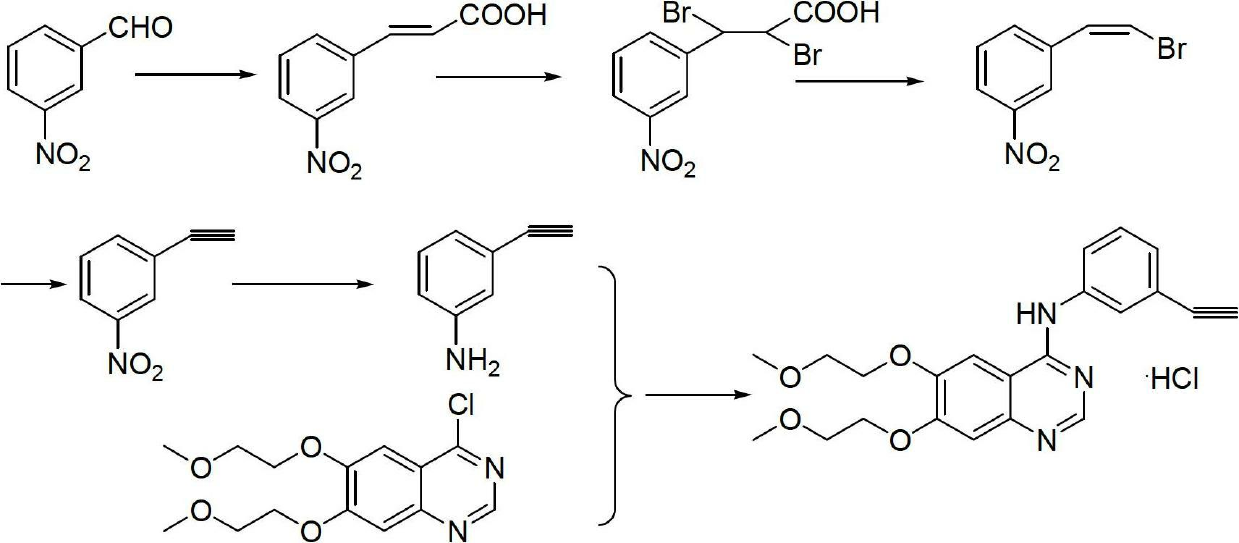
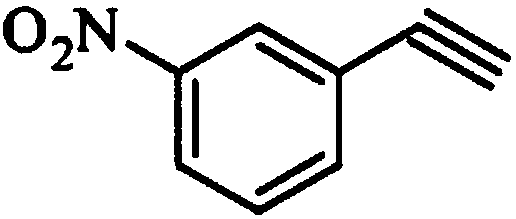
The invention relates to a synthesis method of erlotinib hydrochloride, which comprises the following technological process of: a, synthesizing m-nitrocinnamic acid by taking m-nitrobenzaldehyde as raw material; b, carrying out bromine addition to obtain 2, 3-dibromo-3-(3'-nitrophenyl) propanoic acid; c, carrying out decarboxylation and dehydrobromination to obtain (Z)-beta-bromo-(3'-nitrophenyl) ethylene; d, enabling the (Z)-beta-bromo-(3'-nitrophenyl) ethylene to have reaction with metal hydride to obtain m-nitrobenzene acetylene; e, reducing to obtain m-aminophenyl acetylene; and f, enabling the m-aminophenyl acetylene to have reaction with 4-chloro-6, 7-bi-(2-methoxy-ethoxy)-quinazoline, to obtain the erlotinib hydrochloride. The raw materials of the synthesis method are easy to obtain and low in cost, and the synthesis method is mild in reaction conditions, simple and convenient in operation, higher in yield and suitable for amplification.
Owner:ZHEJIANG SCI-TECH UNIV
Novel preparation method of intermediate namely 3-(1-piperidine methyl)phenol of roxatidine acetate hydrochloride
ActiveCN107698538AAvoid supply shortagesAvoid the situationOrganic chemistrySulfonyl chlorideBenzaldehyde
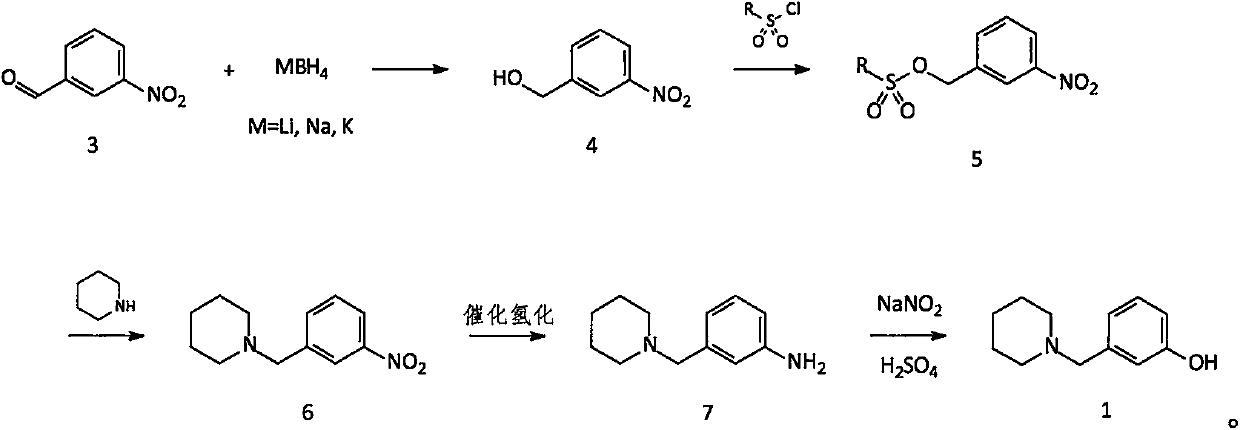
The invention relates to a preparation method of an intermediate namely 3-(1-piperidine methyl)phenol of roxatidine acetate hydrochloride. M-nitrobenzaldehyde is taken as the primary raw material; under the effect of a phase transfer catalyst, m- nitrobenzaldehyde is reduced by metal borohydride to obtain corresponding benzyl alcohol; in the presence of an alkali, benzyl alcohol reacts with organic sulfonyl chloride to generate active organic sulfonate; then in the presence of an alkali, organic sulfonate carries out N-alkylation reactions with piperidine to generate N-substituted piperidine derivatives; reducing the nitro groups of N-substituted piperidine derivatives to obtain corresponding amino compounds; and subjecting the amino compounds to diazotization, hydrolysis, and alkalizationin a sulfuric acid water solution to obtain 3-(1-piperidine methyl)phenol. The problem that the supply of the conventional raw material (m-hydroxyl benzaldehyde) is not enough and the cost is greatlyincreased is solved. The method is simple and safe, the raw materials are cheap and easily available, the reaction yield is high, and the method is very suitable for industrial production of 3-(1-piperidine methyl)phenol.
