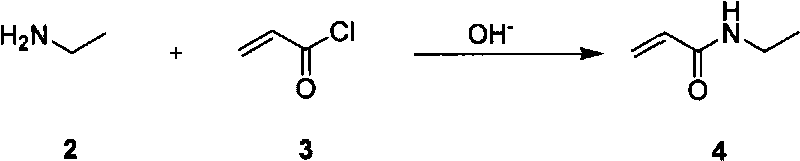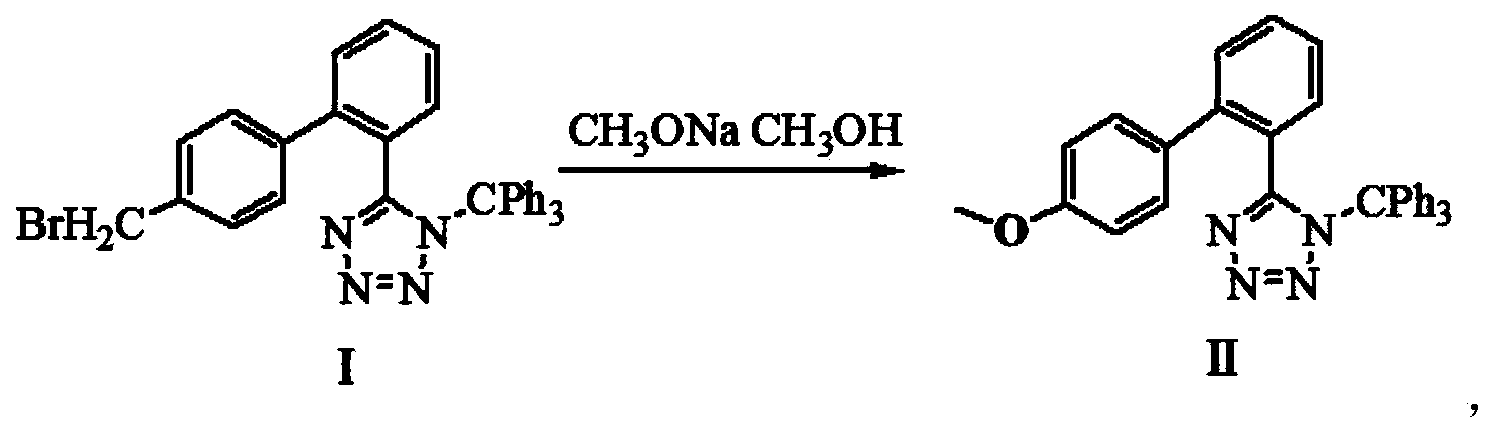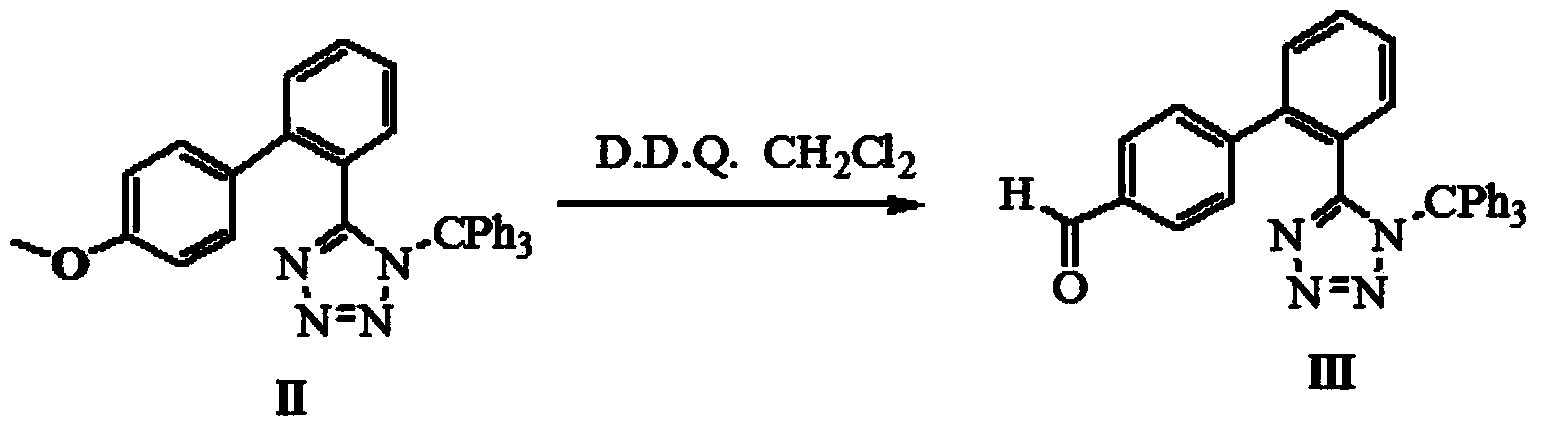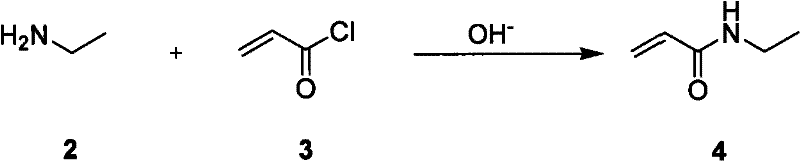Patents
Literature
42results about How to "Guaranteed optical purity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
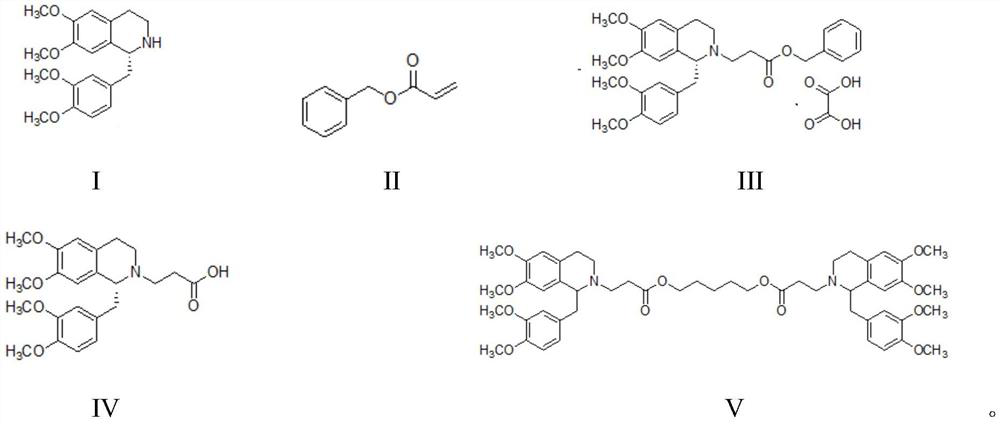
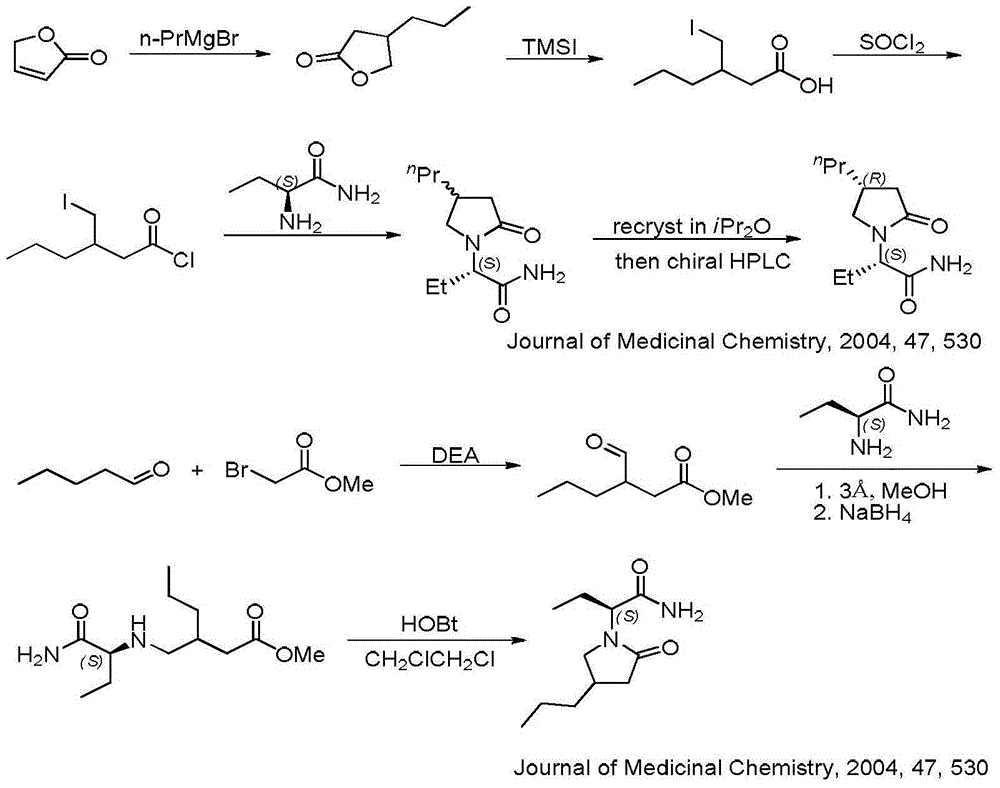
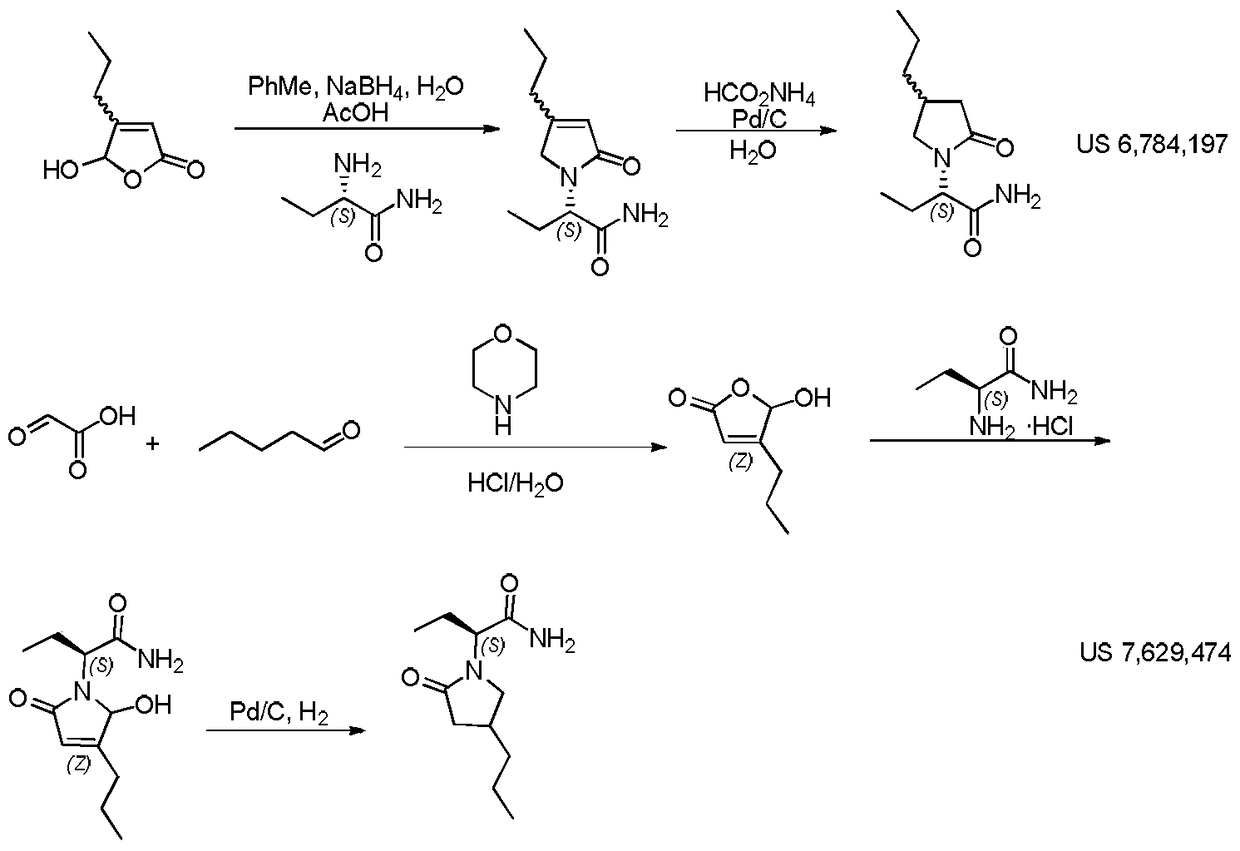
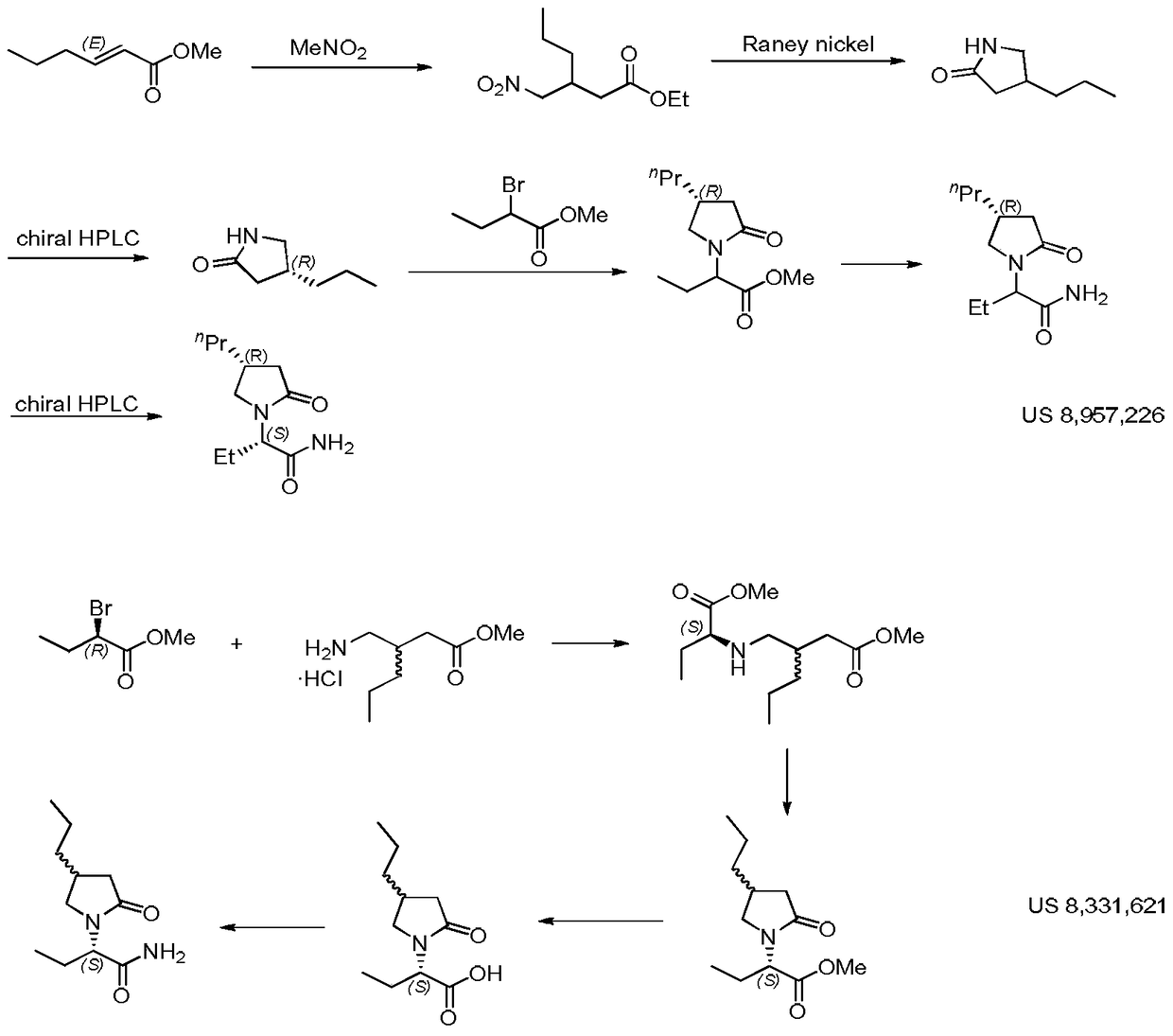
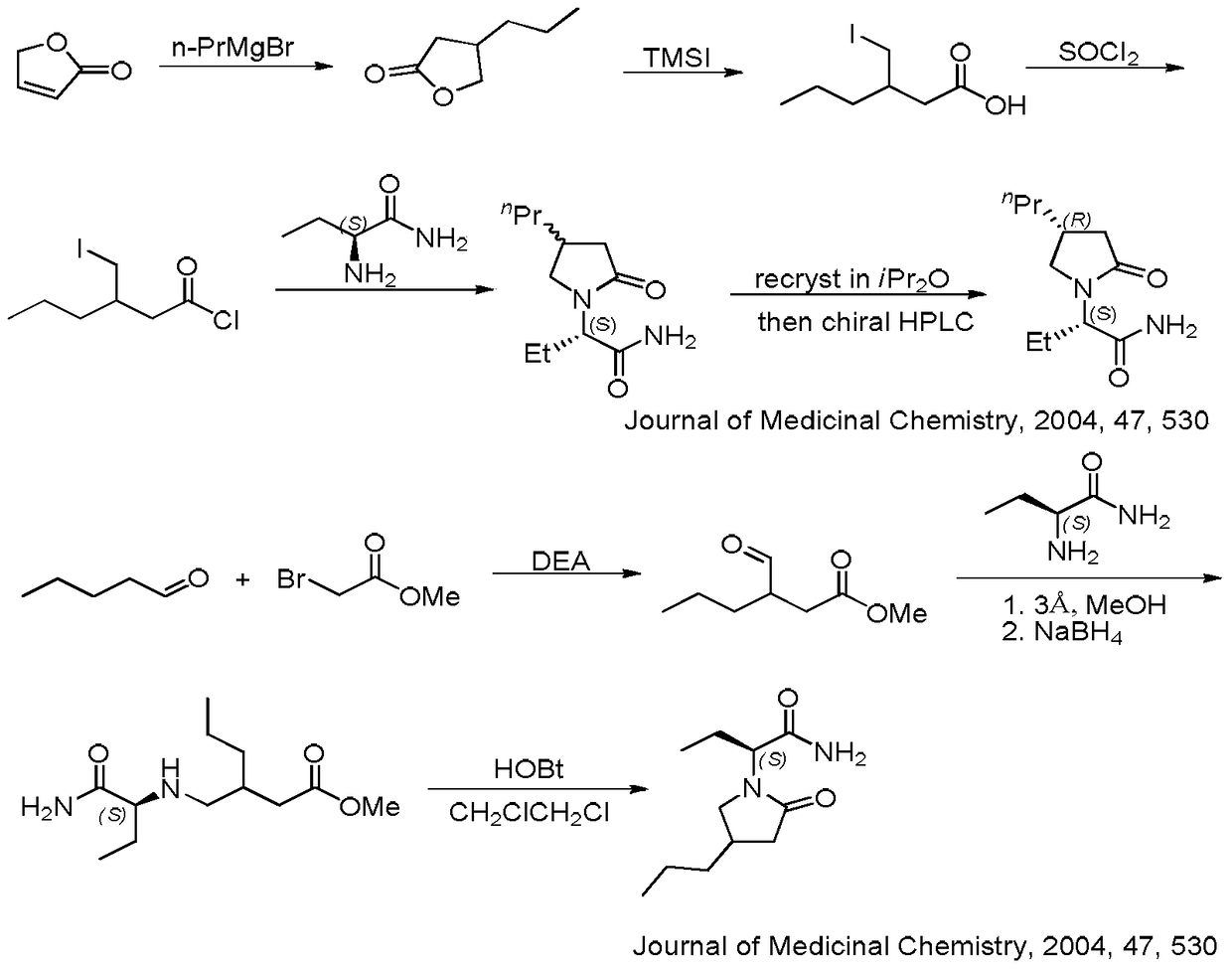
Compound and its preparation method and use in brivaracetam synthesis
ActiveCN106279074ARaw materials are easy to getLow priceOrganic chemistrySynthesis methodsCombinatorial chemistry
The invention provides a compound shown in the formula I and a preparation method thereof. The invention also provides a use of the compound shown in the formula I in brivaracetam synthesis and a synthesis method of brivaracetam. The synthesis method utilizes cheap and easily available raw materials and can produce high optical purity brivaracetam.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
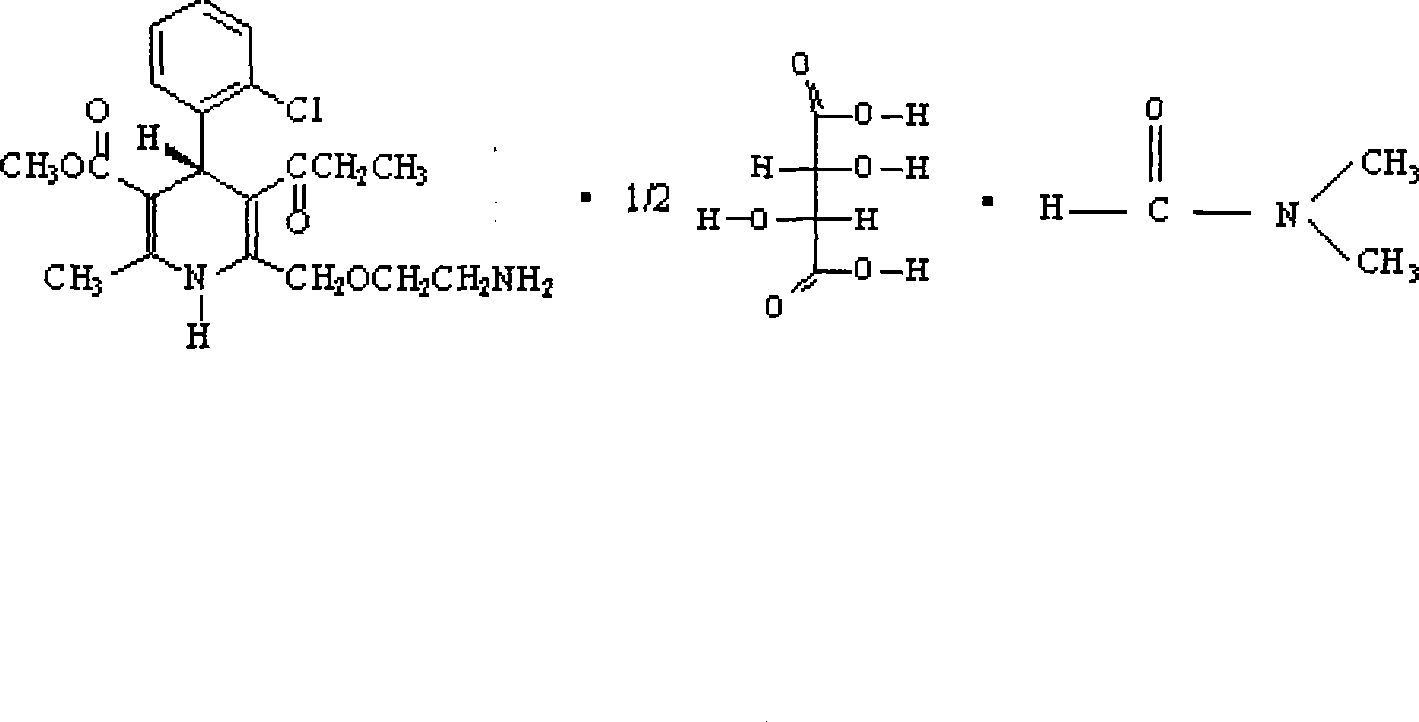
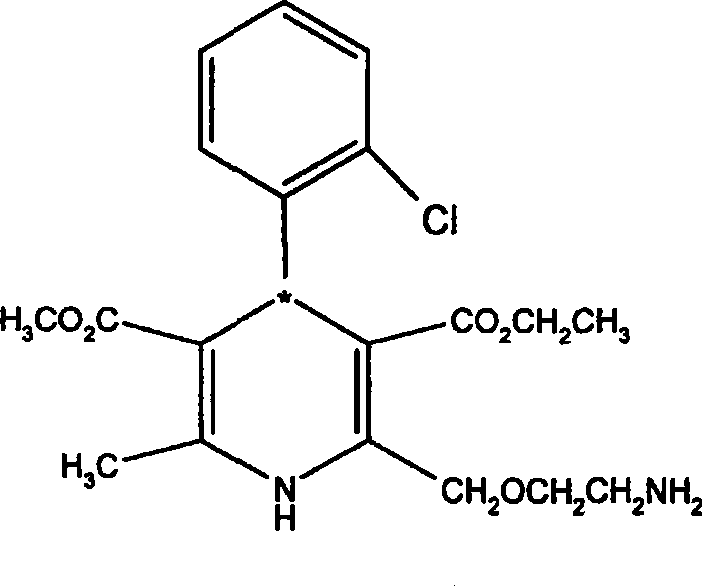
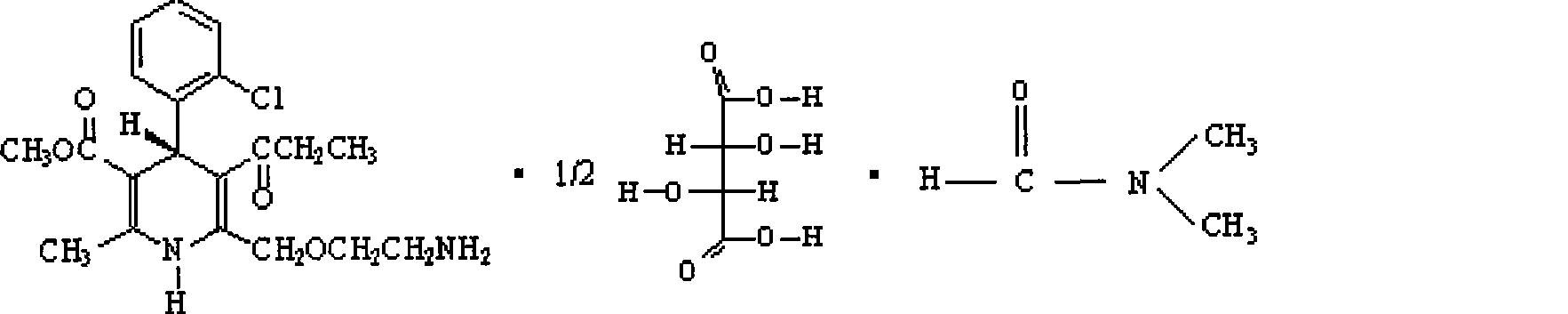
Method for preparing amlodipine
ActiveCN101544597AEasy to recycleEasy to polluteOrganic active ingredientsOptically-active compound separationN dimethylformamideSolvent
The present invention relates to a method for preparing a novel S-(-)-amlodipine, and especially to a novel method for preparing S-(-)-amlodipine through the resolution of racemic amlodipine. The N,N-dimethylformamide (DMF) is used as a dissolvent. The L-(+)-tartaric acid is used as a resolving agent. Not only can the excellent resolving effect be obtained, but also the cost is low and safety is excellent.
Owner:北京天衡药物研究院有限公司
Preparation method of Rivaroxaban midbody and novel synthetic method of Rivaroxaban
The invention discloses a preparation method of (S)-N-glycidol phthalimide in a structural formula 1. The method comprises the following steps of: I, phthalimide salt of a structural formula 9 and a compound of a structural formula 10 are subjected to heating reflux for reaction in an alcohols solvent to obtain the compound of a structural formula 11; II, the compound of the structural formula 11 obtained form the step I is reacted with the compound of a structural formula 12 in an aprotic solvent under the action of alkaline to obtain a (S)-N-glycidol phthalimide crude product of the structural formula 1; and III, the (S)-N-glycidol phthalimide crude product of the structural formula 1 obtained from the step II is refined by ethanol to obtain the (S)-N-glycidol phthalimide with the optical purity larger than or equal to 99.0%. The preparation method is simple to operate and good in safety, the optical purity of the obtained product is high (larger than or equal to 99.0%), and the preparation method is suitable for industrial production. The invention also discloses a novel synthetic method of Rivaroxaban.
