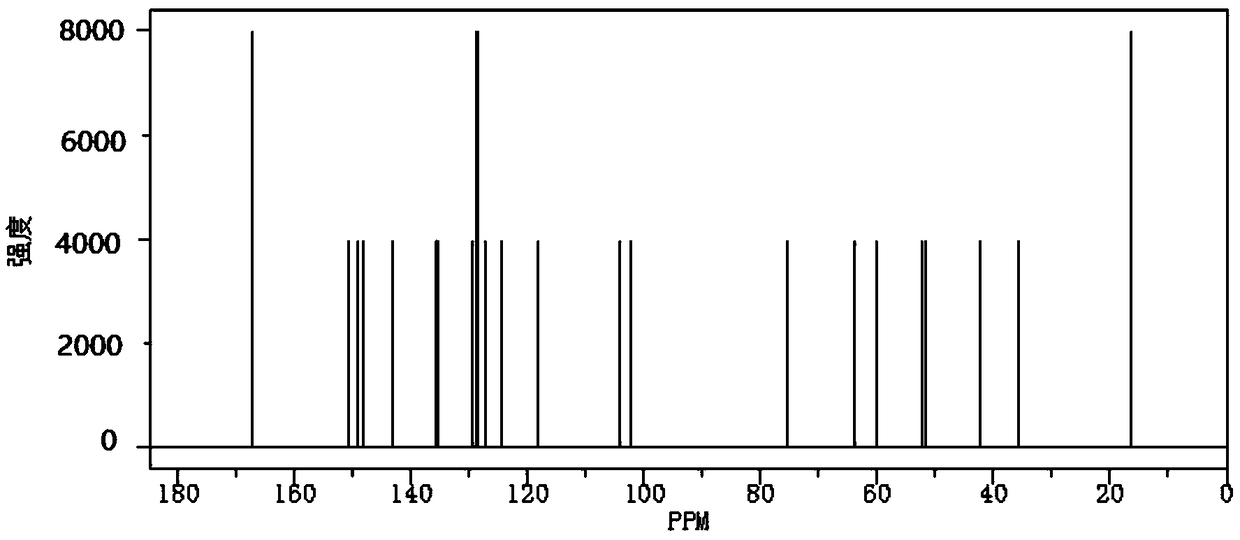
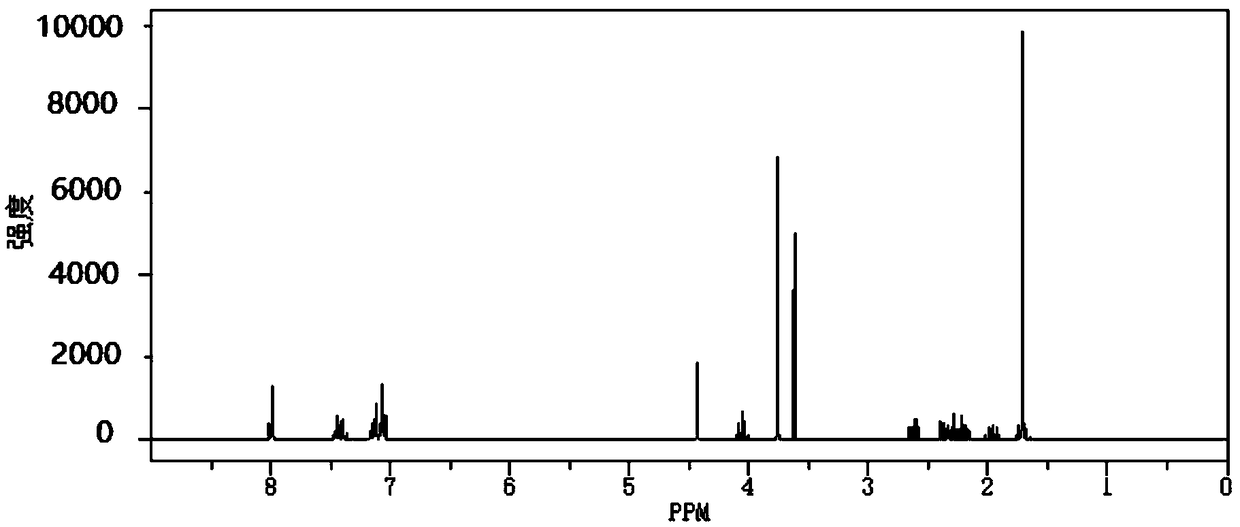
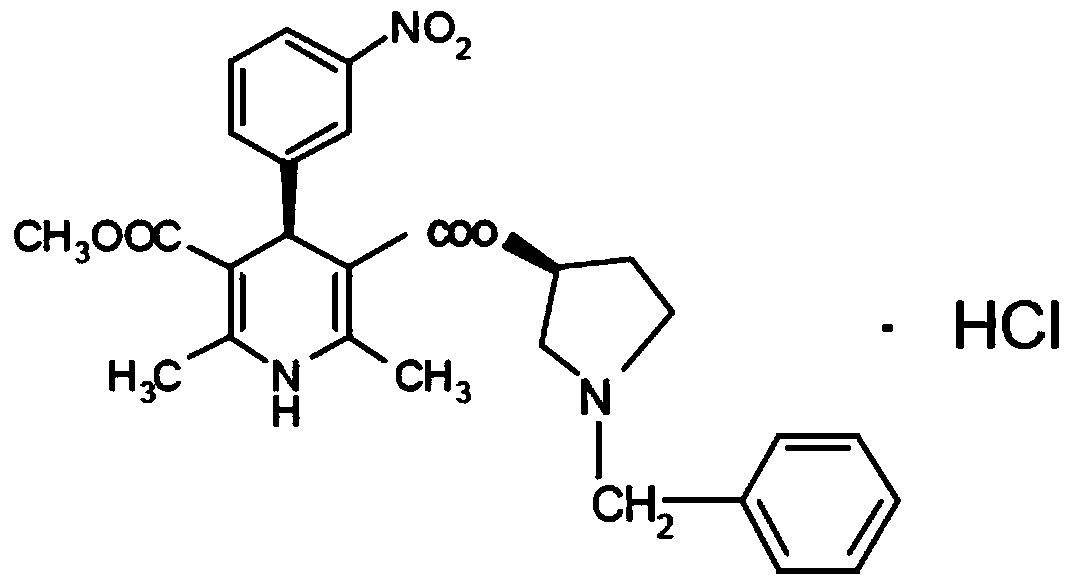
Barnidipine hydrochloride compound and preparation method thereof
A technology of barnidipine hydrochloride and a compound, applied in the direction of organic chemistry and the like, can solve the problems of difficulty in obtaining, low efficiency, difficult industrialization, etc., and achieves the effects of mild reaction conditions, guaranteed optical purity, and short synthetic route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 barnidipine hydrochloride
[0043] (1) Add 600g of 3-hydroxypropionitrile, 400g of ethyl acetate, and 15g of pyridine into the reaction flask, raise the temperature to 80°C, and slowly add diketene (645g) / ethyl acetate solution (1100g) dropwise for about 1 to 2 hours The addition is completed; then the reaction is incubated for 1 to 2 hours, and the TLV detection reaction is completed.
[0044] Cool down to room temperature, add saturated sodium bicarbonate solution dropwise to the reaction solution, stir and separate the liquids, take the organic phase, wash with purified water, dry the organic phase, filter, and concentrate to obtain 776g of intermediate 1 with a yield of 65.2%.