Owner:内蒙古京东药业有限公司
Metaraminol bitartrate synthesis method
InactiveCN109293518ASynthetic reaction raw materials are readily availableEasy to makeOrganic compound preparationCarboxylic acid salt preparationSynthesis methodsSodium nitrite
The invention discloses a metaraminol bitartrate synthesis method, which comprises: S1, raw material preparation: dissolving sodium hydrogen sulfite in water, adding m-nitrobenzaldehyde, stirring anddissolving at a temperature of 40-70 DEG C to obtain an adduct, adding ferrous sulfate to the adduct, heating for 1-3 h at a temperature of 90-120 DEG C while continuously stirring to reduce to obtainan amino product, cooling the reducing solution to a temperature of below 15 DEG C, adding a sodium nitrite solution in a dropwise manner, carrying out diazotization, hydrolyzing at a temperature of100-110 DEG C to obtain m-hydroxybenzaldehyde, decolorizing the m-hydroxybenzaldehyde with active carbon, and carrying out heating crystallization on the decolorized m-hydroxybenzaldehyde solution ata temperature of 120-200 DEG C; S2, addition reaction; S3, preliminary crystallization; S4, salt formation; S5, synthesis reaction; and S6, recrystallization. According to the present invention, the synthesis method has characteristics of easily-available raw materials, simple preparation, less synthesis steps, high preparation efficiency, high enantioselectivity, high yield, and easily-controlled, safe and reliable reaction operation.
Owner:HUBEI TIANSHU PHARMA CO LTD
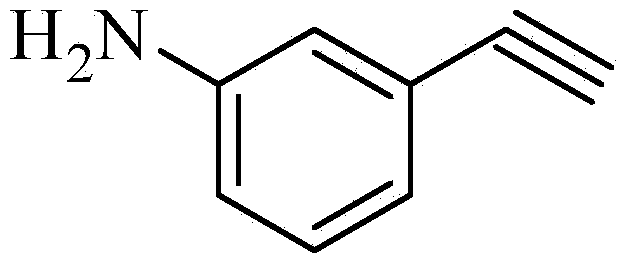
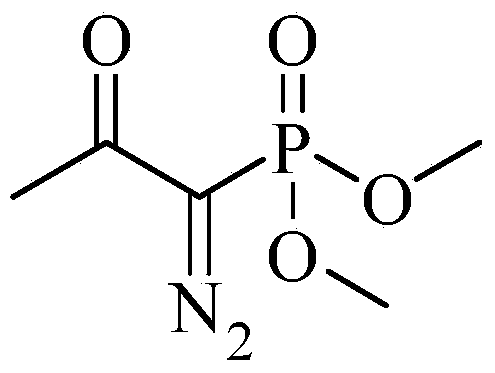
Preparation method for m-aminophenylacetylene
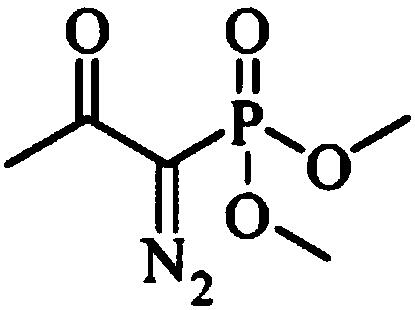
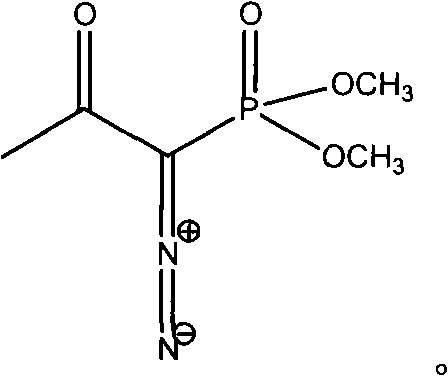
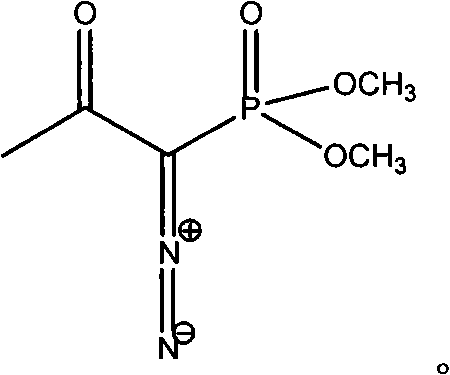
InactiveCN103724211AEfficient responseMild reaction conditionsOrganic compound preparationAmino compound preparationMeta-nitrobenzaldehydeAcetylene
The invention discloses a preparation method of m-aminophenylacetylene. The preparation method comprises the following steps: allowing dimethyl acetylmethylphosphonate to react with 4-methylbenzenesulfonylazide under a strong-alkali condition to obtain (1-diazo-2-oxo-propyl)-phosphonic acid dimethyl ester; allowing the (1-diazo-2-oxo-propyl)-phosphonic acid dimethyl ester to react with m-nitrobenzaldehyde under a catalytic condition to obtain 3-nitrophenylacetylene; finally, reducing the 3-nitrophenylacetylene to obtain the m-aminophenylacetylene. The preparation method has the advantages as follows: the starting raw materials are convenient and easy to obtain; the process route is simple and reasonable; the cost is low; three-waste pollution is small; large scale production is achieved; the product quality is good; the yield is high.
Owner:CHONGQING WORLD HAORUI PHARM CHEM
Preparation method of zaleplon
The invention discloses a preparation method of zaleplon, and belongs to the technical field of medicinal chemistry. According to the method, m-nitrobenzaldehyde and triethylamine which are simple and cheap are adopted as raw materials, and a core skeleton of zaleplon is efficiently and highly selectively constructed through a one-pot cascade reaction without transition metal catalysis, so that the generation of isomers is avoided, the generation of byproducts is reduced, the yield of a target product is increased, and the synthesis cost is reduced; and simple nitro reduction modification is performed to prepare zaleplon. The whole route is short, reaction conditions are mild, operation is easy and convenient, and the method is suitable for industrial production.
Owner:XINXIANG MEDICAL UNIV +1
Synthesis method of methoxy ethyl 2-(3-nitrobenzylidene)acetacetate
InactiveCN102442912AHigh purityPlay the role of removing impuritiesOrganic chemistryOrganic compound preparationAcetic acidSynthesis methods
The invention relates to a synthesis method of a cinildipine intermediate, namely methoxy ethyl 2-(3-nitrobenzylidene)acetacetate. The synthesis method comprises the steps of: (1) dropwise adding concentrated sulfuric acid to methoxy ethyl acetacetate under stirring, and then adding ethyl acetate; (2) adding m-nitrobenzaldehyde, reacting at room temperature, and after the reaction is stopped, standing overnight to separate out solids; (3) adding ethyl acetate until solids are completely dissolved, then washing with water and collecting an organic phase; and (4) evaporating to remove a solvent so as to separate out crystals, filtering and drying. As compared with the traditional preparation method of cinildipine condensation intermediate, the technical scheme provided by the invention has the advantages that: the obtained target product has high purity (more than 99%) and high yield ( more than 70%); and the synthesis method is mild in reaction conditions and simple and convenient to operate.