Owner:JIANGXI SYNERGY PHARMA
Method of synthesizing GHK acetate at low cost
ActiveCN107778349AStrong UV Absorbing PropertiesAvoid cumbersomenessPeptide preparation methodsBulk chemical productionAcetic acidTrifluoroacetic acid
The invention discloses a method of synthesizing GHK acetate at low cost. The method includes steps of: 1) performing a reaction to Trt-Gly-OH with N-hydroxysuccinimide to generate Trt-Gly-OSu; 2) performing a reaction with H-His(Trt)-OH to generate Trt-Gly-His(Trt)-OH; 3) performing a reaction with N-hydroxysuccinimide to generate Trt-Gly-His(Trt)-OSu; 4) performing a reaction with Lys(Trt)-OH togenerate Trt-Gly-His(Trt)-Lys(Trt)-OH, and removing protective groups from the Trt-Gly-His(Trt)-Lys(Trt)-OH in acetic acid to generate the GHK acetate. In the method, all the protective groups are triphenylmethyl group, so that optical purity is guaranteed well; deprotection can be directly carried in acetic acid, so that byproducts generated during deprotection of trifluoroacetic acid can be avoided. The GHK acetate is free of reverse phase chromatograph for purification and ion exchange chromatography for remove trifluoroacetic acid, so that production cost is reduced largely and the methodcan be used in large-scale production.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
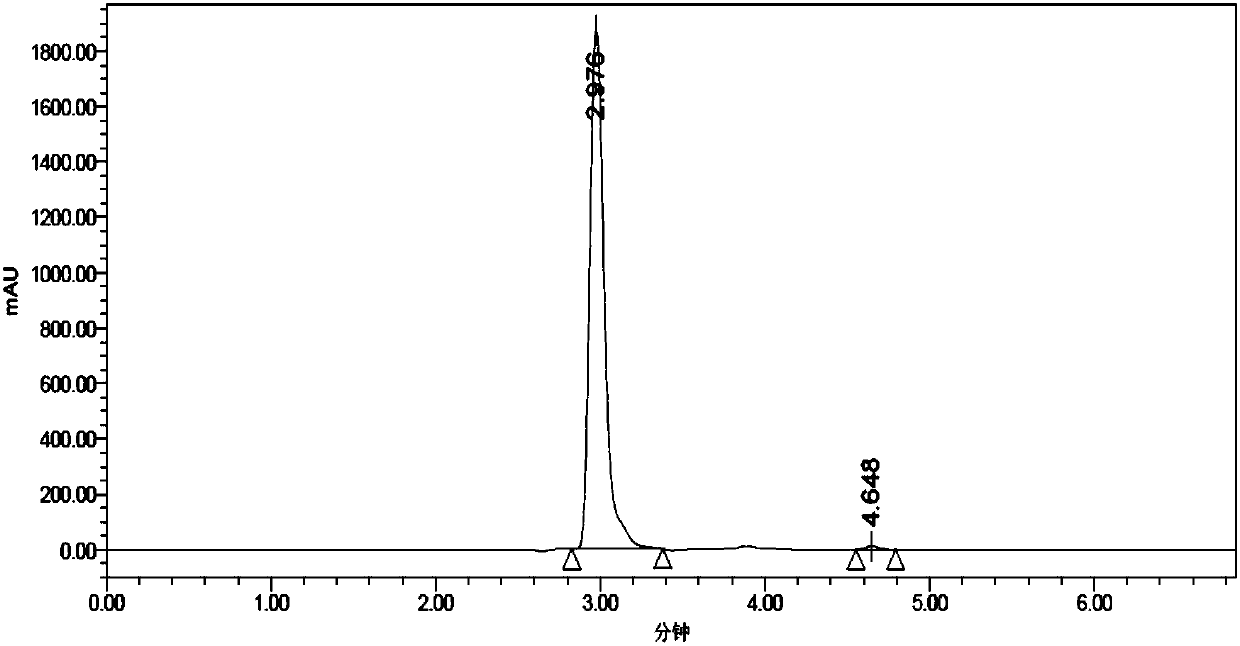
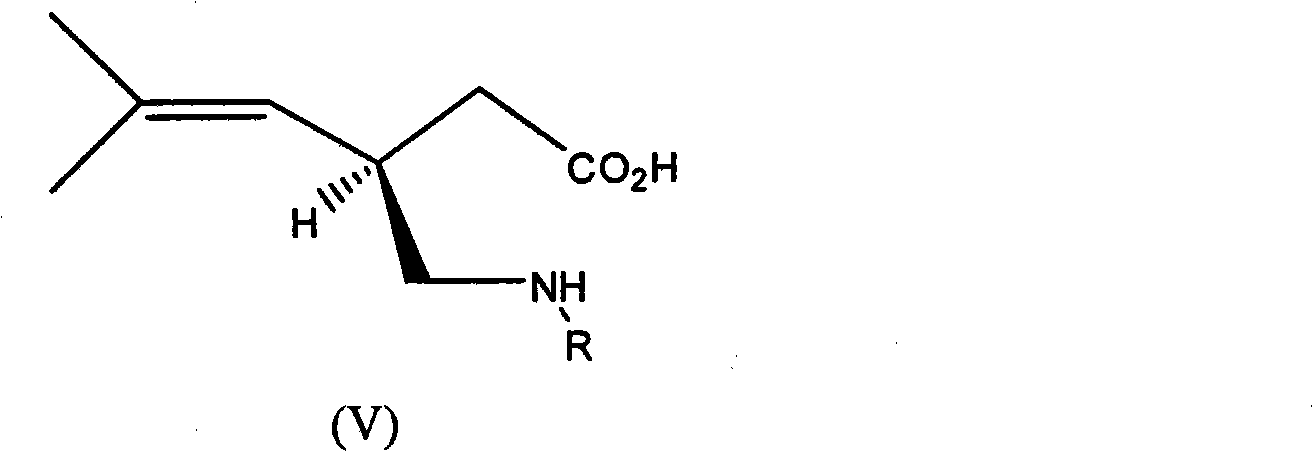
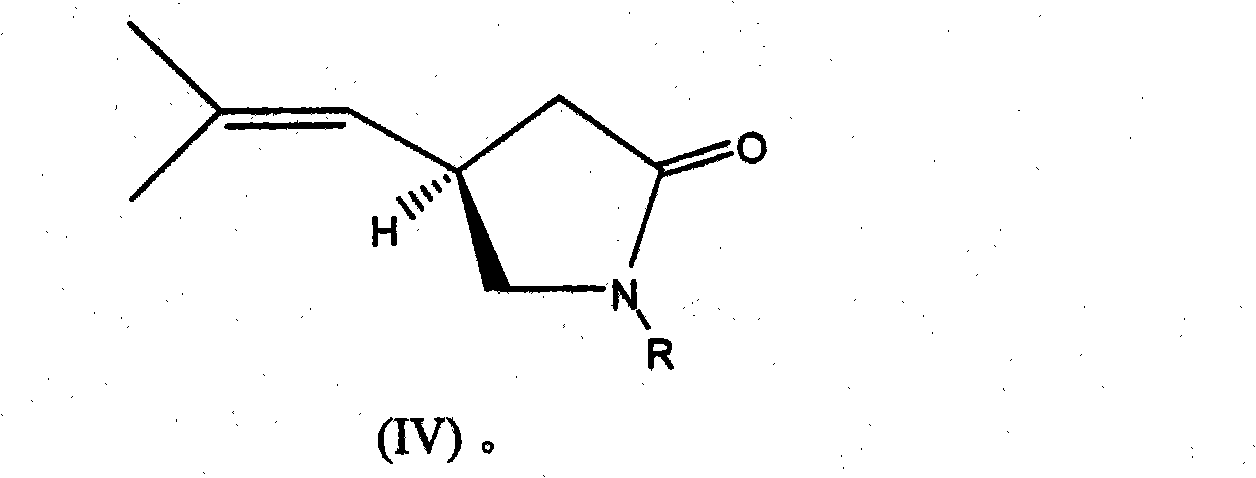
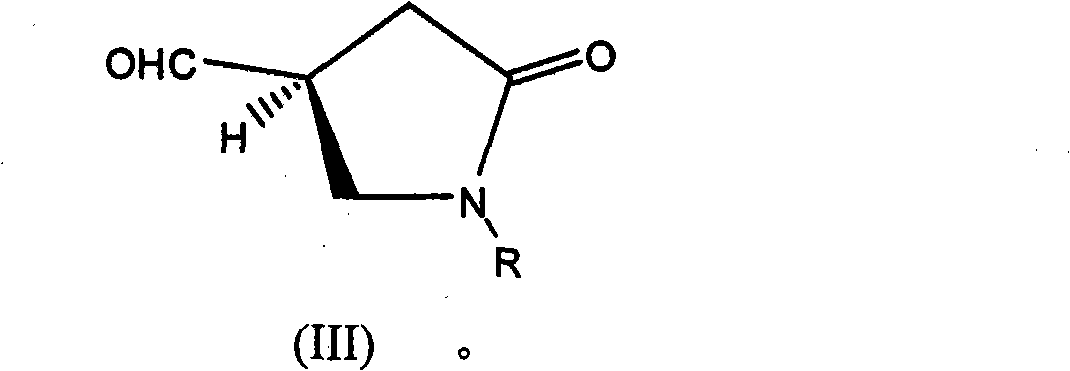
Process for the enantioselective preparation of pregabalin
InactiveCN101848905AGuaranteed optical purityOrganic compound preparationAmino-carboxyl compound preparationPregabalinAcid hydrolysis
The invention provides a new enantioselective process for the preparation of (S) -pregabalin, or a pharmaceutical acceptable addition acid salt comprising: acid hydrolysis of the dioxolan ring of a chiral compound of general formula (I) to obtain compound of general formula (II); oxidation of compound (II) to obtain a compound of general formula (III) and transforming compound (III) into compound of general formula (IV); subjecting compound (IV) to basic hydrolysis to obtain a compound of general formula (V) which is reduced to obtain enantiomerically pure S-pregabalin. The invention also provides new chiral intermediates involved in the process.