[0045](2) Add intermediate 1 (750g), m-nitrobenzaldehyde 730.5g, 3-aminocrotonate methyl ester 556.2g and isopropanol 4500g into the reaction flask, heat up to 70-80°C for 10-14h; cool down to room temperature, then cool down at 0-5°C for 1-2 hours, filter wi...
Embodiment 2
[0056] The preparation of embodiment 2 barnidipine hydrochloride
[0057] (1) Add 600g of 3-hydroxypropionitrile, 400g of ethyl acetate, and 15g of triethylamine into the reaction flask, raise the temperature to 80°C, and slowly add diketene (645g) / ethyl acetate solution (1100g) dropwise at the same time, about 1~ The dropwise addition was completed in 2 hours; then the reaction was incubated for 1 to 2 hours, and the TLV detection reaction was completed.
[0058] Cool down to room temperature, add saturated sodium bicarbonate solution dropwise to the reaction solution, stir and separate the liquids, take the organic phase, wash with purified water, dry the organic phase, filter, and concentrate to obtain 1021g of intermediate 1 with a yield of 85.8%.
[0059] (2) Add intermediate 1 (1000g), m-nitrobenzaldehyde 974g, 3-aminocrotonate methyl ester 741.6g and isopropanol 6000g into the reaction flask, heat up to 70-80°C for 10-14h; cool down to room temperature , then cool down...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com