Owner:BENGBU BBCA MEDICINE SCI DEV
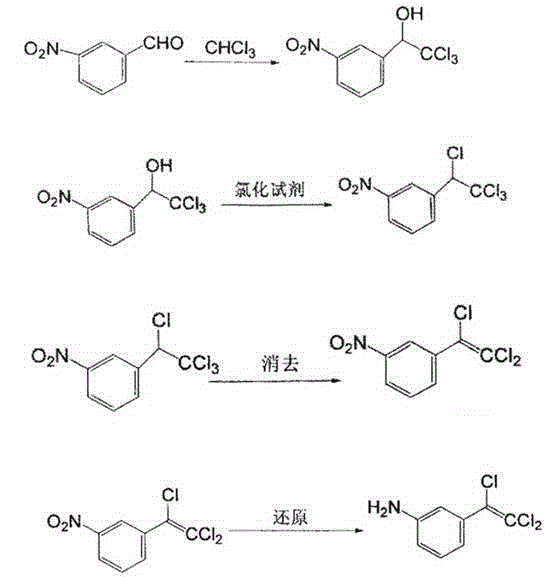
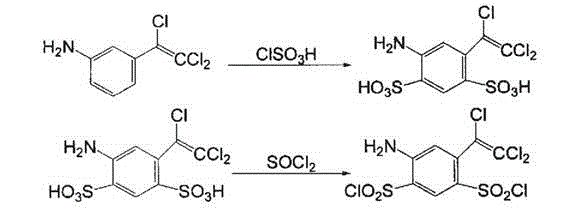
Preparation method of 4-amino-6-(trichloroethenyl)-1, 3-benzene disulfonamide
The invention discloses a preparation method of a veterinary drug 4-amino-6-(trichloroethenyl)-1, 3-benzene disulfonamide. The preparation method comprises the following steps: using m-nitrobenzaldehyde as a raw material, and performing condensation reaction, chlorination reaction, elimination reaction reduction reaction, sulfonation reaction and ammoniation reaction to obtain 4-amino-6-(trichloroethenyl)-1, 3-benzene disulfonamide, wherein a catalyst used in the condensation reaction is cesium carbonate; an iridium complex is used as a catalyst in the reduction reaction. The cesium carbonate is used as the catalyst in the condensation reaction and the iridium complex is used as the catalyst in the reduction reaction, so that the whole reaction process is speeded up, and the prepared veterinary drug is good in quality and high in yield.
Owner:丹阳恒安化学科技研究所有限公司
M-aminophenylacetylene preparation method
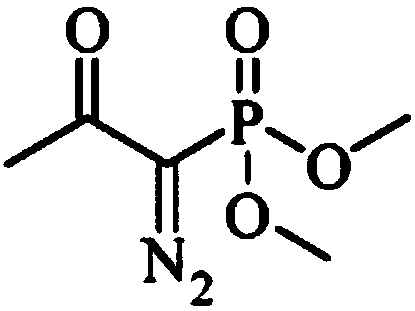
InactiveCN108569969AReduce manufacturing costReduce pollutionOrganic compound preparationGroup 5/15 element organic compoundsSynthesis methodsHigh pressure
The invention discloses an m-aminophenylacetylene preparation method, which comprises: carrying out a reaction on dimethyl acetylmethylphosphonate and tosyl azide under a strong alkali condition to obtain dimethyl(1-diazo-2-oxopropyl)phosphonate; carrying out a reaction on the dimethyl(1-diazo-2-oxopropyl)phosphonate and m-nitrobenzaldehyde under an alkaline catalyst condition to obtain m-nitrophenylacetylene; and reducing the m-nitrophenylacetylene to obtain the m-aminophenylacetylene. According to the present invention, the easily-available raw material m-nitrobenzaldehyde is used so as to avoid the use of the expensive raw materials such as m-iodonitrobenzene, m-bromonitrobenzene or m-nitrophenylacetylene and reduce the production cost; and the synthesis method has advantages of simpleoperation, no requirement of high pressure equipment, no requirement of vacuum rectification, simple requirement on equipment, cost saving and low environment pollution, and is suitable for industrialproduction.
Owner:XIAN GERUIDE NEW CHEM MATERIALS CO LTD
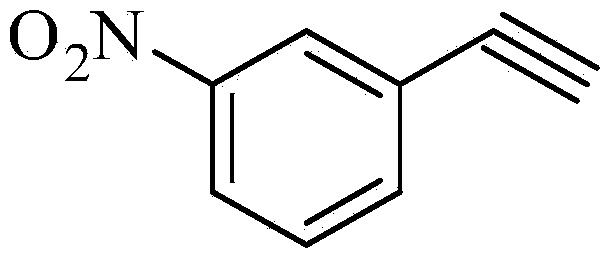
Technique of preparing m-nitrobenzene acetylene
InactiveCN101270053AShort reaction timeMild reaction conditionsOrganic chemistryOrganic compound preparationNitrobenzeneCombinatorial chemistry
The present invention relates to a preparation process of m-nitrobenzene acetylene. The preparation process comprises the following steps: m-nitrobenzaldehyde and Bestmann-Ohira agent react with carbonate or alkoxide, so as to prepare the m-nitrobenzene acetylene. The preparation process starts from the m-nitrobenzaldehyde to prepare the target product through one-step operation; the reaction time is short; the reaction conditions are mild; the post treatment is easy; no column chromatography is required; and the preparation process is simple, economic and practical.
Owner:UNIV OF JINAN
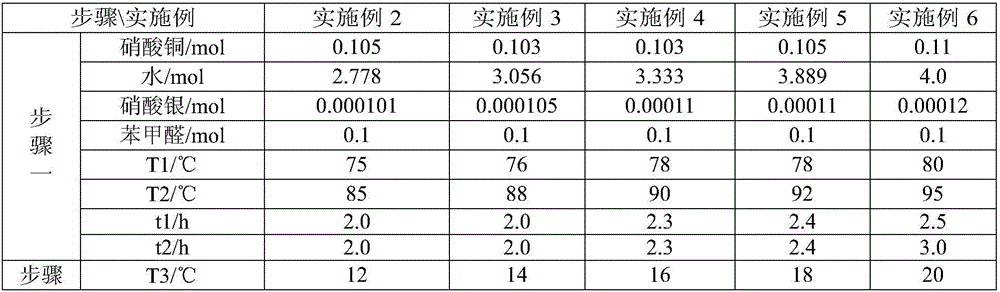
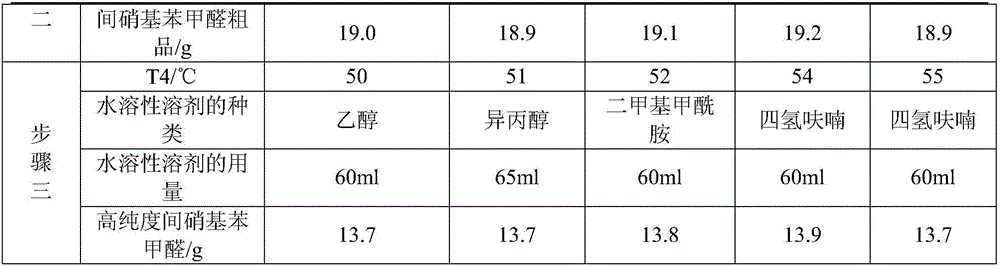
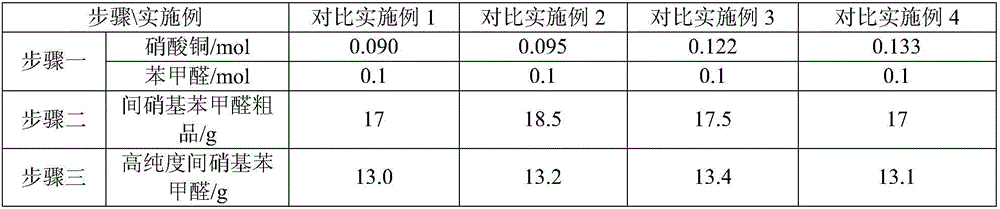
Synthesis method of m-nitrobenzaldehyde
The invention provides a synthesis method of m-nitrobenzaldehyde. The method comprises the following steps: firstly, reacting, concretely carrying out reaction on copper nitrate, water, silver nitrate and benzaldehyde in a molar ratio of (1.03-1.1):(20-40):(0.001-0.0012):1.0, so that reaction mixture is obtained; secondly, preparing an m-nitrobenzaldehyde crude product, concretely, filtering while the reaction mixture is hot, so as to obtain mother liquor, and then cooling the mother liquor, filtering, and taking solid, so that the m-nitrobenzaldehyde crude product is obtained; and thirdly, preparing high-purity m-nitrobenzaldehyde, concretely, adding a water soluble solvent into the m-nitrobenzaldehyde crude product, filtering, and drying, so that high-purity m-nitrobenzaldehyde is obtained. By adopting the method provided by the invention, molar yield of the obtained m-nitrobenzaldehyde is more than 90%, chromatographic purity is more than 99.8%, and content of o-nitrobenzaldehyde is less than 0.1%; and fewer three wastes are produced in a production process, so that the method provided by the invention is environmentally friendly.