Owner:LAB DEL DR ESTEVE SA
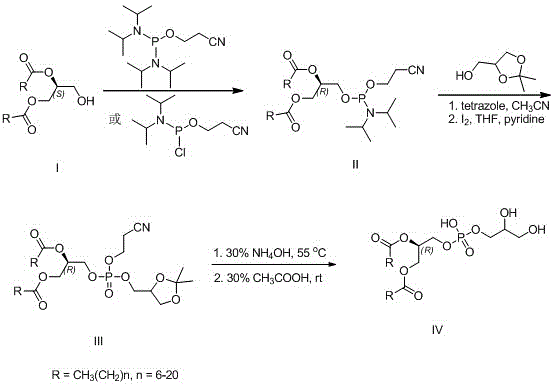
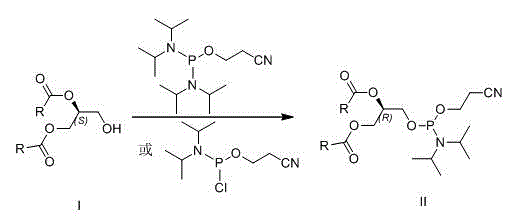
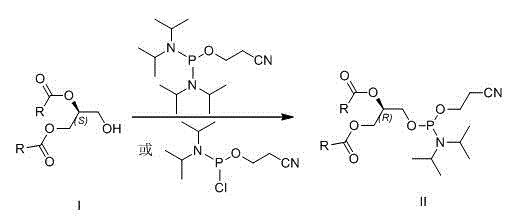
New method for preparing (R)-1,2-di-fatty acid glycerol phosphatidyl glyceride
InactiveCN105985373ARaw materials are easy to getGood optical stabilityPhosphorus organic compoundsBulk chemical productionGlycerolFatty acid glycerol esters
The invention discloses a preparing method for di-fatty acid glycerol phosphatidyl glyceride. The preparing method includes the steps that (S)-1,2-fatty glyceride serves as a raw material, and is reacted with 2-cyano ethyl diisopropyl chloro -amidophosphoric acid or di-(diisopropyl amino) (2-cyano ethyoxyl) phosphine and 2,2-dimethyl-4-hydroxymethyl-1,3-dioxolame in sequence, then phosphate ester is generated under the condition of iodine oxidation, finally all protecting groups are removed under the ammonia water condition and the weak acid condition, and the (R)-1,2-di-fatty acid glycerol phosphatidyl glyceride is obtained. According to the method, the raw materials are low in cost and easy to obtain, the reaction conditions are mild, the steps are simple, and the method is suitable for industrial production.
Owner:江苏东南纳米材料有限公司 +1
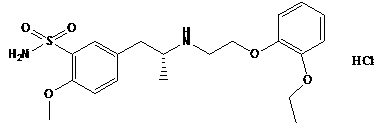
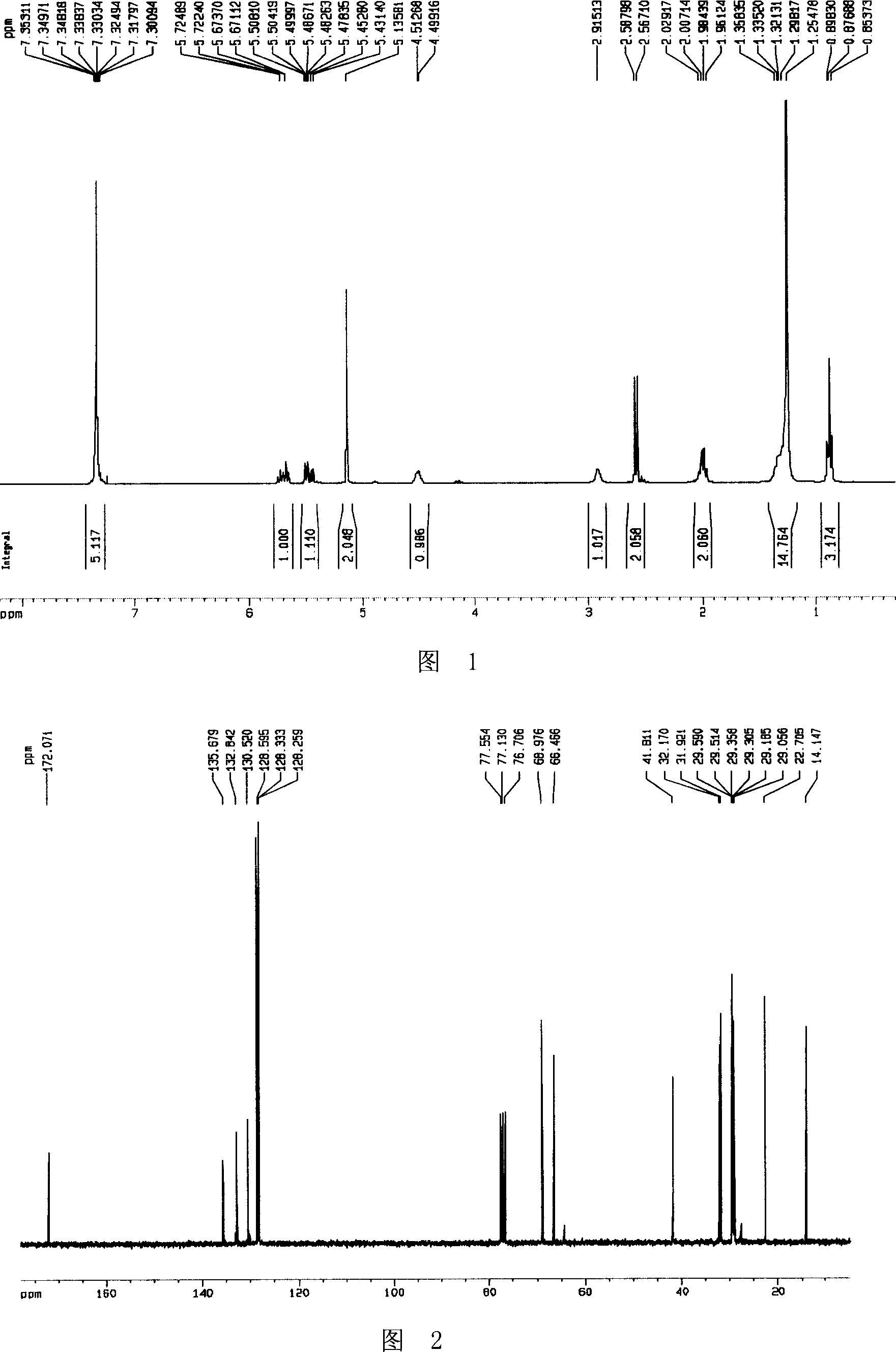
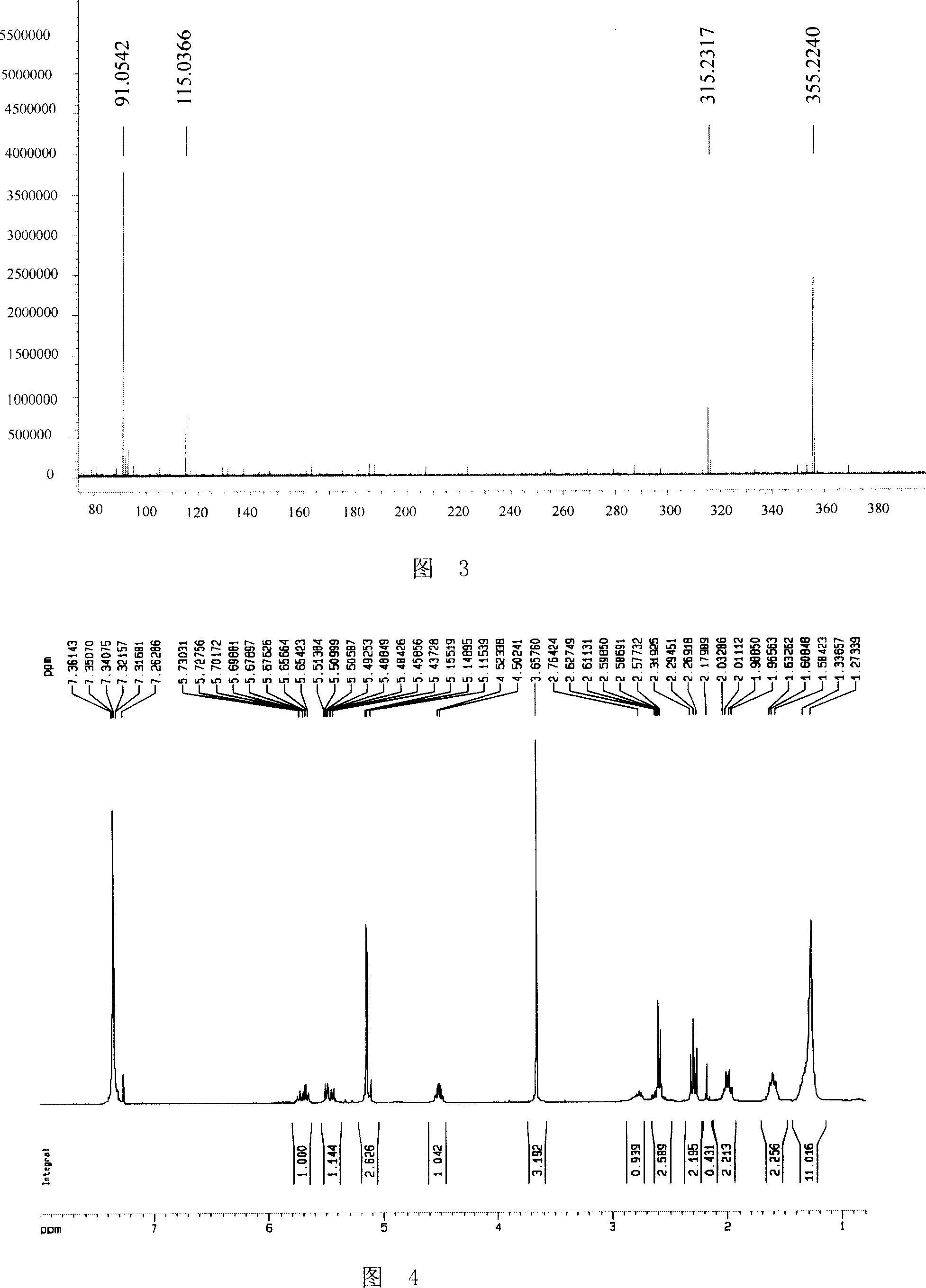
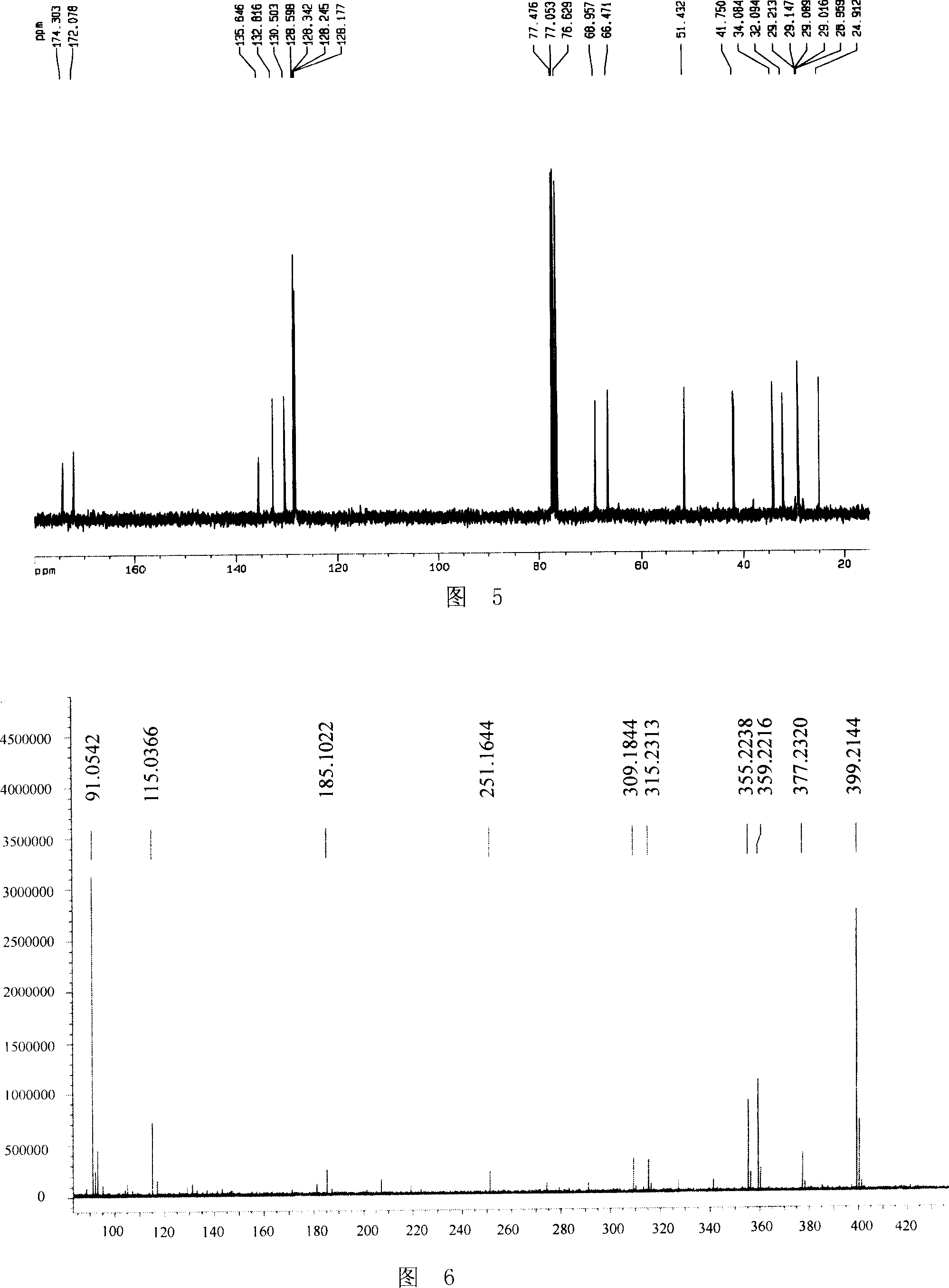
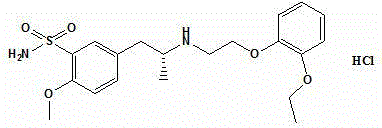
Preparation method of tamsulosin hydrochloride with high optical purity
ActiveCN104926699AGuaranteed optical purityPurification process reductionOrganic chemistryOrganic compound preparationOrganosolvEthyl group
The invention discloses a preparation method of tamsulosin hydrochloride with high optical purity, and belongs to a medicine technology and a chemical field. A recrystallization method is adopted, crude products of (R)-5-(2-(2-(2-ethoxyphenoxy) ethyl amino) propyl)-2-methoxyl phenyl sulfonamide hydrochloride are refined, so that pure products of the (R)-5-(2-(2-(2-ethoxyphenoxy) ethyl amino) propyl)-2-methoxyl group sulfonamide hydrochloride of which the e.e. value is larger than 99.8% is obtained; a crystallizing solvent adopted by the recrystallization method is a mixed solvent consisting of an organic solvent and water, the organic solvent is selected from one of methanol, ethyl alcohol, acetone, acetonitrile and isopropyl alcohol, and the recrystallization temperature is under 15 DEG C. The preparation method disclosed by the invention is simple to operate, short in period, low in cost and good in repeatability, and can solve the inevitable problem of rework for treatment in the industrial production.
