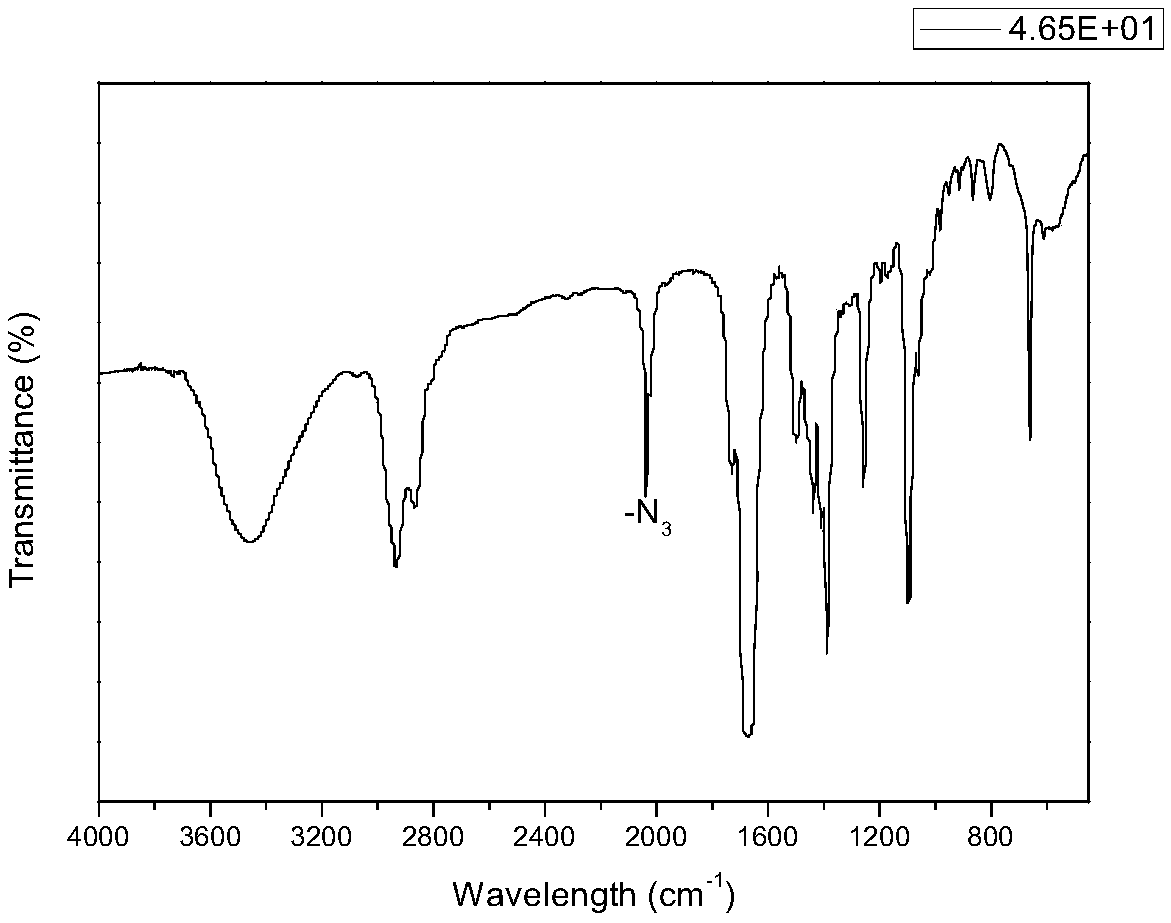
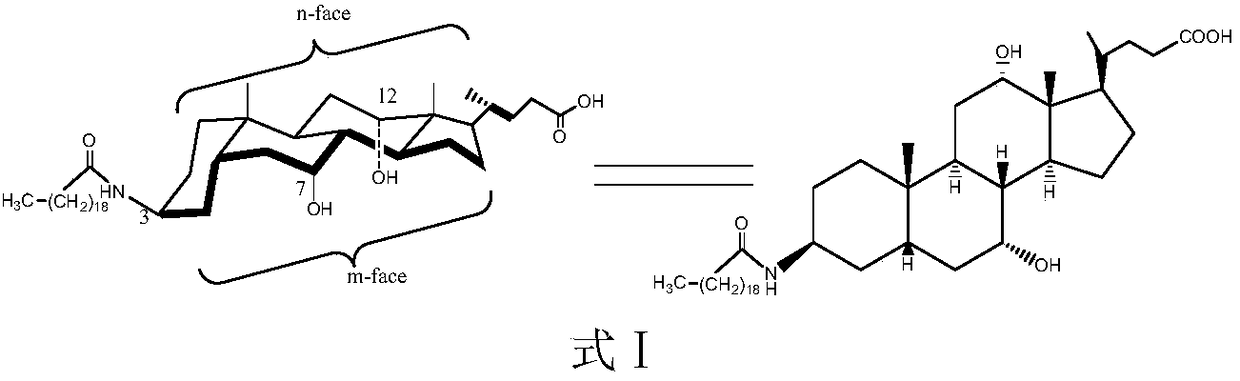
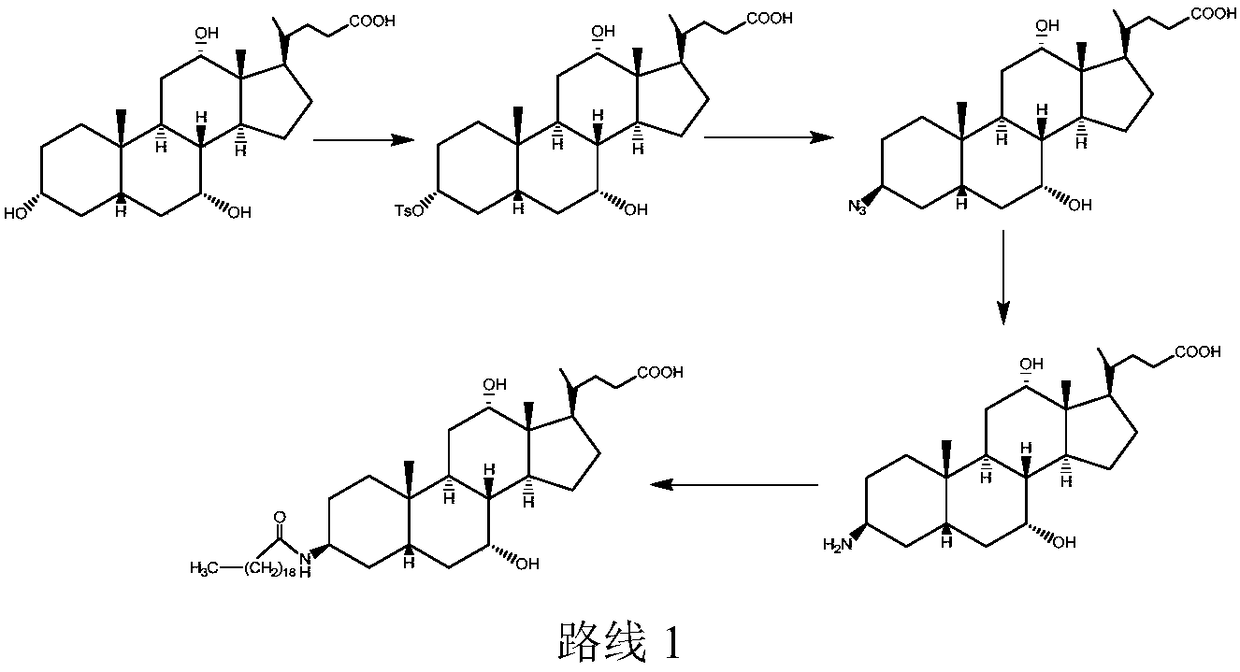
New technology for efficiently synthesizing Aramchol by utilizing cholic acid and arachidic acid as raw materials
A kind of arachidic acid and new process technology, applied in the field of organic synthesis, can solve the problems of difficult material circulation, low yield, and large environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1, the synthesis of intermediate b (methyl cholate)
[0057]
[0058] Slowly add 10mL of cold acetyl chloride dropwise into the cold methanol solution, and continue to stir evenly under the condition of an ice-salt water bath. After the temperature drops, add 10g of cholic acid, continue to stir for 30min, remove the ice bath, return to room temperature naturally, and continue Reaction 12h. After the reaction, use saturated NaHCO 3 After the solution was washed and suction filtered, a white solid product b could be obtained, the purity of which was detected by liquid chromatography >99%, and the yield was 99%, and its structure was confirmed by NMR.