Owner:YUEYANG YETOP FINE CHEM
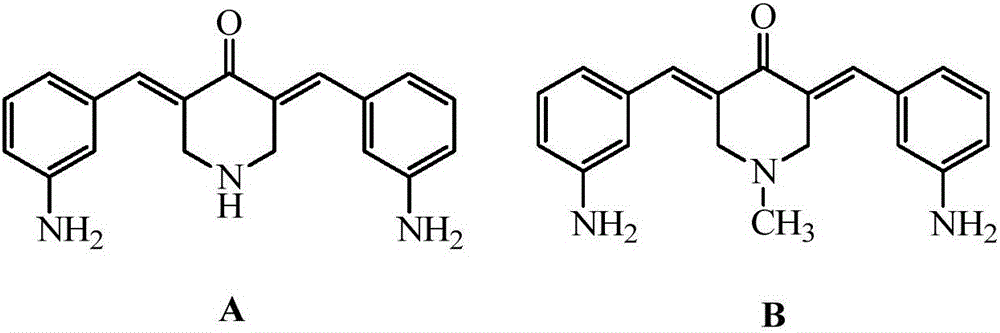
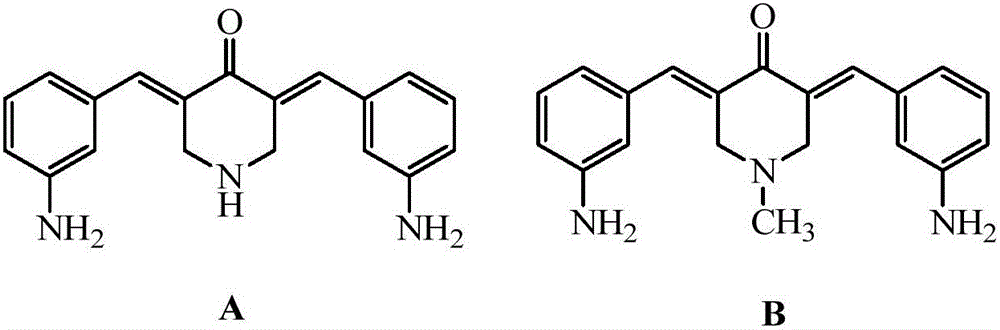
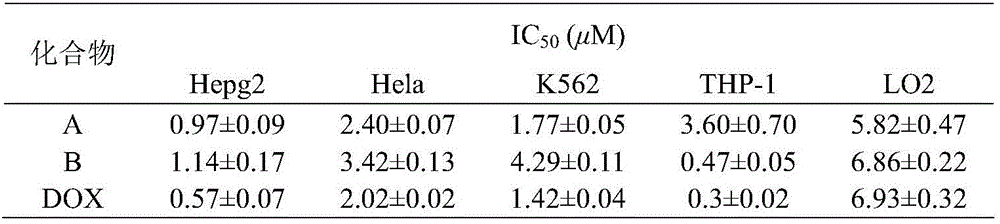
3,5-bis(3-aminobenzylidene)-4-piperidone derivatives with antitumor activity and preparation method thereof
InactiveCN105669537AAvoid genotoxicityLow toxicityOrganic chemistryAntineoplastic agents4-PiperidinoneAntitumor activity
The invention relates to two 3,5-bis(3-aminophenyl)methylene-4-piperidone compounds with antitumor activity and a preparation method thereof, and belongs to the technical field of antitumor drugs and preparation methods thereof.The preparation method of the compounds includes the steps that 4-piperidone and m-nitrobenzaldehyde are subjected to a Clayson-Schmidt condensation reaction to obtain an intermediate, and then the intermediate is subjected to reduction with a reducing agent to obtain the products A and B.The compounds are good in antitumor activity, can avoid the genotoxicity of antitumor drugs used at present and have little toxicity on normal cells.The preparation method is easy and convenient to implement, reaction conditions are mild, the synthesis yield is high, and thus the preparation method can be widely popularized in the antitumor field easily.
Owner:BINZHOU MEDICAL COLLEGE
Preparation method of isopropyl 2-(3-nitrobenzal) acetoacetate
ActiveCN102503833ASame catalytic effectReduce pollutionOrganic chemistryOrganic compound preparationMedicinal chemistryMeta-nitrobenzaldehyde
The invention discloses a preparation method of isopropyl 2-(3-nitrobenzal) acetoacetate. The method comprises the step of: reacting m-nitrobenzaldehyde with isopropyl acetoacetate in the presence of concentrated sulfuric acid as a catalyst to generate isopropyl 2-(3-nitrobenzal) acetoacetate, wherein the molar ratio of m-nitrobenzaldehyde to isopropyl acetoacetate to concentrated sulfuric acid is 1:(1-5):(0.26-0.41). The preparation method of isopropyl 2-(3-nitrobenzal) acetoacetate disclosed by the invention has low catalyst consumption and high yield.
Owner:BEIJING RED SUN PHARMA
Production process of m-nitrobenzaldehyde
The invention relates to a production process of m-nitrobenzaldehyde. The production process is characterized in that based on benzaldehyde used as a raw material, m-nitrobenzaldehyde is prepared by the steps of nitration, addition, alkali precipitation, refining and the like. According to the production process provided by the invention, the content of o-position isomers is controlled below 15% through regulating nitration conditions; and by utilizing the large difference in dissolvability between m-nitrobenzaldehyde and addition products of o-position isomers is large, most of o-position isomers are separated out. By test, the chromatographic purity of the crude product prepared by the production process provided by the invention can reach 99.2%, the chromatographic purity of the refined product can reach above 99.9%, and the content of o-position isomers can be controlled below 0.06%.