Owner:CHENGDU LIKAI CHIRAL TECH
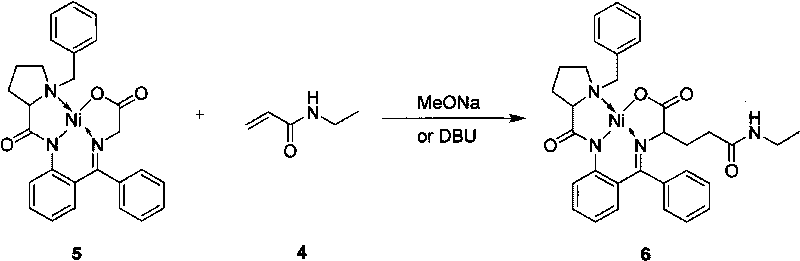
A compound, its preparation method and its use in the synthesis of Buvaracetam
ActiveCN106279074BRaw materials are easy to getLow priceOrganic chemistryCombinatorial chemistryBrivaracetam
The application provides a compound of formula I and a preparation method thereof. The application also provides the use of the compound of formula I for synthesizing buvaracetam and the synthesis method. The raw materials involved in the method described in this application are easy to obtain and cheap, and can prepare brivaracetam with high optical purity.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
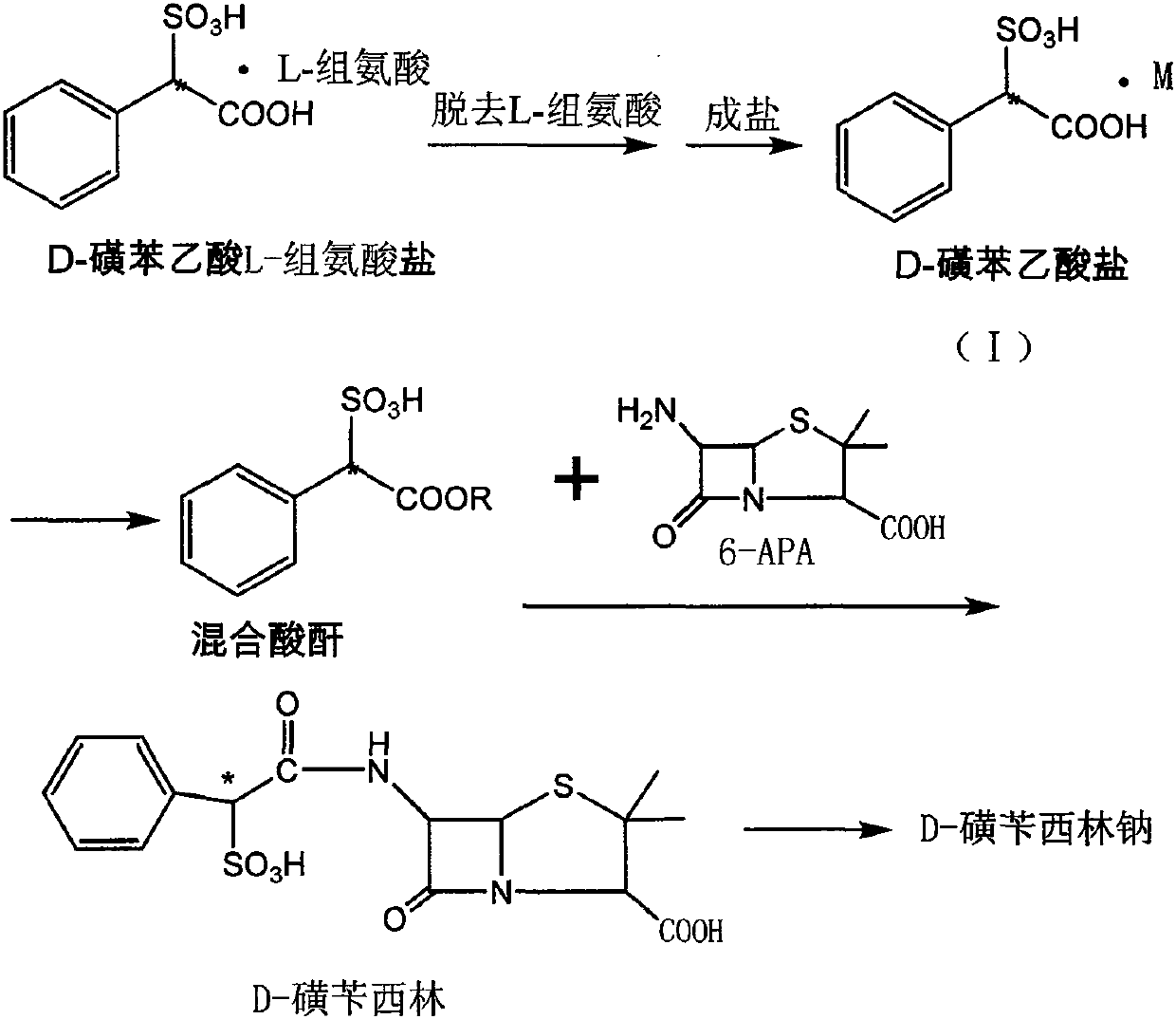
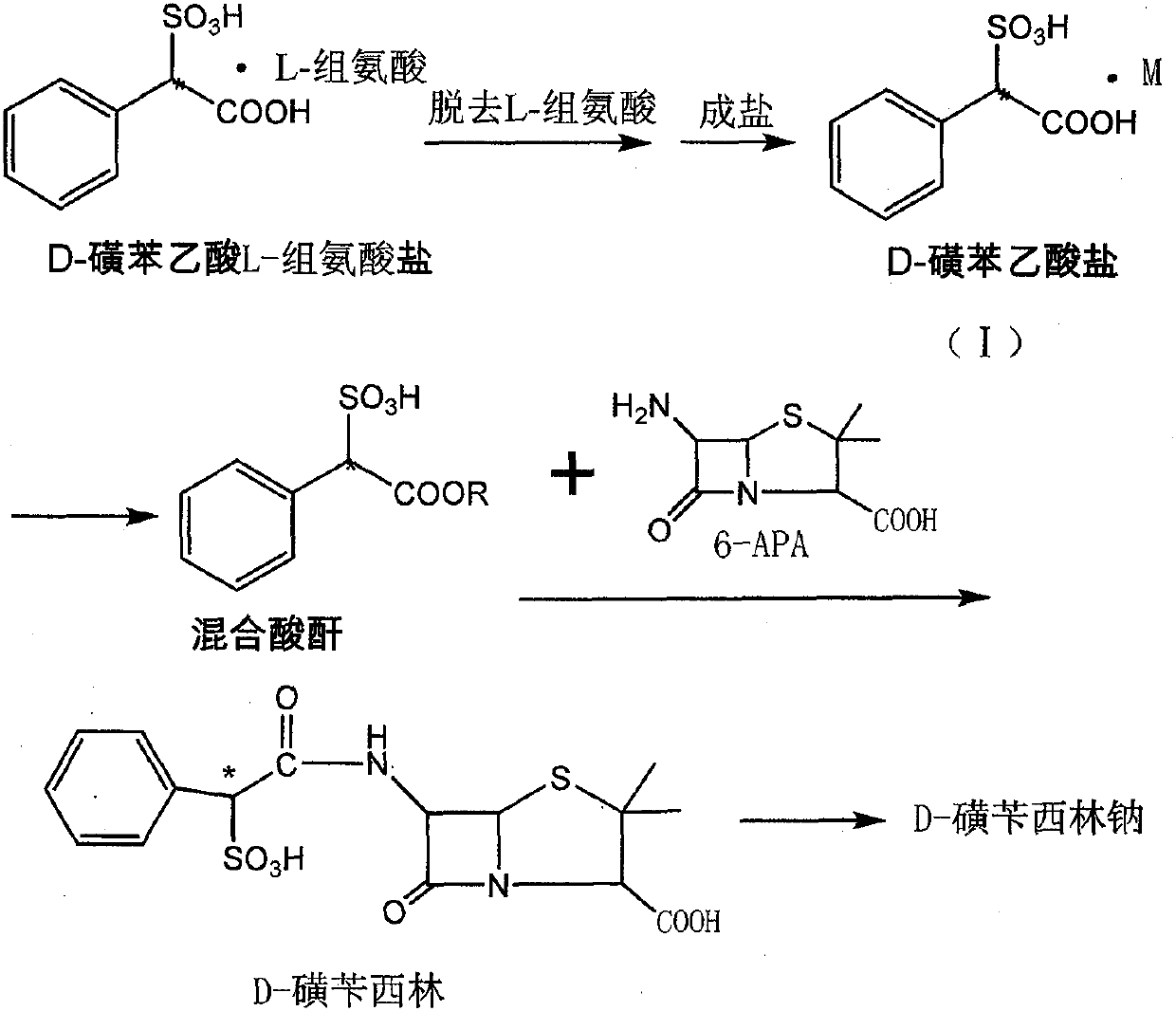
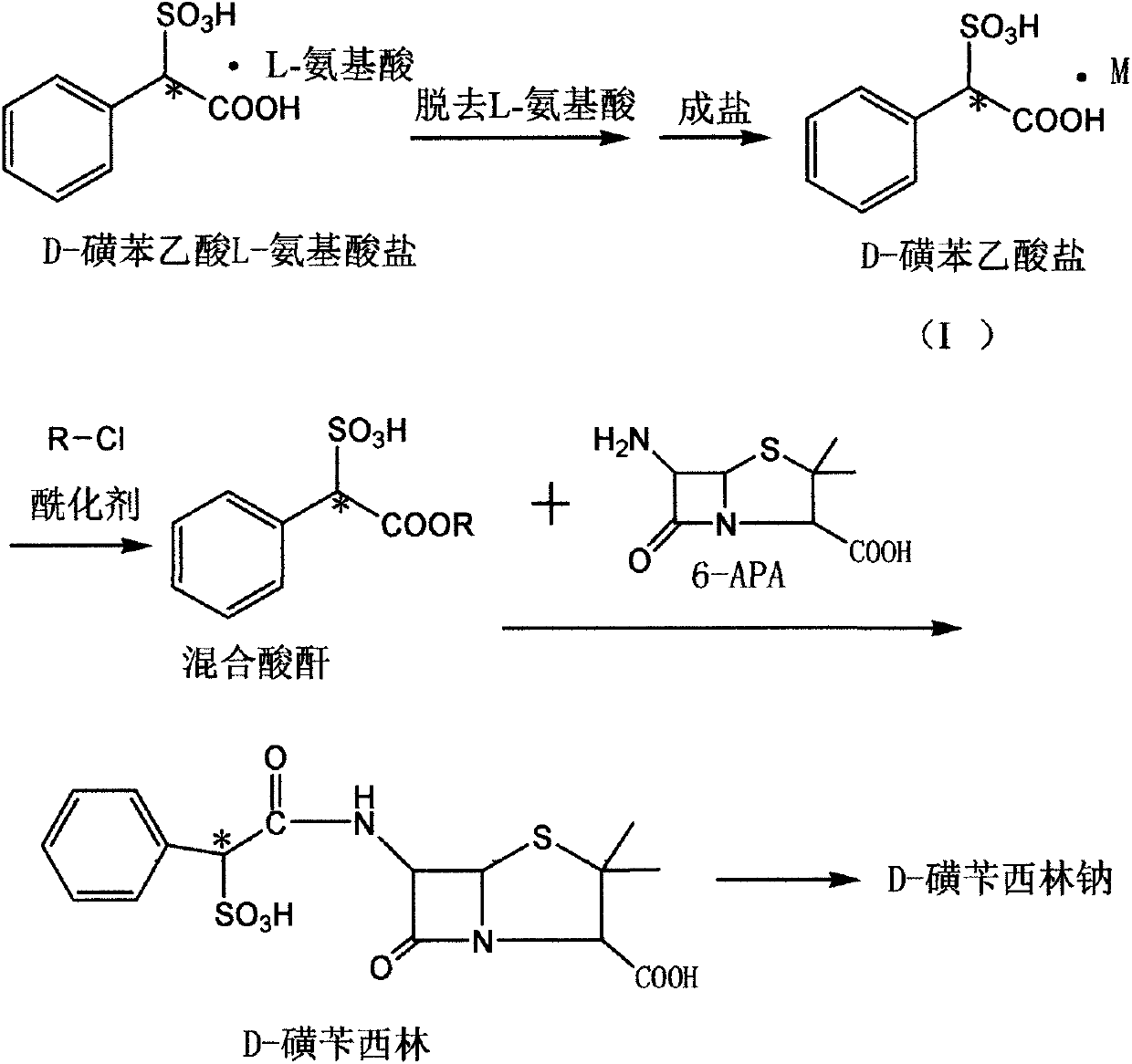
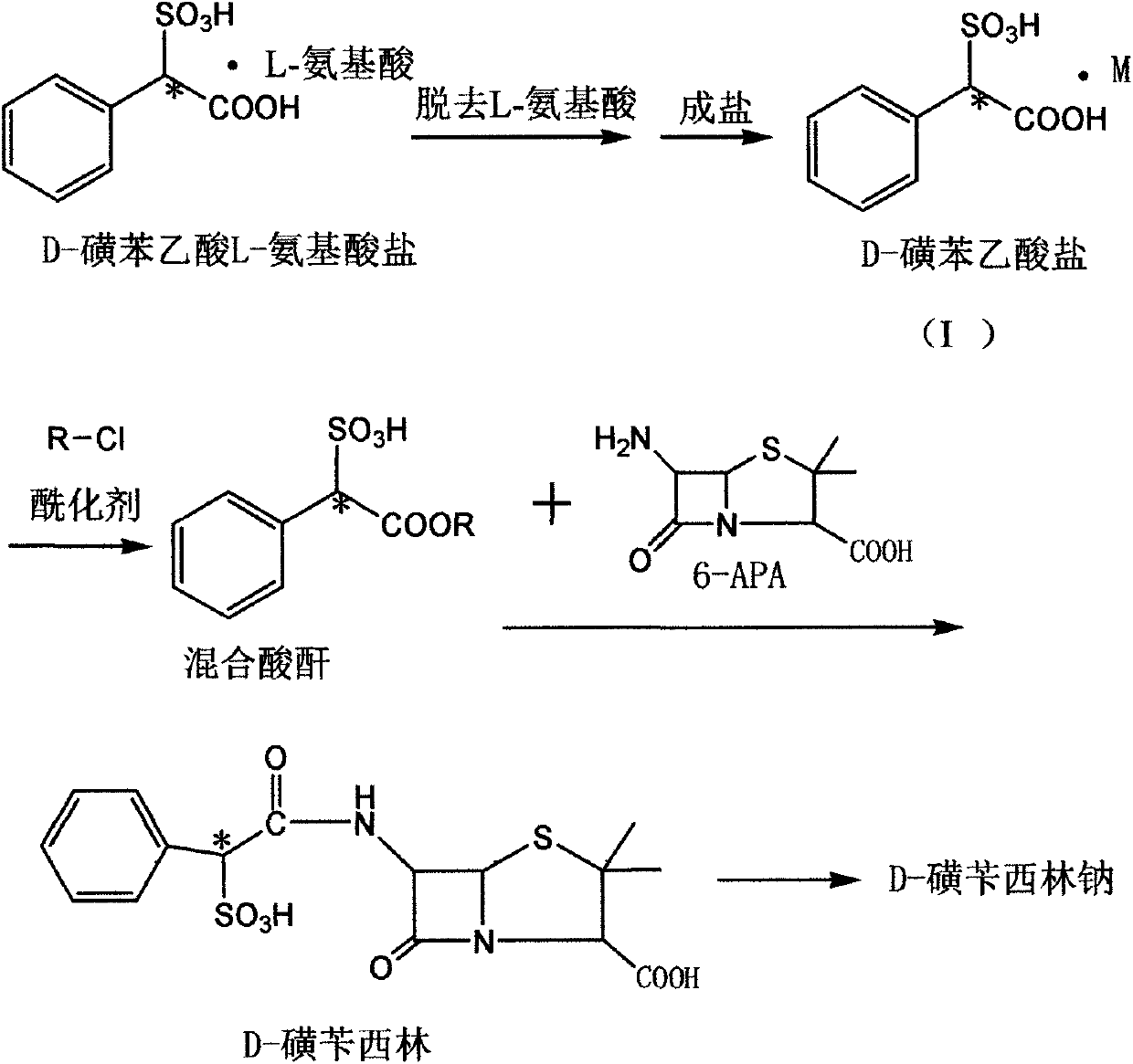
Method for preparing D-sulbenicillin sodium
ActiveCN107641130AAdequate responseIncrease consumptionOrganic chemistrySulbenicillinAfter treatment
The invention discloses a method for preparing D-sulbenicillin sodium. The method includes steps of removing L-amino acid from L-amino acid salt of D-sulfophenylacetic acid in solvents and carrying out salt forming to obtain D-sulfophenylacetic acid salt; carrying out reaction on the D-sulfophenylacetic acid salt and acylating agents to obtain mixed acid anhydride; dissolving 6-APA in organic solvents under the effect of organic alkali; dropwise adding mixed acid anhydride solution into 6-APA solution, and carrying out sufficient reaction and after-treatment to obtain the D-sulbenicillin sodium. The D-sulfophenylacetic acid is an intermediate. The 6-APA is a matrix. The method has the advantages that the D-sulbenicillin sodium is prepared by the aid of novel synthetic routes, conditions are mild, the method includes stable process and is easy to implement, and the problems of instable processes of existing methods or insufficient optical purity of products and the like can be solved bythe aid of the method.
