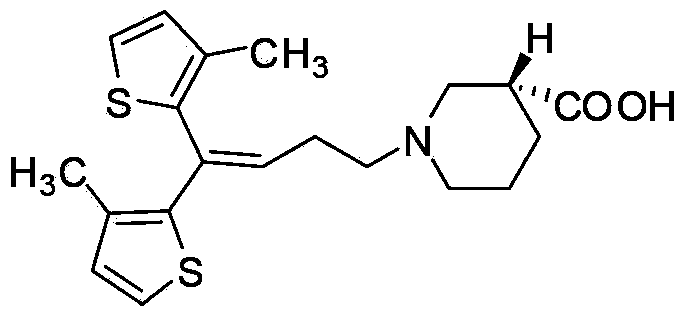
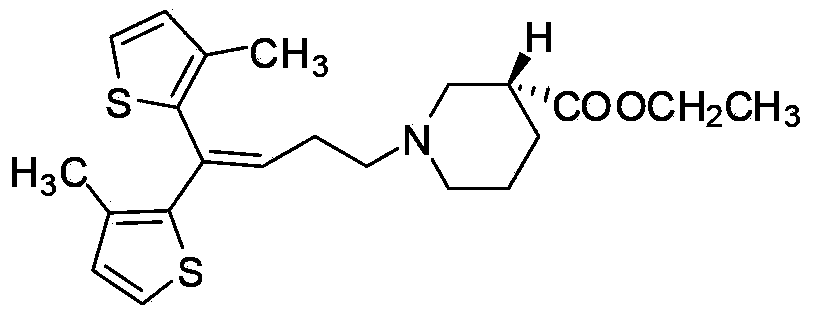
Preparing method for tiagabine hydrochloride
A technology of tiagabine hydrochloride and tiagabine ethyl ester, which is applied in the field of preparation of tiagabine hydrochloride, can solve the problems of waste of materials, increase of production cost, low yield, etc., and achieve the effect of simple operation and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Take 570.00 g of tiagabine ethyl ester (detected by HPLC, the content is 70%) and dissolve it in 2300 mL of ethyl acetate, add 90.00 g of L-alanine, heat to dissolve at 75±5 °C and reflux, and continue to reflux for 30 min after complete dissolution. Stir and cool to room temperature, place at -25±5°C for cooling and crystallization for 5h to 6h, suction filtration to collect crystals; add 570 mL of ethyl acetate to the obtained crystals, heat to dissolve at 75±5°C and reflux, and reflux after complete dissolution 30min, stirred and cooled to room temperature, placed at -5±5°C for cooling and crystallization for 2h to 3h, suction filtered, and the crystals were collected; dried to obtain 456.10g of L-alanine salt of tiagabine ethyl ester (yield 93.64%) , chemical purity 99.97%, optical purity 99.93%). The obtained L-alanine salt of tiagabine ethyl ester was dissolved in 500 mL of ethanol, and an aqueous sodium hydroxide solution (400 mL, 11 M) was added dropwise, and th...
Embodiment 2
[0029] Dissolve 570.00 g of tiagabine ethyl ester (detected by HPLC, the content is 70%) in 800 mL of methanol, add 108.00 g of L-alanine, heat to dissolve at 75±5 °C and reflux, continue to reflux for 30 minutes after complete dissolution, and stir and cool To room temperature, place at -25±5℃ for cooling and crystallization for 5h~6h, suction filtration, and collect the crystals; add 500 mL of ethanol to the obtained crystals, heat and dissolve at 75±5℃, reflux for 30min after complete dissolution, and stir and cool to room temperature, cooled and crystallized at -5±5°C for 2h to 3h, suction filtered to collect crystals; dried to obtain 452.05g of L-alanine salt of tiagabine ethyl ester (yield 92.81%, chemical purity 99.99 %, optical purity 99.97%). The obtained L-alanine salt of tiagabine ethyl ester was dissolved in 500 mL of ethanol, and an aqueous sodium hydroxide solution (400 mL, 11 M) was added dropwise, and the reaction was carried out at room temperature for 8 h. Af...
Embodiment 3
[0031] Take 570.00 g of tiagabine ethyl ester (detected by HPLC, the content is 70%) and dissolve it in 1200 mL of ethyl acetate, add 135.00 g of L-alanine, heat to dissolve at 75±5 °C and reflux, and continue to reflux for 30 min after complete dissolution. Stir and cool to room temperature, place at -25±5°C for cooling and crystallization for 5h to 6h, suction filtration to collect crystals; add 570 mL of ethyl acetate to the obtained crystals, heat to dissolve at 75±5°C and reflux, and reflux after complete dissolution 30min, stirred and cooled to room temperature, placed at -5±5°C for cooling and crystallization for 2h to 3h, suction filtration to collect crystals; dry to obtain 450.64g of L-alanine salt of tiagabine ethyl ester (yield 92.52%). , chemical purity 99.95%, optical purity 99.93%). The obtained L-alanine salt of tiagabine ethyl ester was dissolved in 500 mL of ethanol, and an aqueous sodium hydroxide solution (400 mL, 11 M) was added dropwise, and the reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com