Owner:GUANNAN YISITE CHEM
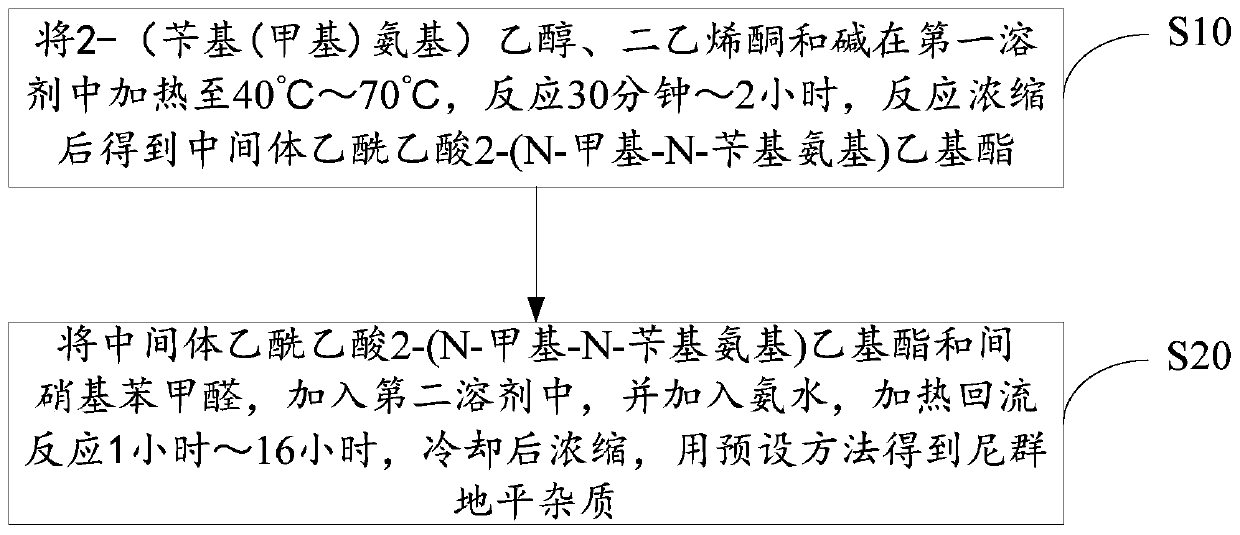
Method for preparing nitrendipine impurities
The invention discloses a method for preparing nitrendipine impurities. The method comprises the following steps that 2-(benzyl(methyl)amino)ethanol, diketene and base are heated in a first solvent to40DEG C-70DEG C for reaction for 30 minute-2 hour, and an intermediate acetoacetate 2-(N-methyl-N-benzylamino)ethyl ester is obtained after reaction concentration; and the intermediate acetoacetate 2-(N-methyl-N-benzylamino)ethyl ester and m-nitrobenzaldehyde are added to a second solvent, ammonia water is added, a heating reflux reaction is performed for 1 hour-16 hour, cooling and then concentration are performed, and the nitrendipine impurities are obtained by a predetermined method. According to the method for preparing the nitrendipine impurities, convenience is provided for impurity analysis and research of nitrendipine bulk drugs and a preparation thereof, and a detection method and a determination basis are provided for the production and drug safety of nitrendipine.
Owner:深圳振强生物技术有限公司
Method for separating benzaldehyde and nitrobenzaldehyde by employing high performance liquid chromatography
The invention discloses a detection method for separating isomers of benzaldehyde and nitrobenzaldehyde by employing high performance liquid chromatography. The method includes steps: C-18 and 5-fluoropheny hybrid bonded silica gel is regarded as a stationary phase, dipotassium phosphate-methanol-organic aqueous alkali is regarded as a mobile phase, and an HPLC spectrogram is recorded with the detection wavelength of less than 240 nm. By employing the method, miscellaneous peak interferences with peak-outlet positions of benzaldehyde, o-nitrobenzaldehyde, m-nitrobenzaldehyde and p-nitrobenzoicacid reference substances can be eliminated, the specificity and the separation degree are superior to those of the conventional method, the durability is good, quality control can be better realized, and the pharmaceutical security is facilitated.
Owner:HINYE PHARM CO LTD
Barnidipine hydrochloride synthesis process
InactiveCN105541797AIncrease profitOvercome the problems of many processes and heavy workloadOrganic chemistryOrganic basePropionitrile
The present invention discloses a barnidipine hydrochloride synthesis process, which comprises that: (1) 3-hydroxy propionitrile (I) and diketene (II) are subjected to a reaction to obtain a compound (VI); (2) the compound (VI) and m-nitrobenzaldehyde (III) are subjected to a reaction to obtain a compound (VII); (3) the compound (VII) and ethyl beta-aminocrotonate (IV) are subjected to a reaction to obtain a compound (VIII); (4) the compound (VIII) is subjected to hydrolysis with a strong base to obtain a compound (IX); (6) the compound (IX) is split with a chiral organic base to obtain a compound (X); (6) the compound (X) and benzyl pyrrole alcohol (V) are subjected to a reaction to obtain a compound (XI); and (7) the compound (XI) is added to a hydrogen chloride solution so as to obtain the barnidipine hydrochloride (XII). According to the present invention, the synthesis process has the following advantages that: (1) the reaction conditions are mild, and the products of every step are easy to separate and purify, and have controlled quality; (2) the yields of each step are high, the used raw materials and the used auxiliary materials are easy to obtain, and the total cost is low; and (3) the column separation is not required, and the process is suitable for industrial production.
Owner:赵建英
Preparation method of high-purity nitrendipine bulk drug
The invention provides a preparation method of a high-purity nitrendipine raw material medicine, which comprises the following steps: preparing a crude product 2-(3-nitrobenzylidene) ethyl acetoacetate at normal temperature by taking ethyl acetoacetate, 98% concentrated sulfuric acid and m-nitrobenzaldehyde as raw materials; then washing, centrifuging and drying to obtain a pure product 2-(3-nitrobenzylidene) ethyl acetoacetate; the preparation method comprises the following steps: adding ethyl 2-(3-nitrobenzylidene) acetoacetate into an organic solvent, then adding methyl 3-aminocrotonate and a moisture scavenger, carrying out heating reaction, after the reaction is finished, removing the moisture scavenger, cooling, stirring, centrifuging, and drying a solid to obtain nitrendipine. The purity of the nitrendipine bulk drug obtained by the preparation method of the high-purity nitrendipine bulk drug is 99.80% or above, and the impurity B and the impurity C are both less than 0.1%.
Owner:天津太平洋化学制药有限公司
Barnidipine hydrochloride compound and preparation method thereof
The invention discloses a barnidipine hydrochloride compound and a preparation method thereof. The preparation method comprises the following steps: (1) using 3-hydroxypropionitrile to react with diketene, to obtain an intermediate 1; (2) enabling the intermediate 1 to react with m-nitrobenzaldehyde and Beta-amino methyl crotonate, to obtain an intermediate 2; (3) enabling the intermediate 2 to behydrolyzed by strong base, to obtain an intermediate 3; (4) enabling the intermediate 3 to be resolved by chiral organic base, to obtain an intermediate 4; (5) enabling the intermediate 4 to react with thionyl chloride, (S)-1-benzyl-3-pyrrolidinol, and HCI ethanol solution, to obtain a crude product of barnidipine hydrochloride; and (6) performing ethyl alcohol pulping and refining, and ethyl alcohol recrystallization on the crude product of the barnidipine hydrochloride, to obtain the barnidipine hydrochloride.