Owner:CHENGDU UNIV
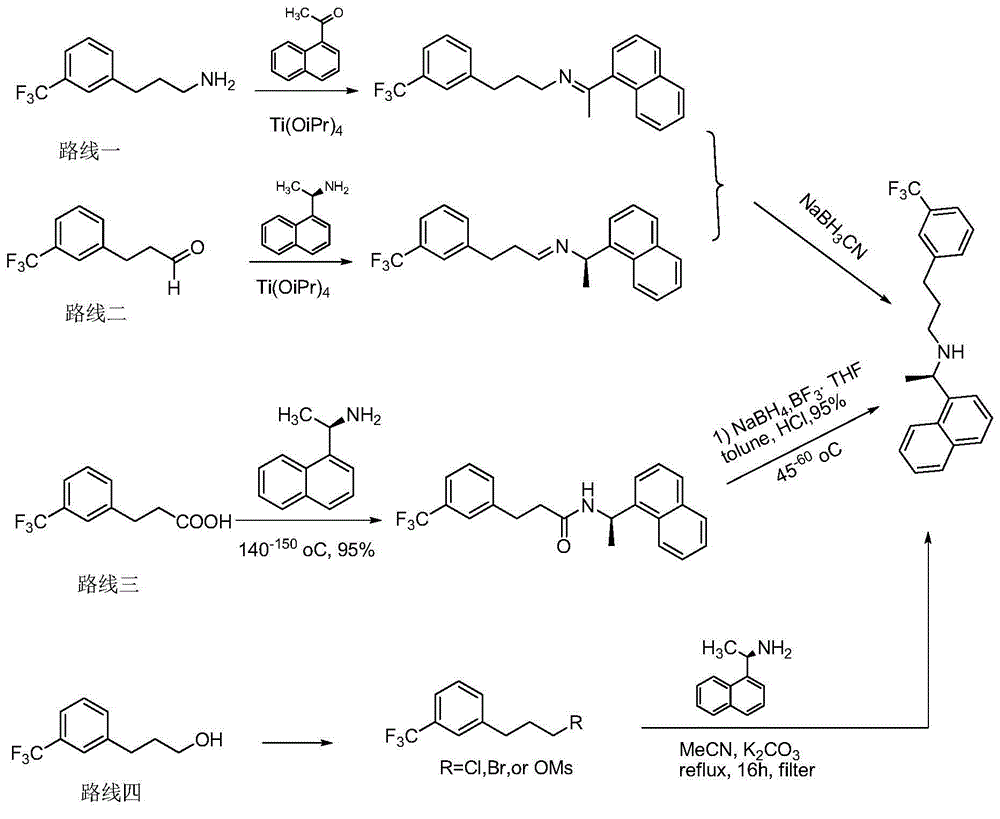
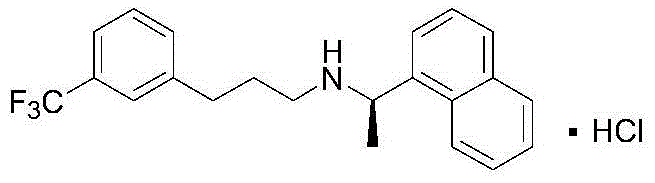
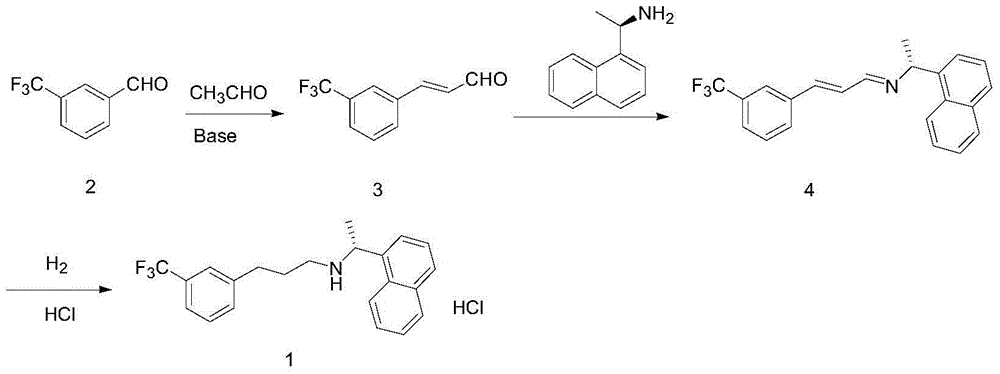
Synthesis method of cinacalcet
ActiveCN104592037AHigh yieldEasy to operateOrganic compound preparationAmino compound preparationP-ChlorophenolSynthesis methods
The invention discloses a synthesis method of cinacalcet. The synthetic route is divided into two parts, namely, (1) preparing a chiral compound as shown in the description from racemic 1-naphthylethylamine as a starting material by virtue of a dynamic kinetic reliquid method in the presence of Pd / LDH-SA serving as a racemic catalyst, p-chlorophenol fatty acyl ester serving as an acyl donor and lipase serving as a biological reliquid catalyst; and (2) reacting m-trifluoromethylbenzaldehyde serving as a starting material and a cheap and easily available material acetaldehyde to obtain m-trifluoromethyl cinnamic aldehyde and carrying out reduced pressure distillation to obtain a pure product; reacting m-trifluoromethyl cinnamic aldehyde and the compound as shown in the description to produce an imine intermediate; dissolving the imine intermediate in ethanol and reacting in the presence of Raney nickel serving as a hydrogenation catalyst to obtain the product cinacalcet. By the synthesis method, the reaction yield and the optical purity of the product are increased, the reaction conditions are milder and the raw materials are easily available.
Owner:ZHEJIANG UNIV
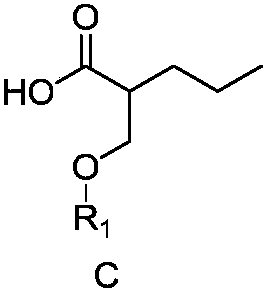
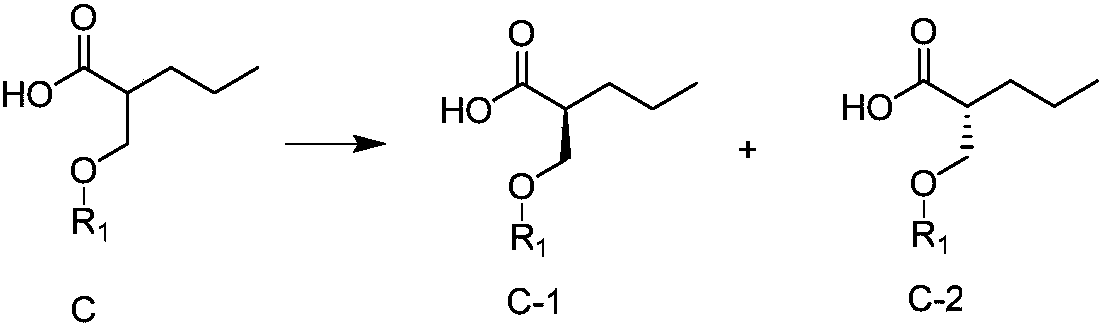
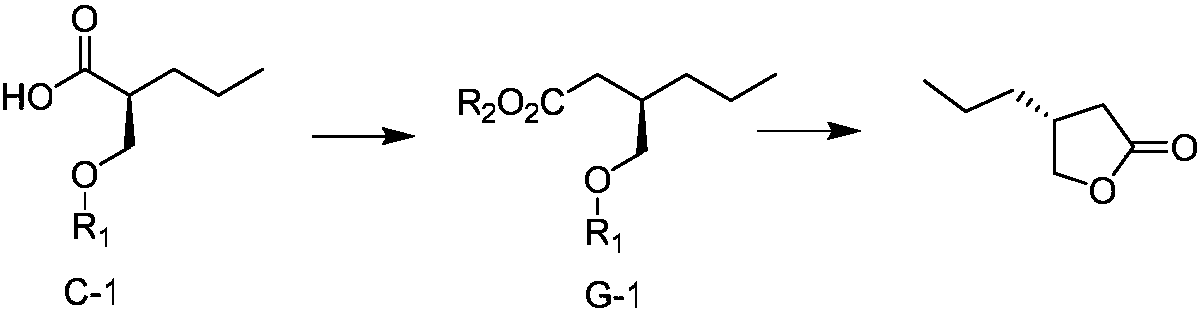
Preparation method for brivaracetam intermediate
ActiveCN108203419AIncrease profitGuaranteed optical purityOrganic chemistry methodsBulk chemical productionCarboxylic acidBrivaracetam
The invention discloses a preparation method for (R)-4-propyl-4,5-dihydrofuran-2-one. The preparation method comprises the following steps: subjecting a carboxylic acid compound to chiral resolution so as to obtain an R-configuration carboxylic acid compound and an S-configuration carboxylic acid compound; and separately subjecting the R-configuration carboxylic acid compound and / or the S-configuration carboxylic acid compound to a reaction so as to obtain (R)-4-propyl-4,5-dihydrofuran-2-one. According to the preparation method, a synthetic route is ingeniously designed, and the two opticallypure isomers obtained through chiral resolution of the carboxylic acid compound are separately utilized by different reaction routes, so the target compound is eventually prepared; and the method improves the utilization rate of the isomers while ensuring optical purity and eliminates the cumbersome steps of racemization and re-splitting of the other half of the isomers.
Owner:ZHEJIANG JINGXIN PHARMA +1
Method for producing optically active 1-bromo-1-[3,5-bis(trifluoromethyl)phenyl] ethane
InactiveCN102985395AGuaranteed optical purityPreparation by halogen additionAsymmetric synthesesArylN-Bromosuccinimide
Disclosed is a method that is for producing 1-bromo-1-[3,5-bis(trifluoromethyl)phenyl] ethane having high optical purity, and that is a method containing a step for brominating optically active 1-[3,5-bis(trifluoromethyl)phenyl] ethanol using, as a brominating agent, either a) the combination of a phosphorus halide and hydrogen bromide, b) the combination of 1,2-dibromo-1,1,2,2-tetrachloroethane and the organophosphorus compound represented by general formula I, which is P(R1)(R2)(R3) (in the formula: R1, R2 and R3 each independently indicate a C6-10 aryl group, a C6-10 aryloxy group, a C1-10 alkyl group, a C1-10 alkoxyl group, a C3-6 cycloalkyl group, or a C3-6 cycloalkoxyl group), or c) the combination of N-bromosuccinimide and a dialkyl sulphide.
Owner:KOWA CO LTD
New technology for efficiently synthesizing Aramchol by utilizing cholic acid and arachidic acid as raw materials
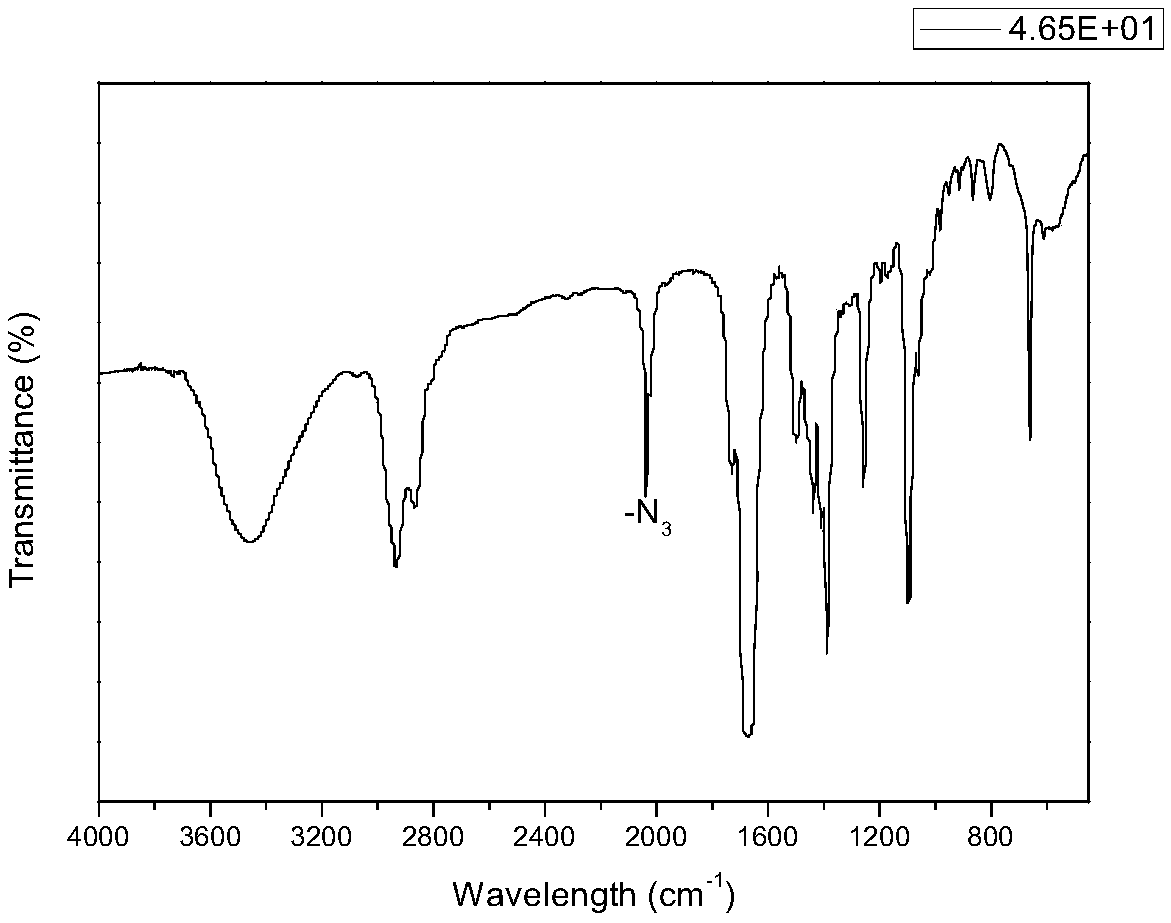
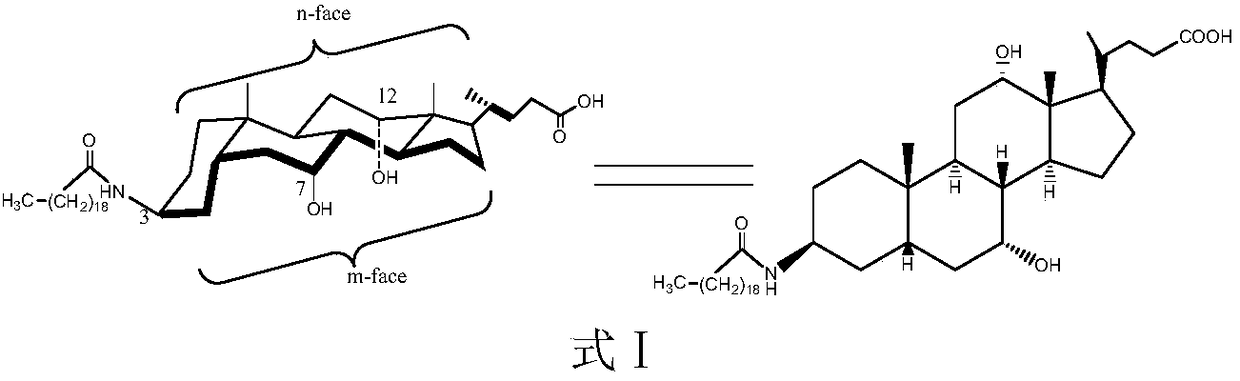
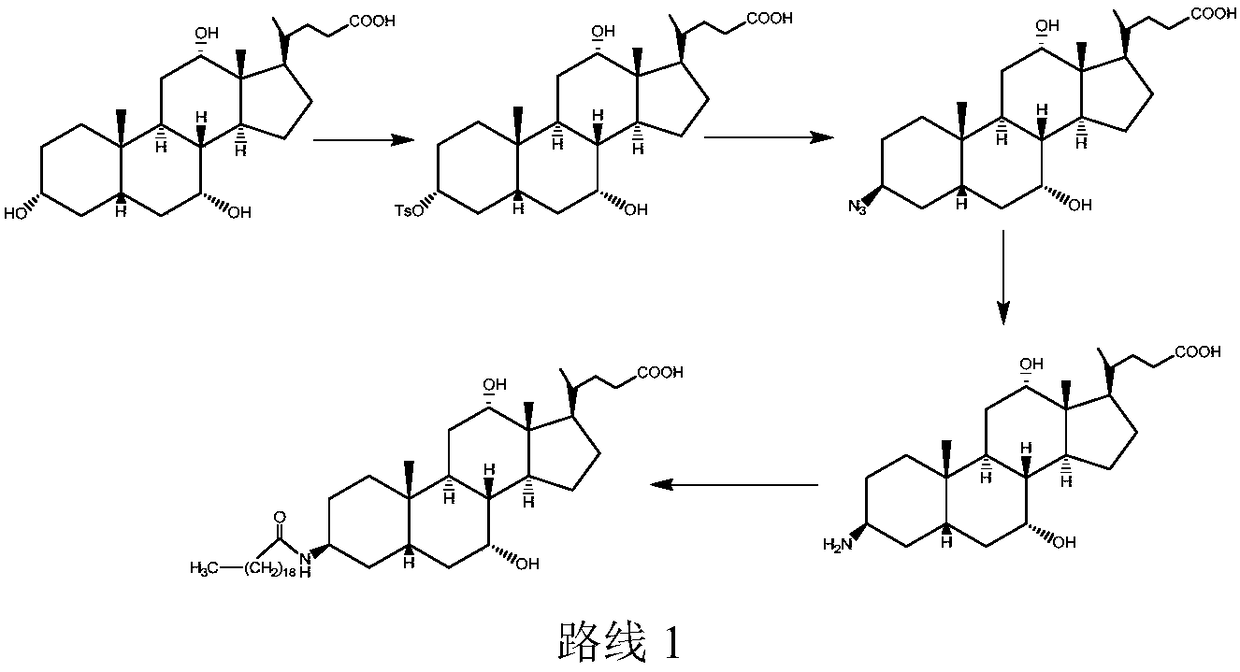
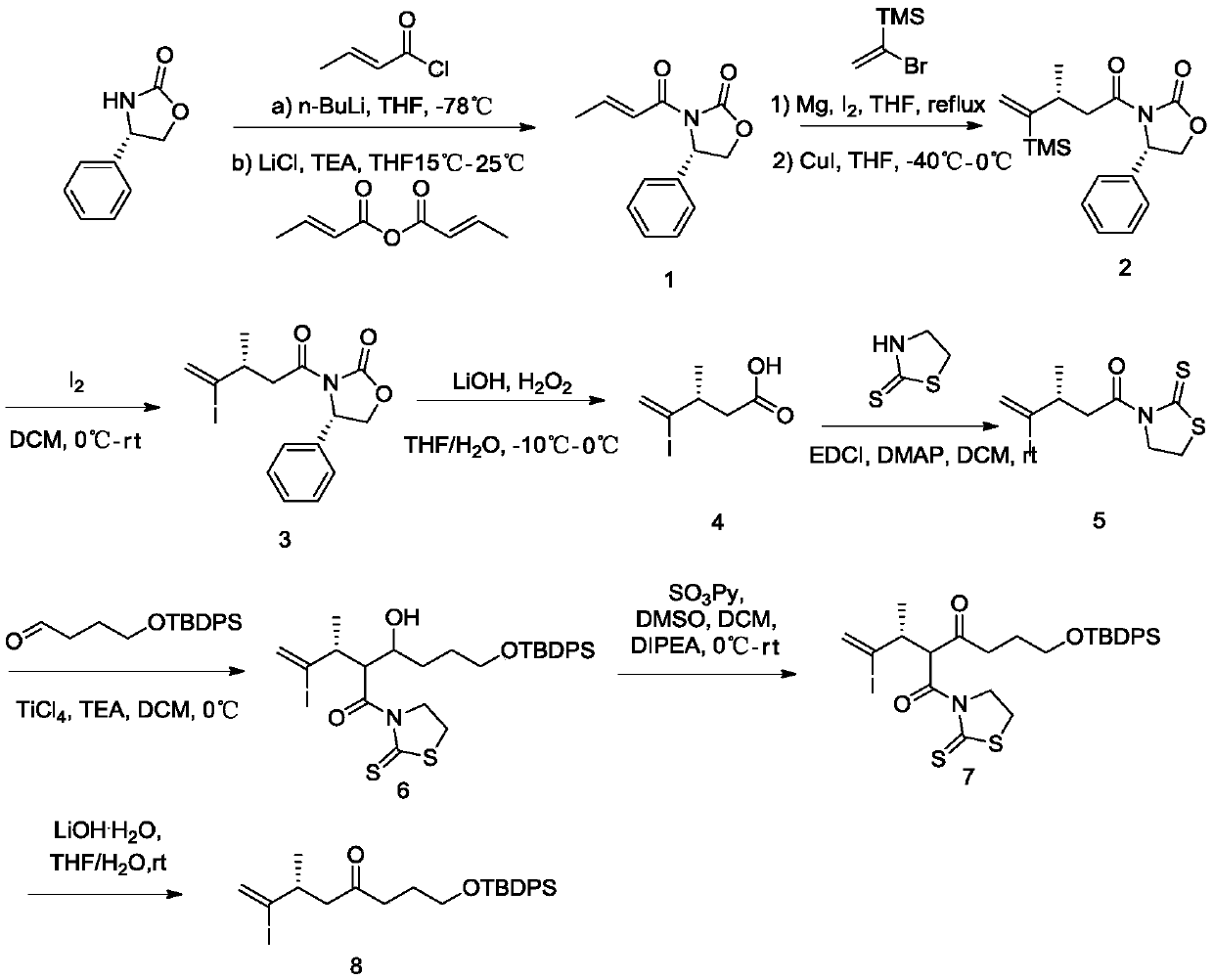
The invention discloses a new technology for efficiently synthesizing Aramchol by utilizing cholic acid and arachidic acid as raw materials. According to the new technology disclosed by the invention,a 3- hydroxyl group of cholic acid is activated by selecting para-butyl benzene sulfonyl chloride in larger steric hindrance and the like, 3alpha-O-para-butyl benzene sulfonyl methyl cholate, chiral3-C can be attacked by an azide group just through a direction against an alkylbenzene sulfonyloxy group of a leaving group during a nucleophilic substitution reaction process of the 3alpha-O-para-butyl benzene sulfonyl methyl cholate and sodium azide, inversion of 3-C configuration of a product-3-azido methyl cholate is ensured, and a firm foundation is laid for controlling the optical purity ofa final product-Aramchol.