[0059] 1 H NMR (600MHz, cdcl 3 )δ3.94(s,1H),3.82(s,1H),3.64(s,3H),3.42(t,J=10.8Hz,1H),2.35(ddd,J=14.6,9.8,4.5Hz,1H ),2.26–2.12(m,3H),2.03(d,J=8.5Hz,1H),1.94–1.21(m,18H),1.09(ddd,J=24.0,14.9,8.9Hz,1H),0.96( d,J=6.2Hz,3H),0.88(s,3H),0.66(s,3H).
[0060] 2. Synthesis of intermediate c (3α-O-p-tert-butylbenzenesulfony...
Embodiment 2
[0081] Intermediate b is synthesized with embodiment 1;
[0082] Synthesis of intermediate c: Dissolve 2g of b (methyl cholate) into 25mL of carbon tetrachloride, and ice-bath the resulting solution to subzero with brine, and dissolve 1.9g of m-dimethylphenylsulfonyl chloride into 7.5mL Pyridine solution, and then the resulting solution was slowly added dropwise to the pyridine solution of b, stirring was continued for 1 h, the ice bath was removed, and the reaction was carried out at 40°C for 4 h. After the reaction was finished, the pH was adjusted, and then washed three times with saturated brine, separated, dried, and the solvent was evaporated to obtain a white solid. The crude product was separated by column chromatography with petroleum ether: ethyl acetate = 3:1, and the yield was 75%.
[0083] 1 H NMR (600MHz, cdcl 3 )δ7.82(s,1H),7.55(d,J=8.5Hz,2H),4.37(ddd,J=16.2,11.0,5.2Hz,1H),3.97(s,1H),3.83(t,J =13.2Hz,1H),3.68(s,3H),2.54(dd,J=25.2,13.1Hz,2H),2.39(ddd,J=16.0,14...
Embodiment 3
[0086] Intermediate b is synthesized with embodiment 1;
[0087] Synthesis of intermediate c: Dissolve 2g b (methyl cholate) and 0.5g triethylamine into 20mL of dichloroethane solution, and ice-bath the resulting solution to subzero with brine, take 1.7g p-tert-butylbenzene Sulfonyl chloride was dissolved in 5 mL of dichloroethane solution, and the resulting solution was slowly added dropwise to the dichloromethane solution of b, and reacted at 30°C for 4h. After the reaction was completed, it was washed three times with saturated brine, separated, dried, and dichloroethane was evaporated to obtain a white solid. The crude product was separated by column chromatography with petroleum ether: ethyl acetate = 3:1, and the yield was 50%.
[0088] 1 H NMR (600MHz, cdcl 3 )δ7.81(d, J=8.6Hz, 2H), 7.52(d, J=8.6Hz, 2H), 3.96(s, 1H), 3.83(d, J=2.6Hz, 1H), 3.66(s, 3H), 2.40–2.32(m,2H), 2.28–2.01(m,2H), 1.95–1.38(m,16H), 1.35(s,9H), 1.30–1.23(m,2H), 1.12(ddd, J=24.5,12.1,6.1Hz,2H),0.9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com