Owner:森淼(山东)药业有限公司
Preparation method of hypotensive drug (R)-cilnidipine
InactiveCN107879971AHigh selectivityHigh yieldOrganic chemistry methodsHypotensive drugHypotensive agents
The invention relates to the preparation field of a single configuration of a hypotensive drug (R)-cilnidipine, in particular to a preparation method of a hypotensive drug (R)-cilnidipine. The preparation method comprises the following steps: 1) adding ethyl cyano acetoacetate, m-nitrobenzaldehyde, 3-amino-2-methoxyethyl butenoate into isopropanol for a reflux reaction to obtain a racemized product; 2) adding the racemized product into an aqueous solution, adding lipase as a catalyst to stir for a catalytic reaction, and after reaction, acidifying and filtering a reaction liquid to obtain (R)-1,4-dihydrogen-2,6-dimethyl-4-(3-nitryl phenyl)-3,5-dipicolinic acid-5-(2-methoxyl ethyl); and 3) carrying out an esterification reaction with cinnamyl alcohol to obtain (R)-cilnidipine. The method provided by the invention obtains (R)-cilnidipine with high selectivity and high yield, and ee% reaches up to 99.7%.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
Preparation process of hypotensive drug optically pure cilnidipine
InactiveCN107879970AHigh yield in three stepsHigh chemoselectivityOrganic chemistry methodsHypotensive drugCinnamic alcohol
The invention discloses a preparation process of a hypotensive drug optically pure cilnidipine. The preparation process comprises the following steps: 1) in the presence of (S)-BINAP and a ferric ironcompound, adding ethyl cyano acetoacetate, m-nitrobenzaldehyde and 3-amino-2-methoxyethyl butenoate into isopropanol to react to obtain (R)-1,4-dihydro-2,6-dimethul-4-(3-nitryl phenyl)-3,5-dipicolinic acid-3-(2-cyano ethyl ester)-5-(2-methoxyl ethyl ester); 2) stirring the product in a tetrahydrofuran aqueous solution of alkali to react to obtain (R)-1,4-dihydro-2,6-dimethyl-4-(3-nitryl phenyl)-3,5-dipicolinic acid-5-(2-methoxyl ethyl ester); and 3) carrying out an esterification reaction with cinnamyl alcohol to obtain the optically pure (R)-cilnidipine. The three-step yield of the (R)-cilnidipine reaches up to 41%, ee% reaches up to 99.5%, and the preparation process is simple and suitable for industrial production.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
Environment-friendly production method of benzaldehyde
InactiveCN110551029AHigh yieldAvoid damageOrganic compound preparationNitro compound preparationBenzaldehydeCopper nitrate
The invention relates to the technical field of organic compound synthesis, in particular to an environment-friendly production method of benzaldehyde, and aims to improve the product yield, reduce the production cost and reduce environmental damage. The preparation method is characterized by comprising the following steps: S1, adding copper nitrate, water, silver nitrate and catalyst potassium permanganate into a reaction device, heating, dropwise adding benzaldehyde, continuously heating, and carrying out heat preservation to react, so as to obtain a reaction mixed solution; and S2, filtering the reaction mixed solution to remove copper hydroxide precipitates to obtain a mother solution, cooling the mother solution, and filtering to obtain a solid m-nitrobenzaldehyde crude product with the content of o-nitrobenzaldehyde less than 0.5%; and S3, putting the m-nitrobenzaldehyde crude product into a reaction device, adding 3.0-3.5 volume times of a water-soluble solvent of the m-nitrobenzaldehyde crude product, heating, filtering to remove insoluble impurities, adding water of the same volume of that of the water-soluble solvent, filtering, taking out a precipitated solid, and drying to obtain the high-purity m-nitrobenzaldehyde.
Owner:枣阳市众成化工有限公司
Preparation method of isopropyl 2-(3-nitrophenylmethylene)acetacetate
InactiveCN102557957AMild reaction conditionsHigh yieldOrganic chemistryOrganic compound preparationN dimethylformamideTetrahydrofuran
The invention relates to a preparation method of isopropyl 2-(3-nitrophenylmethylene)acetacetate, particularly a preparation method of an azelnidipine intermediate isopropyl 2-(3-nitrophenylmethylene)acetacetate, which comprises the following steps: taking 130-170 parts by weight of nitrobenzaldehyde and 130-160 parts by weight of isopropyl acetacetate, dissolving in 500-1000 parts by volume of solvent, adding 1.45-145 parts by weight of catalytic amount of piperidine acetate, stirring at -20-45 DEG C for 1-24 hours, standing, and filtering to obtain the isopropyl 2-(3-nitrophenylmethylene)acetacetate. The solvent is one of C1-C4 alcohol, acetone, butanone, tetrahydrofuran, dioxane, benzene, methylbenzene, xylene, dichloromethane, chloroform, n-hexane, cyclohexane, acetonitrile and DMF (N,N-dimethylformamide).
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Preparation method for key intermediate of Barnidipine
ActiveCN110283118ASimple preparation processEasy to splitOrganic chemistryLewis acid catalysisCarboxylic acid
he preparation method of the intermediate is characterized by comprising the following steps: with chiral hydroxy acid as a starting material, the chiral hydroxy acid reacts with isopropanol under the catalysis of lewis acid, then reacts with an acetoacetic acid reagent, and is directly cyclized with m-nitrobenzaldehyde and methyl 3-aminocrotonate in an alcohol solvent, then crystallization is performed in a low temperature environment for realizing chiral resolution, hydrolysis is performed by sodium hydroxide, and then acidization is performed by hydrochloric acid to obtain a product. the intermediate is characterized by being prepared by the following steps: with chiral hydroxy acid as a starting material,the chiral hydroxy acid reacts with isopropanol under the catalysis of lewis acid, then reacts with an acetoacetic acid reagent, and is directly cyclized with m-nitrobenzaldehyde and methyl 3-aminocrotonate in an alcohol solvent, crystallization and chiral resolution are realized in a low temperature environment, hydrolysis is performed by sodium hydroxide, and then acidization is performed by hydrochloric acid to obtain a product. The preparation method disclosed by the invention has the advantages that the preparation technology is simple, the resolution is easy, the product yield is high, the optical purity is good, the quality is stable, and the large-scale industrial production is easy.
Owner:贵州中森医药有限公司
Industrial production method of benzaldehyde
InactiveCN110551030AHigh yieldAvoid damageOrganic compound preparationNitro compound preparationBenzaldehydeCopper nitrate
The invention relates to the technical field of organic compound synthesis, in particular relates to an industrial production method of benzaldehyde, and aims to improve the product yield, reduce theproduction cost and reduce environmental damage. The preparation method is characterized by comprising the following steps: S1, adding copper nitrate, water, silver nitrate and catalyst potassium chlorate into a reaction device, heating, dropwise adding benzaldehyde, continuously heating, and carrying out heat preservation to react, so as to obtain a reaction mixed solution; and S2, filtering thereaction mixed solution to remove copper hydroxide precipitates to obtain a mother solution, cooling the mother solution, and filtering to obtain a solid m-nitrobenzaldehyde crude product with the content of o-nitrobenzaldehyde less than 0.5%; and S3, putting the m-nitrobenzaldehyde crude product into a reaction device, adding 3.0-3.5 volume times of a water-soluble solvent of the m-nitrobenzaldehyde crude product, heating, filtering to remove insoluble impurities, adding water of the same volume of that of the water-soluble solvent, filtering, taking out a precipitated solid, and drying to obtain the high-purity m-nitrobenzaldehyde.