Owner:HEFEI UNIV OF TECH
Intermediate for preparing halichondrin compound and preparation method thereof
ActiveCN111285894AGuaranteed optical purityHigh purityGroup 4/14 element organic compoundsPtru catalystBiochemical engineering
The invention relates to an intermediate for preparing a halichondrin compound and a preparation method thereof. The invention particularly relates to an intermediate for preparing halichondrin, eribulin or analogues thereof, and a preparation method and application of the intermediate. The intermediate as well as the preparation method and application thereof are used for constructing C20-C26 structural fragments of the halichondrin compound. The initial raw materials of the synthesis route are cheap, easy to obtain, stable in source and reliable in quality; the structural characteristics ofreactants are fully utilized in the selection of a chiral center construction method, so that the synthesis efficiency is practically improved, and the difficulty and risk of product quality control are reduced; and the use of a high-toxicity and expensive organic tin catalyst is avoided, so that the cost and the environmental friendliness are remarkably improved.
Owner:BEIJING TIENYI LUFU PHARMATECH CO LTD
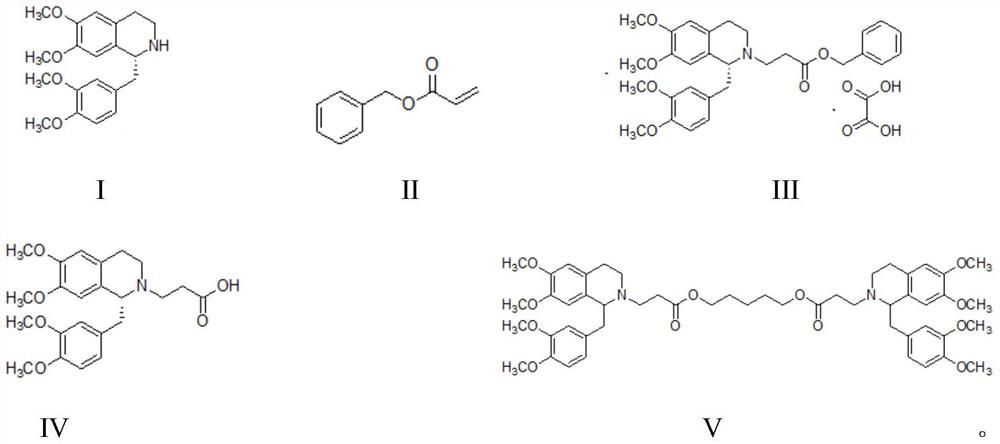
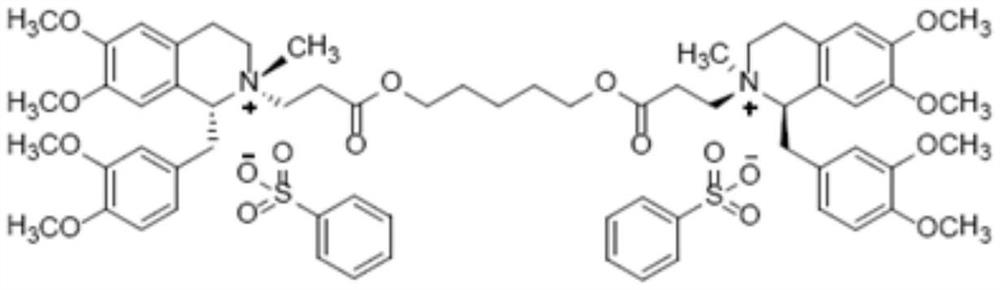
Method for synthesizing cisatracurium besilate
InactiveCN111777554AEasy maintenanceGuaranteed optical puritySulfonic acids salts preparationCombinatorial chemistryBenzenesulfonic acid
The invention discloses a method for synthesizing cisatracurium besilate. The method comprises the following steps of: reacting a compound shown as formula I, a compound shown as formula II and oxalicacid dihydrate to generate a compound shown as formula III; reacting the compound shown as formula III under the catalysis of Pd / C to generate a compound shown as formula IV; reacting the compound shown as formula IV with 1, 5-pentanediol to generate a compound shown as formula V; and reacting the compound shown as formula V with methyl benzenesulfonate to prepare cisatracurium besilate; whereinthe compounds shown as the formula I, the formula II, the formula III, the formula IV and the formula V are respectively represented as the specification. The synthesis method provided by the invention can significantly improve the yield and purity of cisatracurium besilate and reduce the synthesis cost of cisatracurium besilate.
Owner:SHANDONG RUIAN PHARMA CO LTD
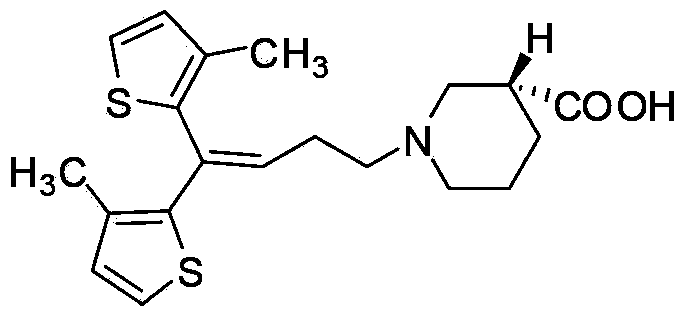
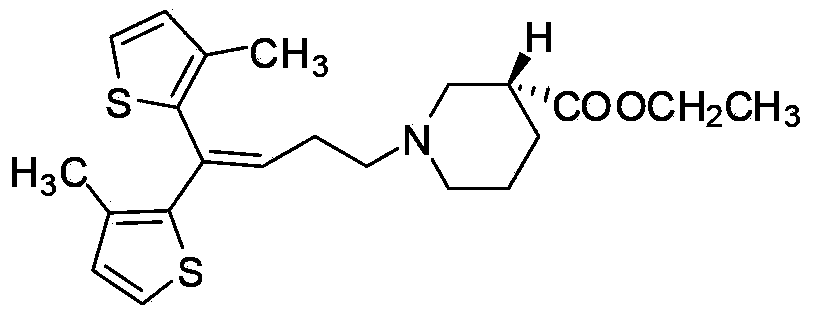
Preparing method for tiagabine hydrochloride
The invention provides a preparing method for tiagabine hydrochloride, which includes the following operating steps: firstly, taking tiagabine ethyl ester to be dissolved in solvent, adding L-configuration organic acid, performing heating reflux until the tiagabine ethyl ester is completely dissolved, then performing reflux for 30-60 min, cooling to the room temperature, cooling and crystallizing at the temperature of minus 30 -minus 20 DEG C, and collecting a solid matter A; secondly, adding solvent in the solid matter A, performing heating reflux until the tiagabine ethyl ester is completely dissolved, then performing reflux for 30-60 min, cooling to the room temperature, cooling and crystallizing at the temperature of minus 10-0 DEG C, and collecting a solid matter B; thirdly, dissolving the solid matter B, sequentially adding sodium hydroxide and hydrochloric acid to react, extracting and recrystallizing, so as to obtain the tiagabine hydrochloride. The preparing method provided by the invention not only remarkably improves the productivity by 90% above, but also guarantees the 99.9% of chemical and optical purity, is simple and convenient to operate, has no special requirement for equipment, and is more suitable for large-scale industrial production.
Owner:凯默斯医药科技上海有限公司
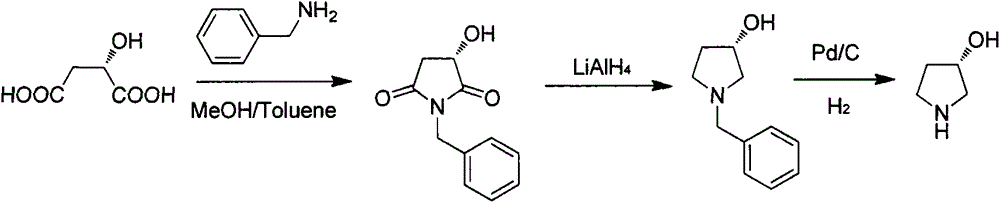
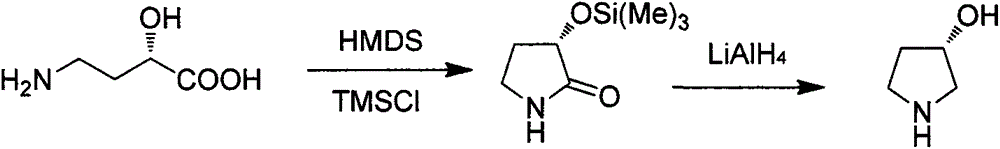
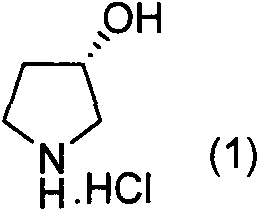
Preparation method of (S)-3-hydroxypyrrolidine hydrochloride
InactiveCN105646321AConvenient sourceLow costOrganic chemistry methodsBulk chemical productionTert-Butyloxycarbonyl protecting groupMitsunobu reaction
The invention relates to the field of chemistry, particularly a preparation method of a key intermediate (S)-3-hydroxypyrrolidine hydrochloride of darifenacin for treating overactive bladder syndrome and an antihypertensive drug barnidipine. The preparation method comprises the following steps: carrying out Mitsunobu reaction on (R)-1-N-tert-butyloxycarbonyl-3-hydroxypyrrolidine so as to be condensed with acid to obtain an upturned-structure ester, hydrolyzing ester bond under alkaline conditions to obtain (S)-1-N-tert-butyloxycarbonyl-3-hydroxypyrrolidine, and removing Boc protecting groups under acidic conditions, thereby finally obtaining the key intermediate. The method is simple and easy to implement, has the advantages of cheap and accessible raw materials, lower cost and high yield, and has potential production value.
Owner:CHINA PHARM UNIV
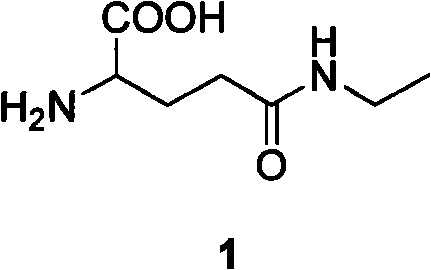
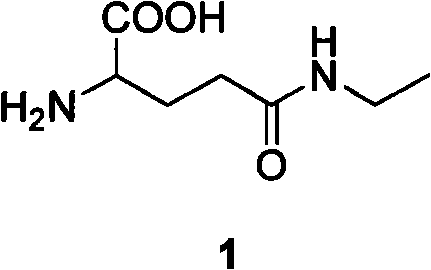
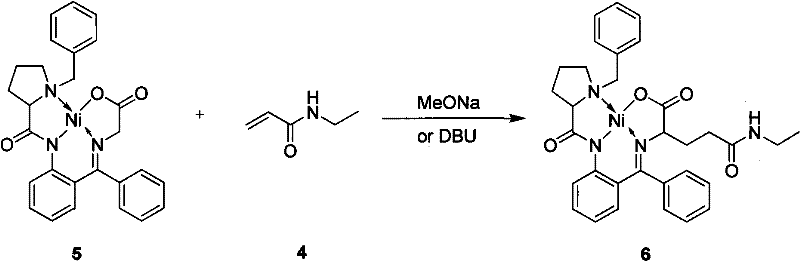
Method for preparing L-theanine by chemical method
InactiveCN101717347AGuaranteed optical purityImprove economyOrganic compound preparationCarboxylic acid amides preparationGlycineAcid hydrolysis
The invention discloses a method for preparing L-theanine by a chemical method. The method comprises the following steps: firstly, reacting ethylamine with acryloyl chloride in the presence of an alkali to obtain N-ethylacrylamide; secondly, perform Michael addition reaction on chiral auxiliary BPB based glycine Schiff base Ni(II) coordination compound and the N-ethylacrylamide in the presence of the alkali to obtain theanine Schiff base Ni(II) coordination compound; and finally, performing acid hydrolysis on the theanine Schiff base Ni(II) coordination compound to prepare the L-theanine. The method adopts the chiral auxiliary chemical method to prepare the L-theanine, and constructs a complete theanine fragment through the Michael addition reaction so as to prepare the theanine Schiff base Ni(II) coordination compound, wherein the rigid space structure of the coordination compound can maintain the L-configuration of the theanine so as to ensure the optical purity of the L-theanine. The method can reclaim the chiral auxiliary in high yield, and can elute Ni(II) ions through ion exchange for recycling.
Owner:NANJING UNIV OF TECH
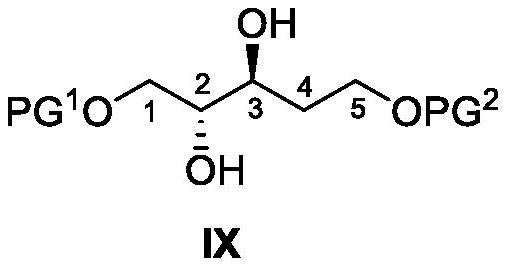
Eribulin intermediate and synthesis method and application thereof
ActiveCN111848369AControllable optical purityHigh optical puritySilicon organic compoundsOrganic compound preparationEribulinHalichondrin B
The invention belongs to the field of drug synthesis, and particularly relates to an eribulin intermediate and a synthesis method and application thereof. The invention provides an intermediate whichcan be used for synthesizing halichondrin B, eribulin or analogues thereof, particularly C27-C35 structural fragments thereof, as well as a preparation method and application of the intermediate. Theinitial raw materials of the synthetic route are cheap and easy to obtain, and the optical purity of the synthetic route can be guaranteed so that the optical purity of C27-C35 structural fragments inhalichondrin, eribulin or analogues thereof is guaranteed; according to the method, the chiral center of the C27-C35 structural fragment is constructed, the diastereoselectivity and yield are high, and especially the preparation method of the compounds shown in the formula (X), the formula (XI), the formula (XVI) and the formula (XV) is provided; partial reaction by-products can be removed only by recrystallization so that purification is facilitated, and the cost is greatly reduced.