Owner:枣阳市众成化工有限公司
Method for preparing m-nitrobenzaldehyde by using microchannel reactor
PendingCN114478260AHigh purityHigh yieldOrganic compound preparationChemical/physical/physico-chemical microreactorsBenzaldehydeNitration
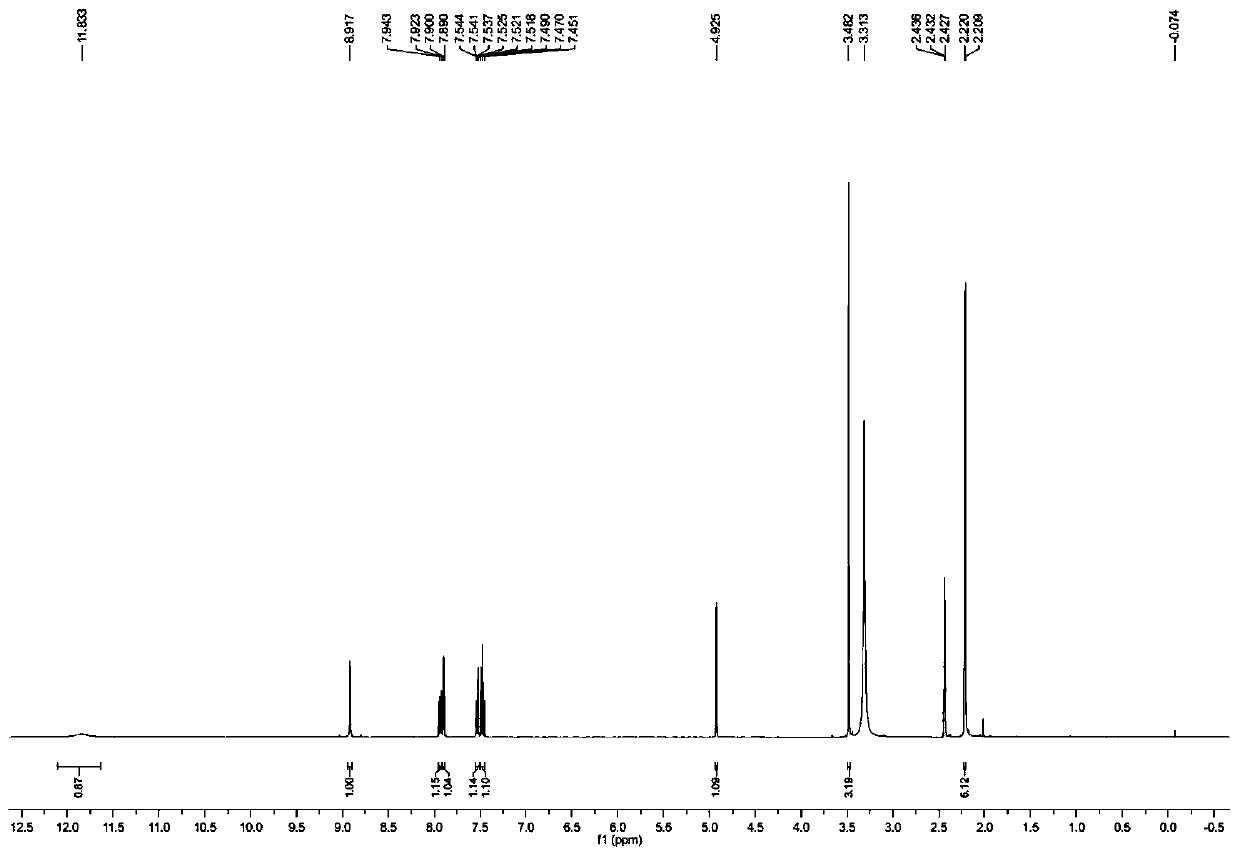
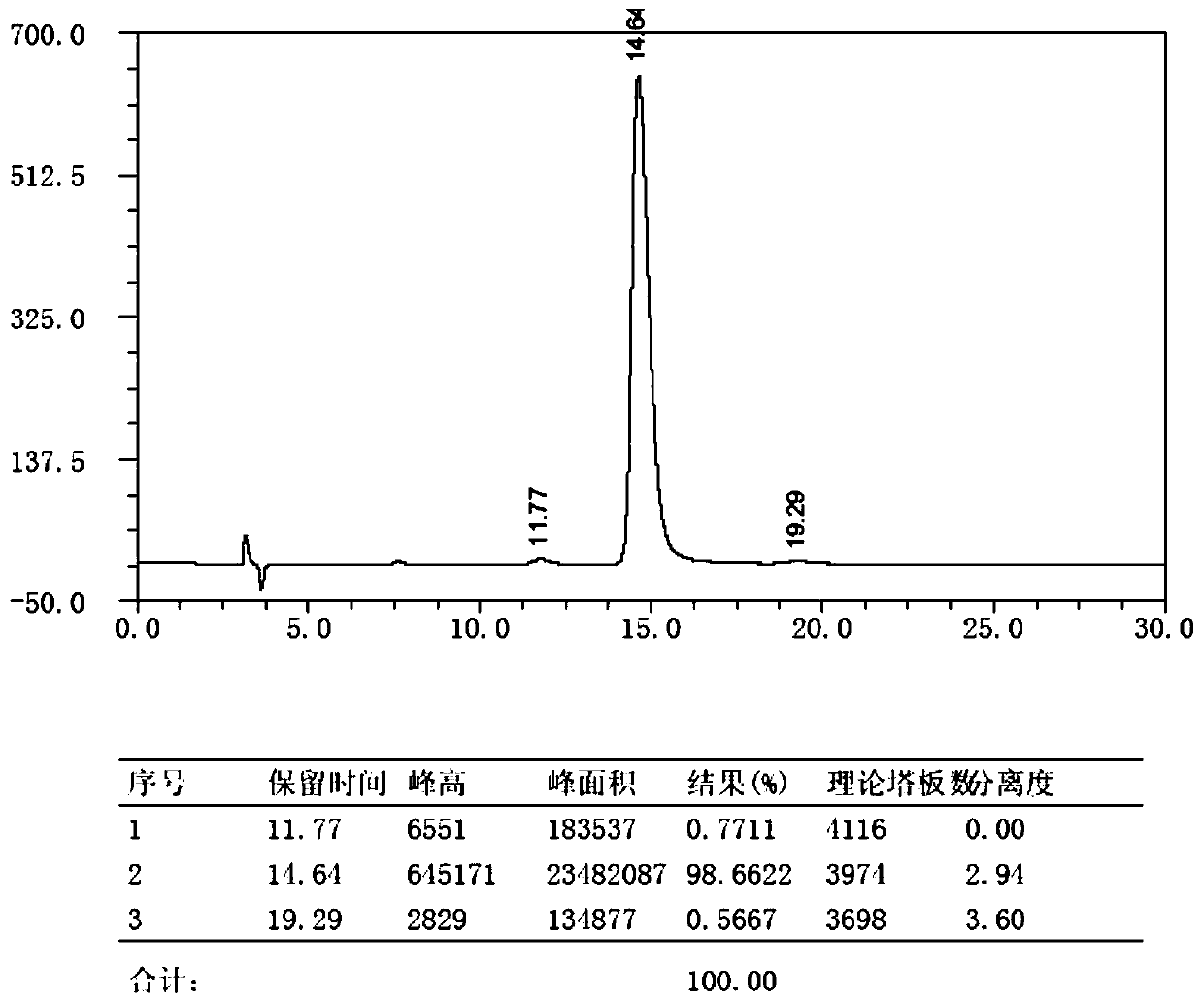
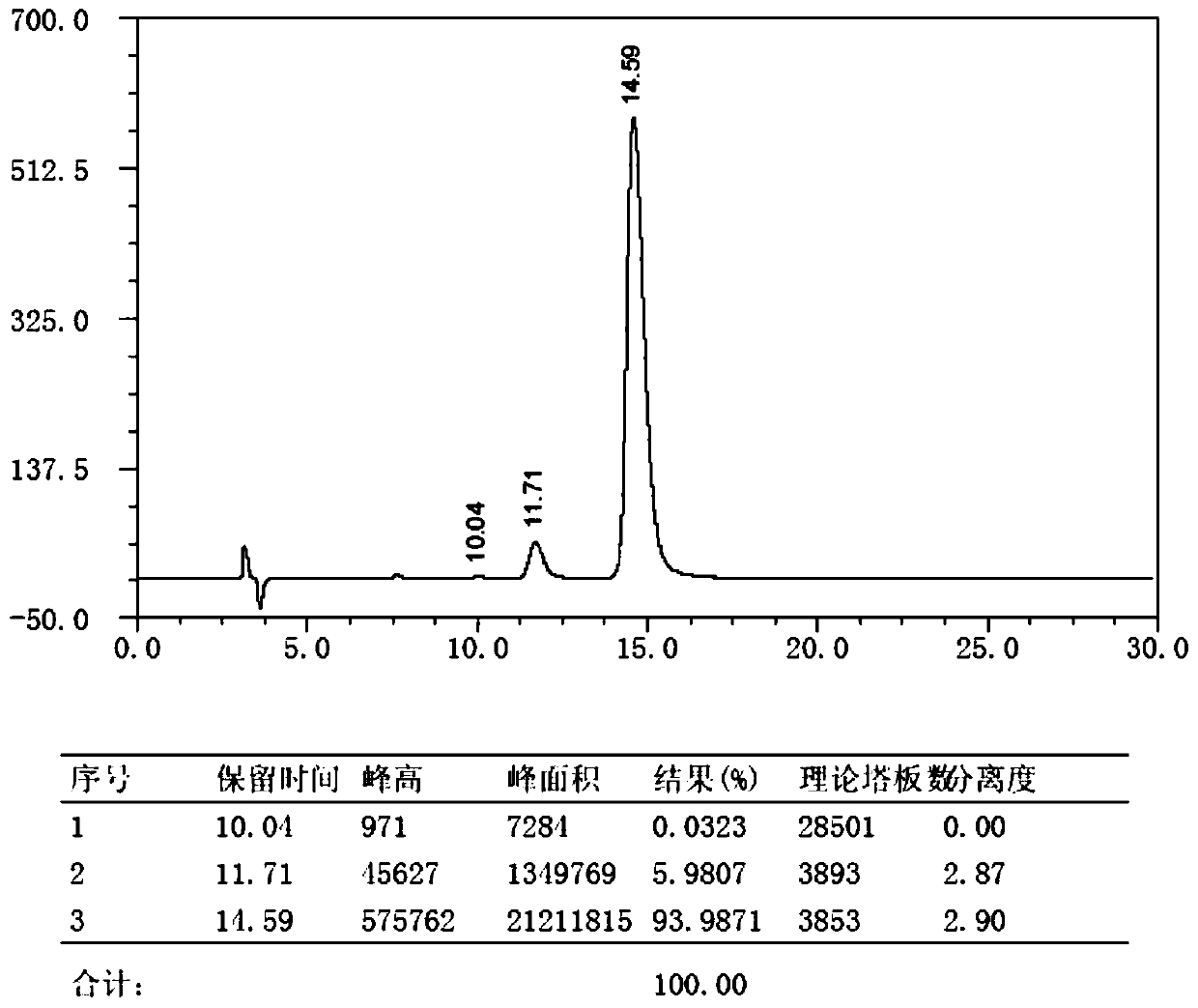
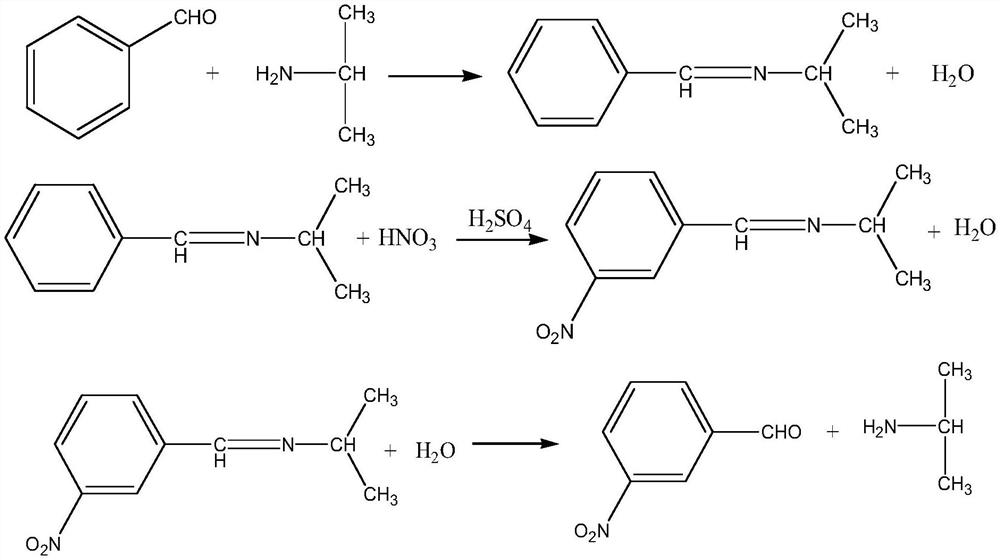
The invention discloses a method for preparing m-nitrobenzaldehyde by using a microchannel reactor, which comprises the following steps: carrying out condensation reaction on benzaldehyde and isopropylamine at 10-20 DEG C to generate Schiff base; carrying out nitration reaction on the Schiff base at 5-35 DEG C under the action of mixed acid of concentrated sulfuric acid and concentrated nitric acid; and after the nitration reaction is finished, carrying out hydrolysis and post-treatment to obtain the m-nitrobenzaldehyde. Wherein the condensation reaction and the nitration reaction are respectively carried out in the microchannel reactor. According to the preparation method for preparing m-nitrobenzaldehyde provided by the invention, the yield and purity of the final product m-nitrobenzaldehyde can be remarkably improved by controlling the temperatures of the condensation reaction and the nitration reaction aiming at the specific reaction substrate benzaldehyde. In addition, the preparation method is low in raw material cost, simple to operate and easy to control, convenient in post-treatment, high in safety and suitable for industrial continuous production.