Owner:BEIJING TIENYI LUFU PHARMATECH CO LTD
A kind of preparation method of rivaroxaban intermediate and new synthesis method of rivaroxaban
The invention discloses a preparation method of (S)-N-glycidol phthalimide in a structural formula 1. The method comprises the following steps of: I, phthalimide salt of a structural formula 9 and a compound of a structural formula 10 are subjected to heating reflux for reaction in an alcohols solvent to obtain the compound of a structural formula 11; II, the compound of the structural formula 11 obtained form the step I is reacted with the compound of a structural formula 12 in an aprotic solvent under the action of alkaline to obtain a (S)-N-glycidol phthalimide crude product of the structural formula 1; and III, the (S)-N-glycidol phthalimide crude product of the structural formula 1 obtained from the step II is refined by ethanol to obtain the (S)-N-glycidol phthalimide with the optical purity larger than or equal to 99.0%. The preparation method is simple to operate and good in safety, the optical purity of the obtained product is high (larger than or equal to 99.0%), and the preparation method is suitable for industrial production. The invention also discloses a novel synthetic method of Rivaroxaban.
Owner:JIANGXI SYNERGY PHARMA
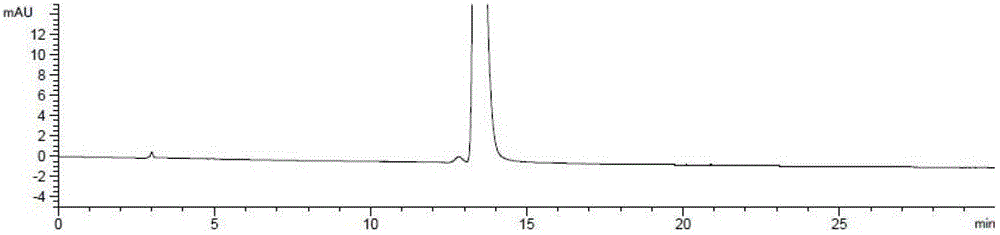
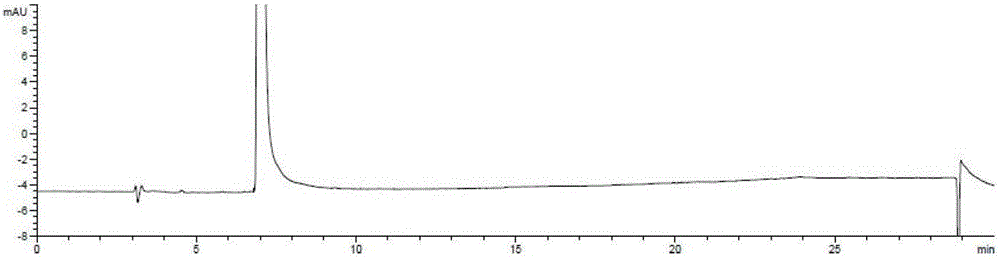
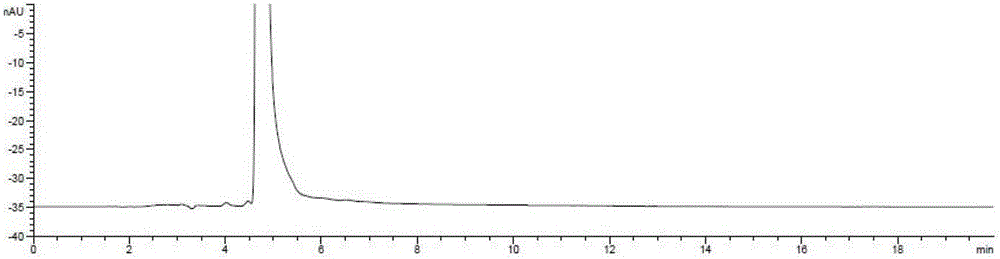
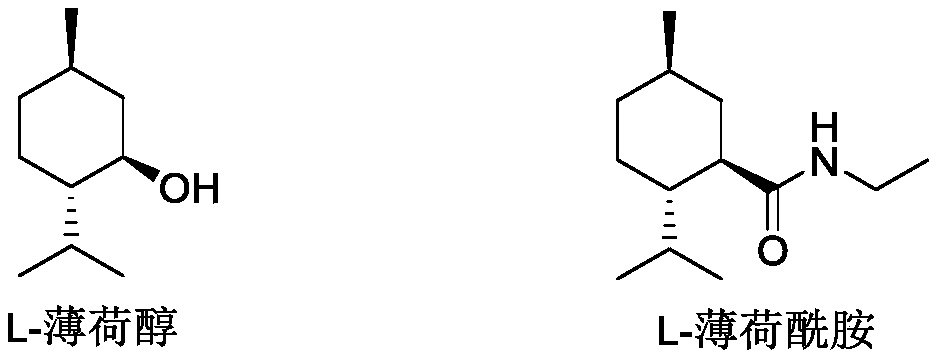
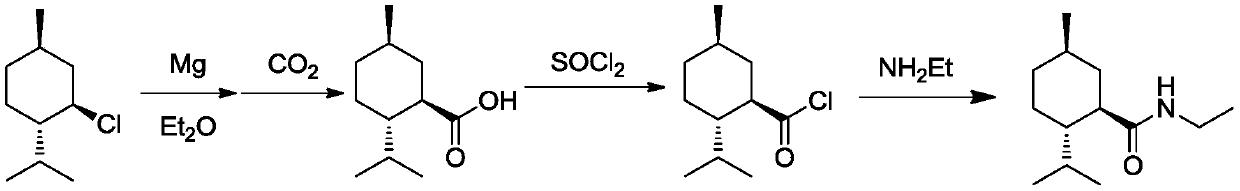
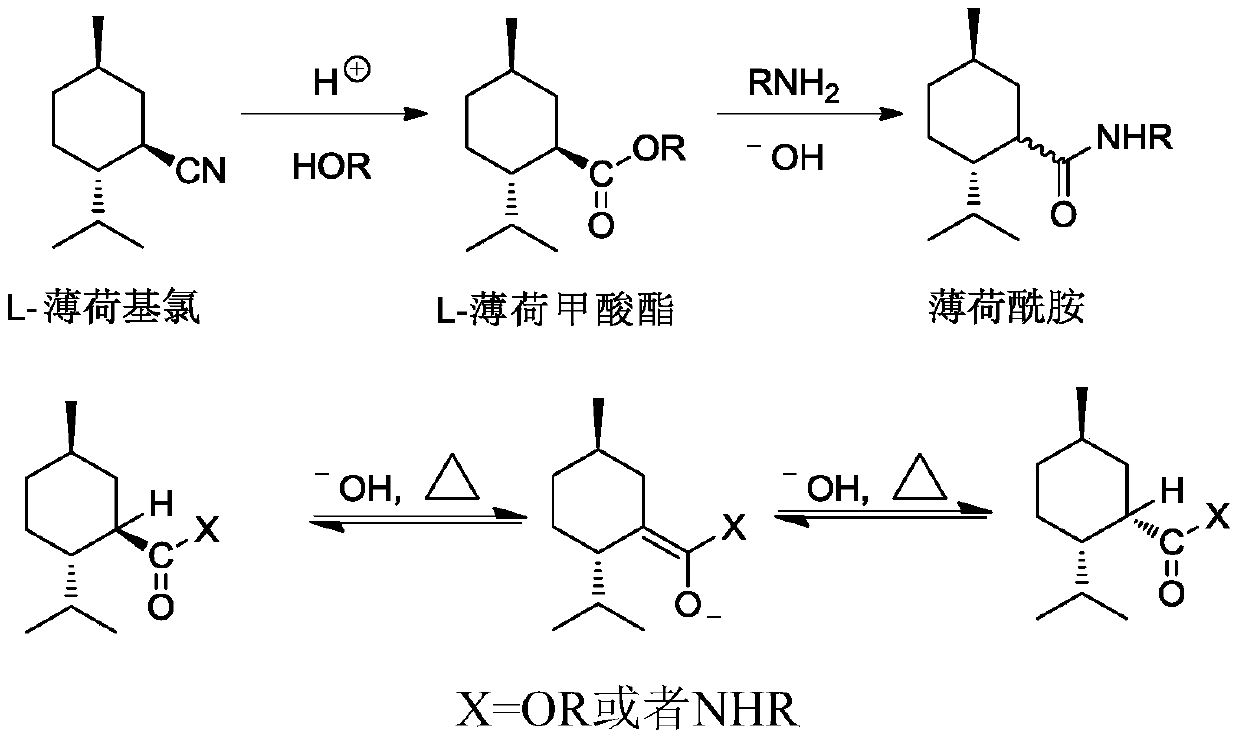
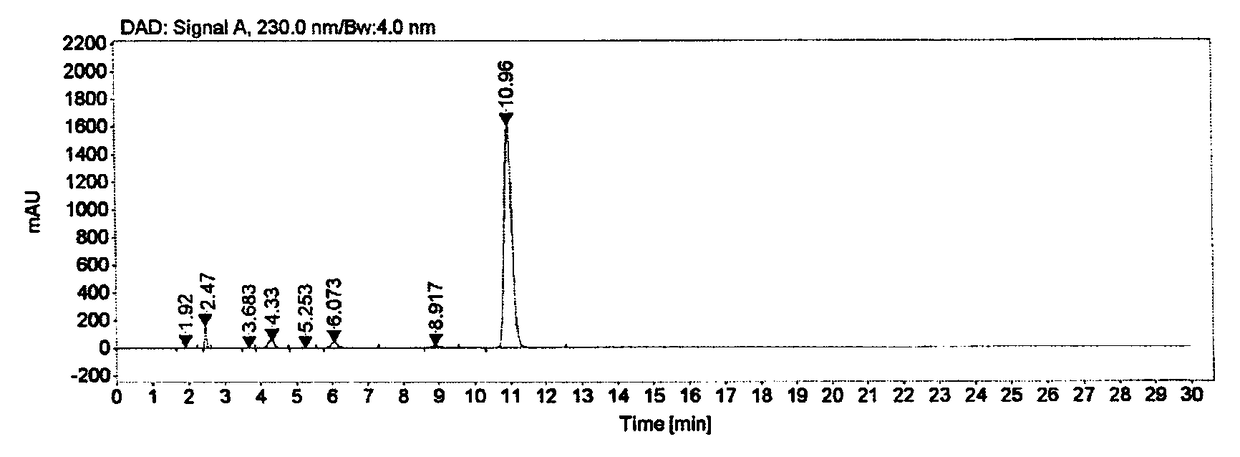
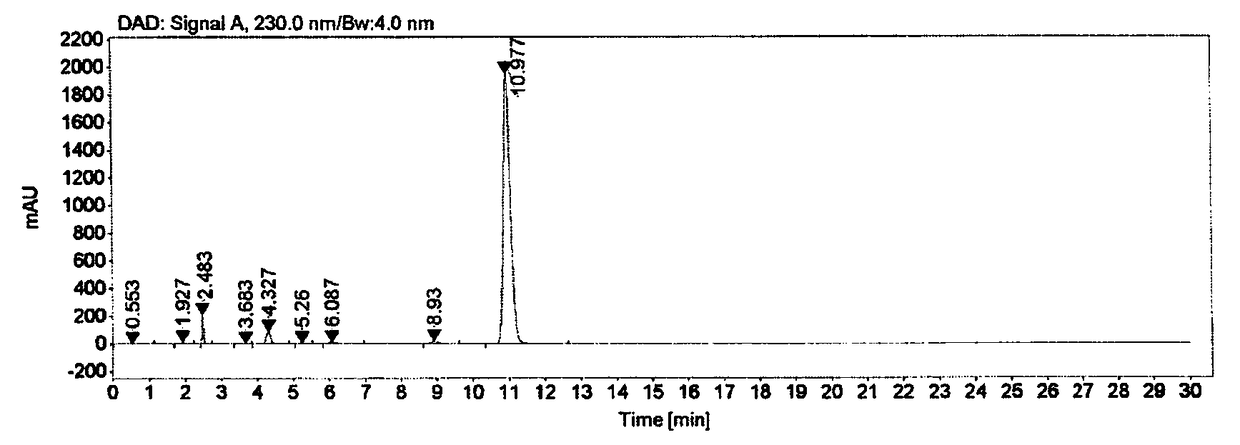
Ester ammonolysis reaction catalyst composition and preparation method of L-menthane carboxamide
ActiveCN111393293ALow costConfiguration retentionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystChloroformate
The invention discloses an ester ammonolysis reaction catalyst composition and a preparation method of L-menthane carboxamide. The ester ammonolysis reaction catalyst comprises a catalyst and a ligand, wherein the catalyst is cuprous salt CuX, the ligand is a (R)-N, N, N, N-tetraalkyl binaphthylamine compound shown as the specification, wherein R1, R2, R3 and R4 are independently selected from thegroups shown as the specification. Menthyl chloride is used as the starting raw material in the method to react with metal magnesium to prepare menthyl magnesium chloride, then reaction with chloroformate with different substituent groups is carried out to obtain menthyl formate, and then ammonolysis under the catalysis of an ester ammonolysis catalyst composition is conducted to obtain the L-menthane carboxamide. The yield is improved, the production cost is reduced, the optical purity is high, and environmental protection and no wastewater discharge are realized.
Owner:WANHUA CHEM GRP CO LTD
Detection and analysis method for quality control of raltitrexed synthesis
ActiveCN109283263AGuaranteed contentGuaranteed optical purityComponent separationEnantiomerContent determination
The invention discloses a detection and analysis method for quality control of raltitrexed synthesis. The method includes purity detection of a starting material 6-bromomethyl-3,4-dihydro-2-methyl-quinazoline-4-one, purity detection of a starting material 5-nitro-2-thiophenecarboxylic acid, raltitrexed content determination, content detection of the 6-bromomethyl-3,4-dihydro-2-methyl-quinazoline-4-one, 6-hydroxymethyl-3,4-dihydro-2-methyl-quinazoline-4-one and the 5-nitro-2-thiophenecarboxylic acid in raltitrexed, and raltitrexed enantiomer detection. The method of the invention has the characteristics of high sensitivity and strong specificity, and can rapidly and accurately perform quality control on a synthesis process of the raltitrexed, thereby ensuring the quality of raltitrexed bulk medicine.
Owner:NANJING CHIA TAI TIANQING PHARMA
Method for preparing and detecting intermediate and corresponding isomer of afatinib
ActiveCN106442793AGuaranteed accuracyGuaranteed optical purityComponent separationCelluloseIsocratic elution
The invention discloses a method for simultaneously detecting a key intermediate (formula 2) and a corresponding isomer (formula 3) of afatinib. According to the method, isocratic elution is performed on a high performance liquid chromatograph; a chromatographic column which takes cellulose-tris(3,5-dimethyl phenylcarbamate) as a filling agent is adopted; a flow phase is a mixed solution of n-hexane, isopropyl alcohol and acetonitrile. Through the method, the key intermediate (formula 2) and the corresponding isomer (formula 3) of the afatinib can be effectively separated and detected, and the separation degree can reach 2.0 or higher.
Owner:SHINEWAY PHARMA GRP LTD
A kind of preparation method of tamsulosin hydrochloride with high optical purity
ActiveCN104926699BGuaranteed optical purityPurification process reductionOrganic chemistryOrganic compound preparationAlcoholOrganic solvent
Owner:CHENGDU LIKAI CHIRAL TECH
3-hydroxy olefine acid derivative and its prepn and application
InactiveCN101020639AReactiveGuaranteed optical purityGroup 4/14 element organic compoundsAcid derivativeColneleic acid
The present invention discloses one kind of 3-hydroxy olefine acid derivative in the structure as shown and its preparation process and application. By means of the inherent chirality of the material and the cross-metathesis of olefine, the product of the present invention, 3-hydroxy olefine acid, has high optical purity. The present invention lays foundation for the industrial preparation of 3-hydroxy olefine acid derivative.
Owner:INST OF CHEM CHINESE ACAD OF SCI
3-hydroxy olefine acid derivative and its preparation and application
InactiveCN100491326CReactiveGuaranteed optical purityGroup 4/14 element organic compoundsAcid derivativeAcyl group
The present invention discloses one kind of 3-hydroxy olefine acid derivative in the structure as shown and its preparation process and application. By means of the inherent chirality of the material and the cross-metathesis of olefine, the product of the present invention, 3-hydroxy olefine acid, has high optical purity. The present invention lays foundation for the industrial preparation of 3-hydroxy olefine acid derivative.