Owner:廊坊市北辰创业树脂材料股份有限公司 +1
Preparation method of benzaldehyde
InactiveCN110551028AHigh yieldAvoid damageNitro compound preparationSimple Organic CompoundsBenzaldehyde
The invention relates to the technical field of organic compound synthesis, in particular to a preparation method of benzaldehyde, and aims to improve the product yield, reduce the production cost andreduce environmental damage. The preparation method is characterized by comprising the following steps: S1, adding copper nitrate, water, silver nitrate and catalyst concentrated nitric acid into a reaction device, heating, dropwise adding benzaldehyde, continuously heating, and carrying out heat preservation to react, so as to obtain a reaction mixed solution; and S2, filtering the reaction mixed solution to remove copper hydroxide precipitates to obtain a mother solution, cooling the mother solution, and filtering to obtain a solid m-nitrobenzaldehyde crude product with the content of o-nitrobenzaldehyde less than 0.5%; and S3, putting the m-nitrobenzaldehyde crude product into a reaction device, adding 3.0-3.5 volume times of a water-soluble solvent of that of the m-nitrobenzaldehyde crude product, heating, filtering to remove insoluble impurities, adding water of the same volume of the water-soluble solvent, filtering, taking out precipitated solids, and drying to obtain the high-purity m-nitrobenzaldehyde.
Owner:枣阳市众成化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com