Owner:INST OF CHEM CHINESE ACAD OF SCI
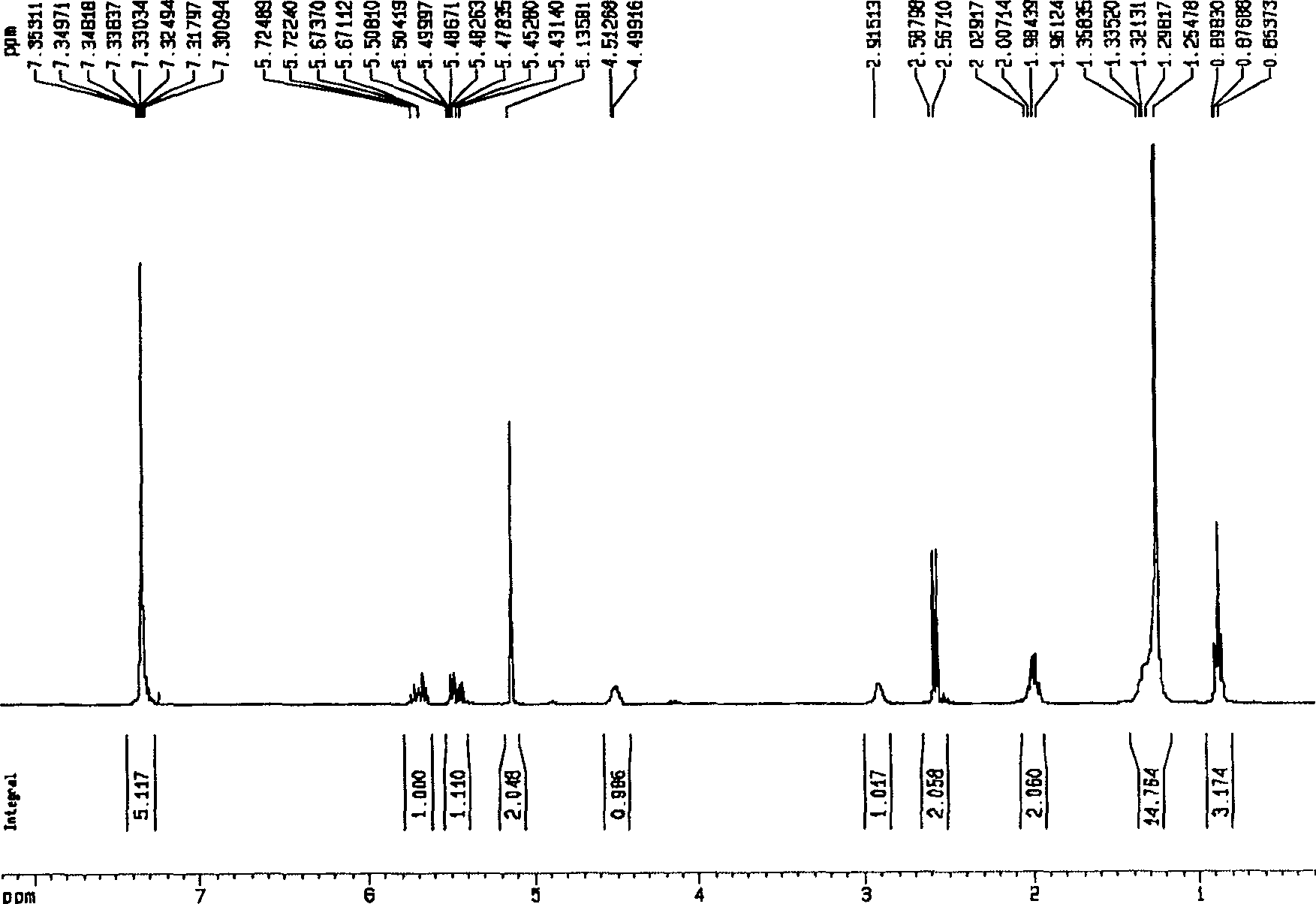
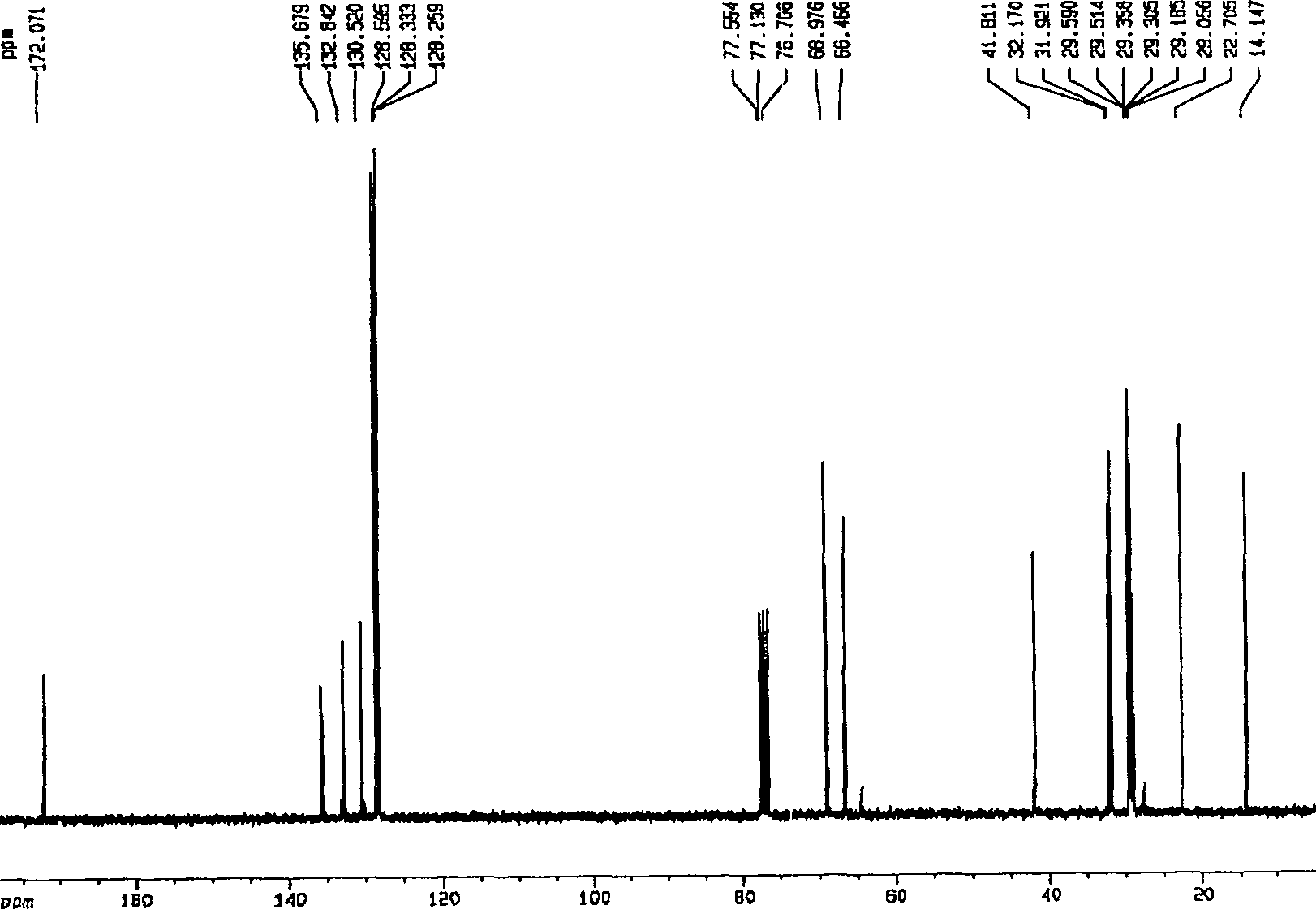
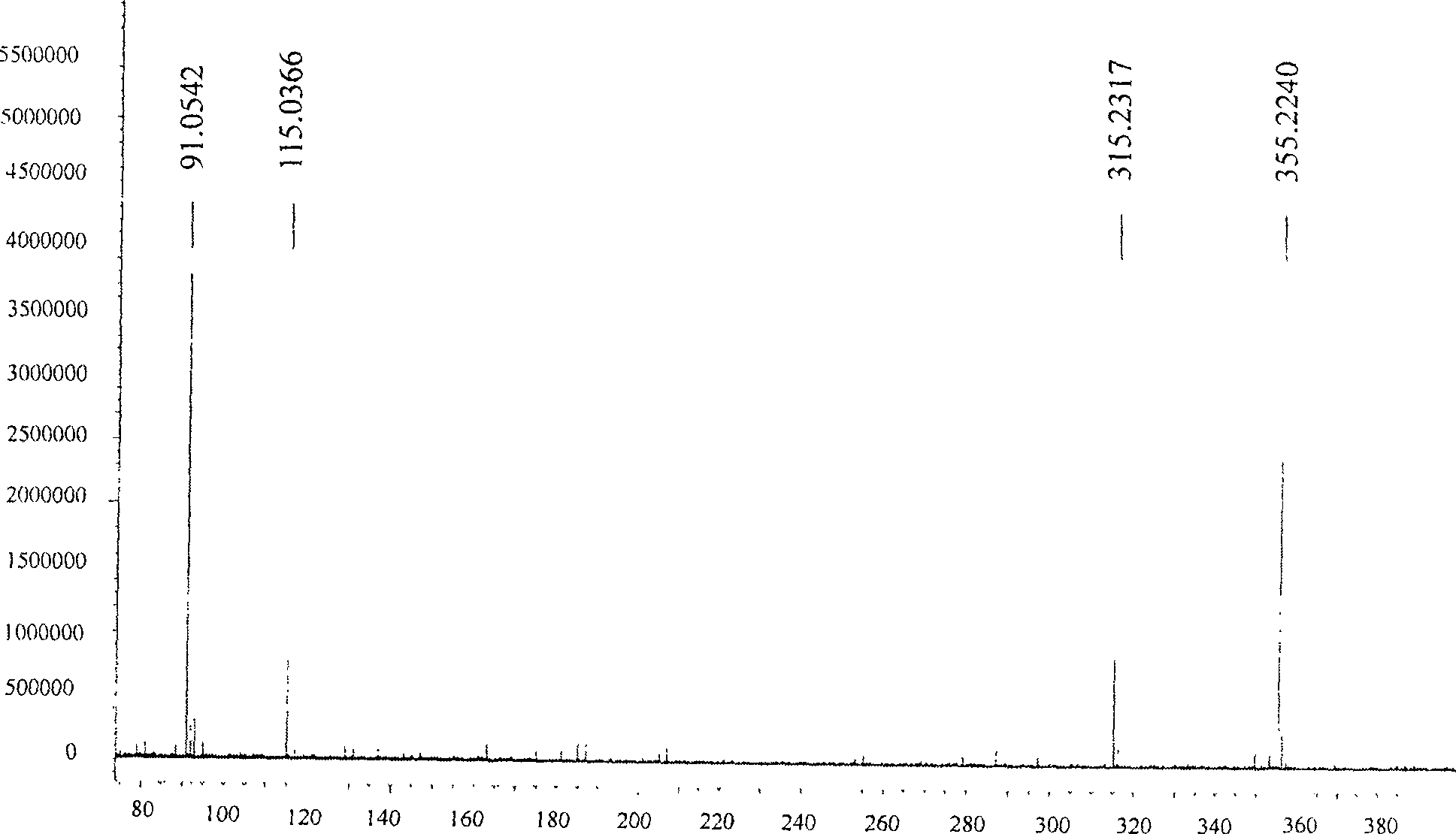
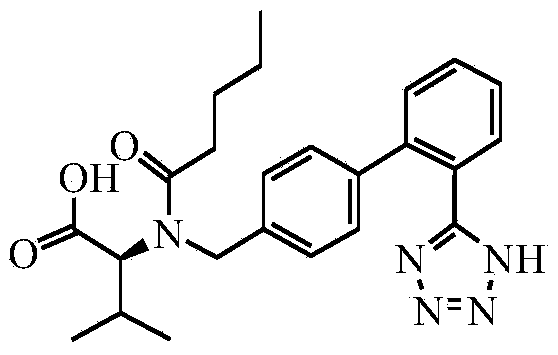
Method for preparing valsartan
InactiveCN104292174AAvoid racemizationGuaranteed optical purityOrganic chemistrySodium methoxideValsartan
The invention provides a method for preparing valsartan. The method comprises the following steps: firstly, reacting N-(triphenylmethyl)-5-(4'-formyl-biphenyl-2-yl) tetrazolium with sodium methoxide, secondly, adding dichlorodicyanobenzoquinone (DDQ) and oxidizing, thirdly, reacting with L-valine methyl ester, fourthly, alkylating with valeryl chloride, fifthly, removing triphenylmethyl group and sixthly removing ester group and acidifying to obtain the target product valsartan. According to the method, a novel method for preparing valsartan is provided by virtue of the Williamson ether synthesis step and DDQ oxidation step, since potassium dihydrogen phosphate is adopted as a buffering reagent in the triphenylmethyl group removal process and a 1.3mol / L sodium hydroxide solution is used in the hydrolysis process, the problem of racemization of the product generated caused by too strong alkaline is avoided and the method is of important significance in maintaining the optical purity of the valsartan product.
Owner:EAST CHINA UNIV OF SCI & TECH
A kind of highly active bifunctional catalyst and its preparation method and application
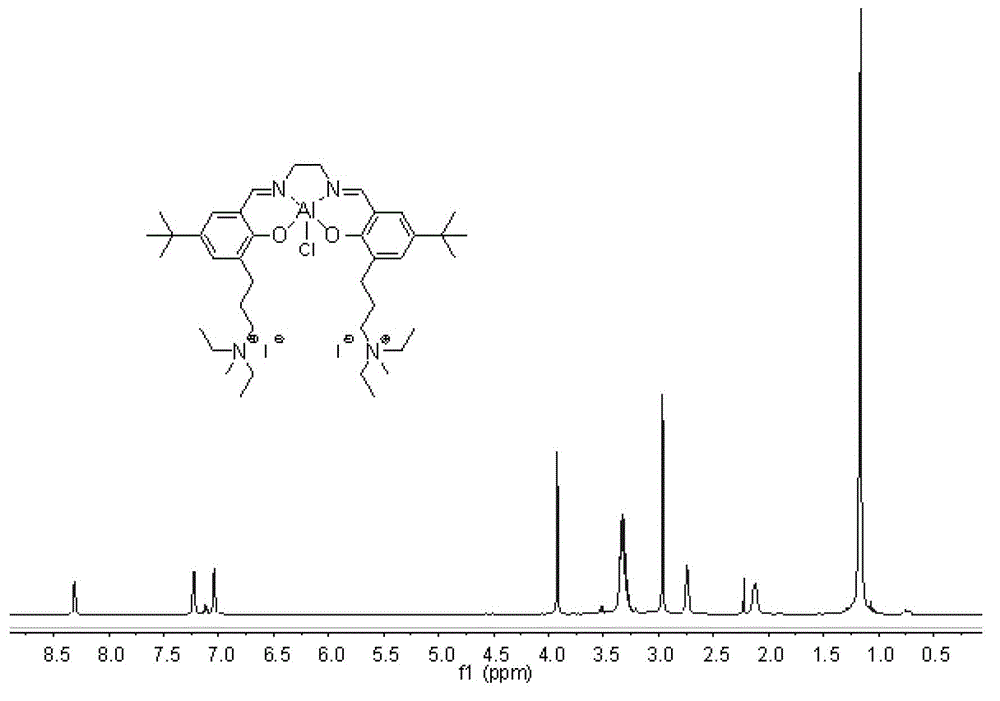
ActiveCN103170365BHigh catalytic activityMild reaction conditionsOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsOrganic solventPhosphonium salt
The invention relates to a high-activity double-function catalyst as well as a preparation method and application of the high-activity double-function catalyst. The catalyst is a tetradentate schiff base aluminum complex and the molecule contains more than one quaternary ammonium salt or quaternary phosphonium salt radical. Under a low catalyst concentration, the high-activity double-function catalyst still has the higher catalytic activity; reaction conditions are relatively moderate and a process is simple and convenient; the catalytic efficiency is high and the product selectivity is high; any organic solvent does not need to be added; and when chiral epoxyalkane is used as a reactant, the optical purity of a product can be completely kept.
Owner:SHENYANG GOLD JYOUKI TECH
Method for preparing L-theanine by chemical method
InactiveCN101717347BGuaranteed optical purityImprove economyOrganic compound preparationCarboxylic acid amides preparationGlycineAcid hydrolysis
The invention discloses a method for preparing L-theanine by a chemical method. The method comprises the following steps: firstly, reacting ethylamine with acryloyl chloride in the presence of an alkali to obtain N-ethylacrylamide; secondly, perform Michael addition reaction on chiral auxiliary BPB based glycine Schiff base Ni(II) coordination compound and the N-ethylacrylamide in the presence ofthe alkali to obtain theanine Schiff base Ni(II) coordination compound; and finally, performing acid hydrolysis on the theanine Schiff base Ni(II) coordination compound to prepare the L-theanine. Themethod adopts the chiral auxiliary chemical method to prepare the L-theanine, and constructs a complete theanine fragment through the Michael addition reaction so as to prepare the theanine Schiff base Ni(II) coordination compound, wherein the rigid space structure of the coordination compound can maintain the L-configuration of the theanine so as to ensure the optical purity of the L-theanine. The method can reclaim the chiral auxiliary in high yield, and can elute Ni(II) ions through ion exchange for recycling.
Owner:NANJING TECH UNIV
A kind of preparation method of d-sulbenicillin sodium
ActiveCN107641130BAdequate responseIncrease consumptionOrganic chemistrySulbenicillinOrganic solvent
The invention discloses a method for preparing D-sulbenicillin sodium. The method includes steps of removing L-amino acid from L-amino acid salt of D-sulfophenylacetic acid in solvents and carrying out salt forming to obtain D-sulfophenylacetic acid salt; carrying out reaction on the D-sulfophenylacetic acid salt and acylating agents to obtain mixed acid anhydride; dissolving 6-APA in organic solvents under the effect of organic alkali; dropwise adding mixed acid anhydride solution into 6-APA solution, and carrying out sufficient reaction and after-treatment to obtain the D-sulbenicillin sodium. The D-sulfophenylacetic acid is an intermediate. The 6-APA is a matrix. The method has the advantages that the D-sulbenicillin sodium is prepared by the aid of novel synthetic routes, conditions are mild, the method includes stable process and is easy to implement, and the problems of instable processes of existing methods or insufficient optical purity of products and the like can be solved bythe aid of the method.
Owner:CHENGDU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for producing optically active 1-bromo-1-[3,5-bis(trifluoromethyl)phenyl] ethane Method for producing optically active 1-bromo-1-[3,5-bis(trifluoromethyl)phenyl] ethane](https://images-eureka.patsnap.com/patent_img/42ccbbe9-5d2a-42c9-aa31-5dc40bc60b1d/133338DEST_PATH_IMAGE005.PNG)
![Method for producing optically active 1-bromo-1-[3,5-bis(trifluoromethyl)phenyl] ethane Method for producing optically active 1-bromo-1-[3,5-bis(trifluoromethyl)phenyl] ethane](https://images-eureka.patsnap.com/patent_img/42ccbbe9-5d2a-42c9-aa31-5dc40bc60b1d/160517DEST_PATH_IMAGE008.PNG)
![Method for producing optically active 1-bromo-1-[3,5-bis(trifluoromethyl)phenyl] ethane Method for producing optically active 1-bromo-1-[3,5-bis(trifluoromethyl)phenyl] ethane](https://images-eureka.patsnap.com/patent_img/42ccbbe9-5d2a-42c9-aa31-5dc40bc60b1d/297101DEST_PATH_IMAGE009.PNG)