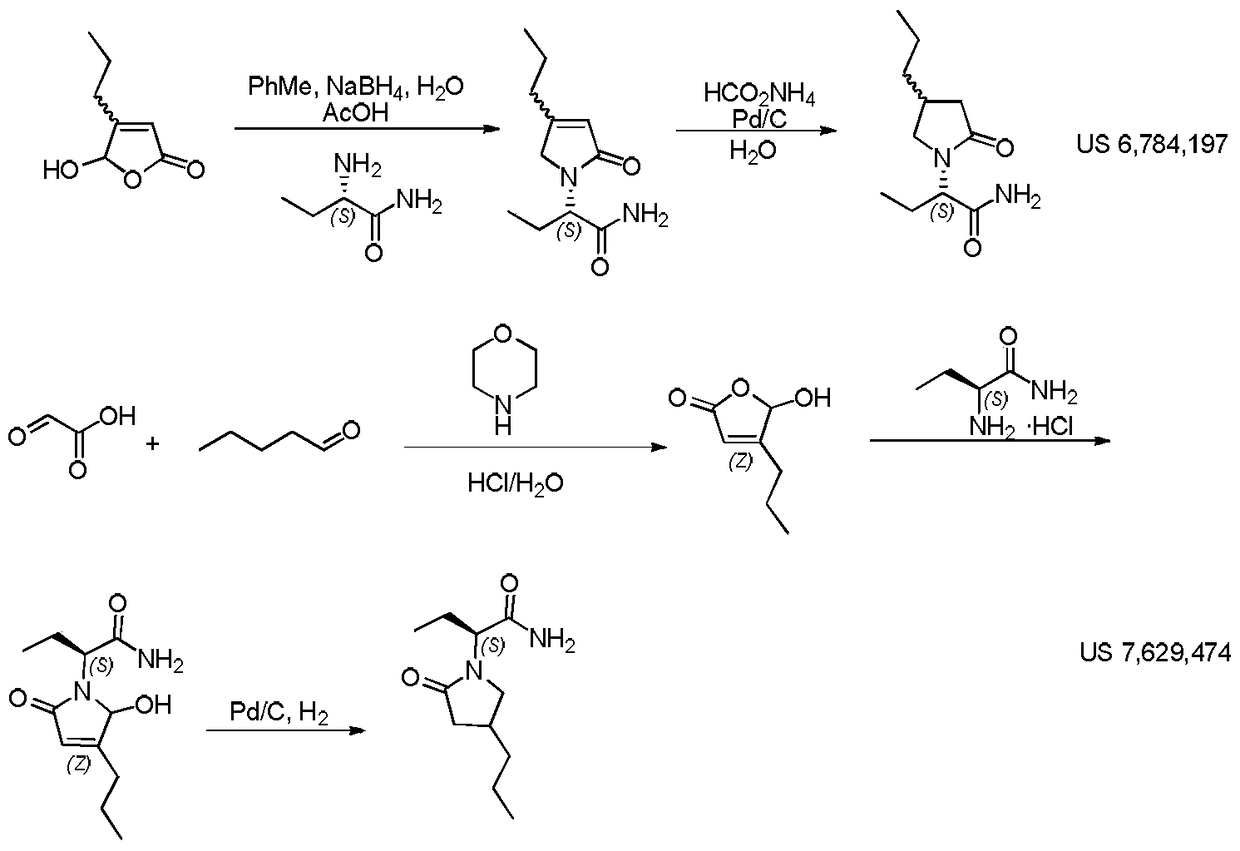
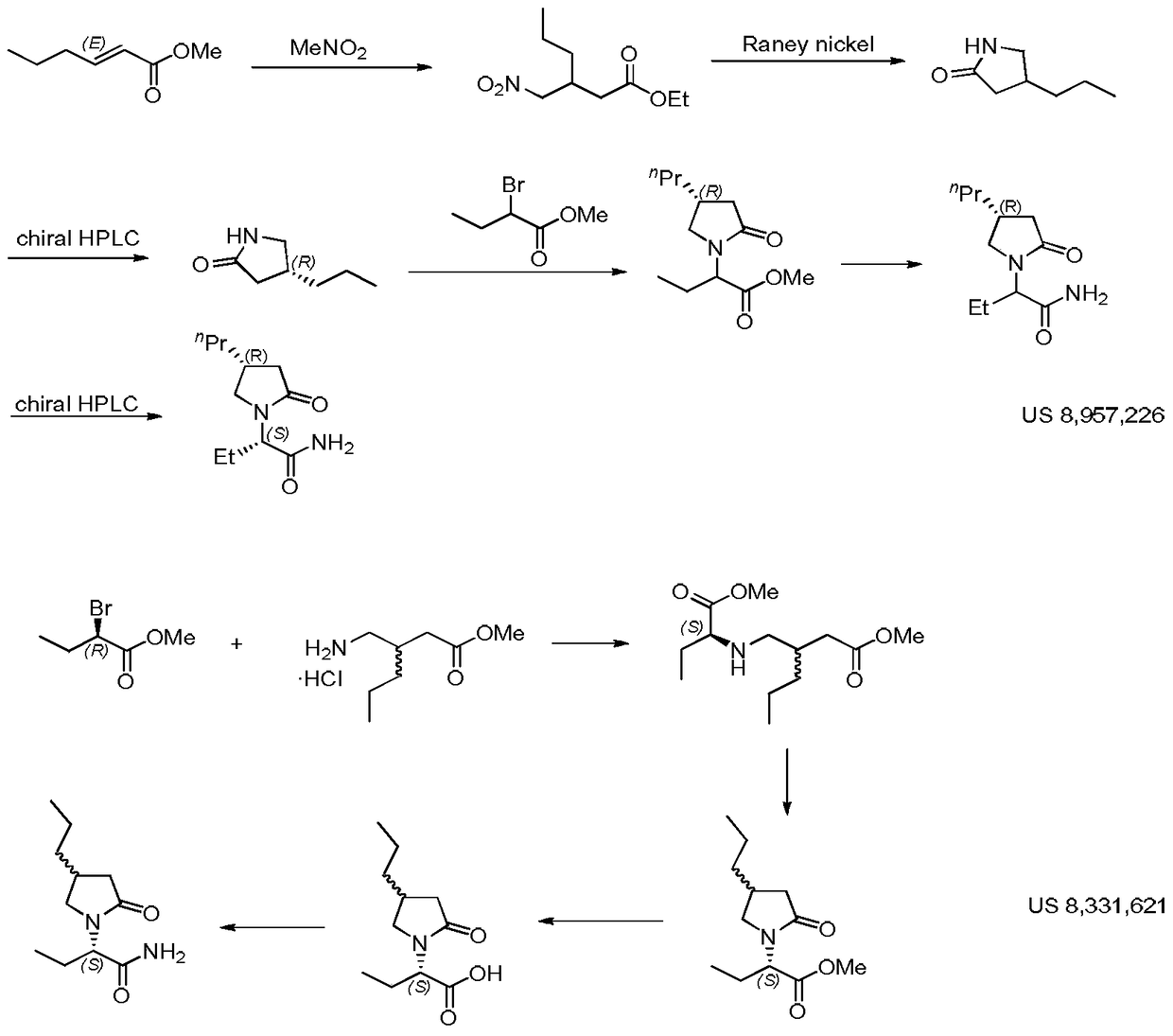
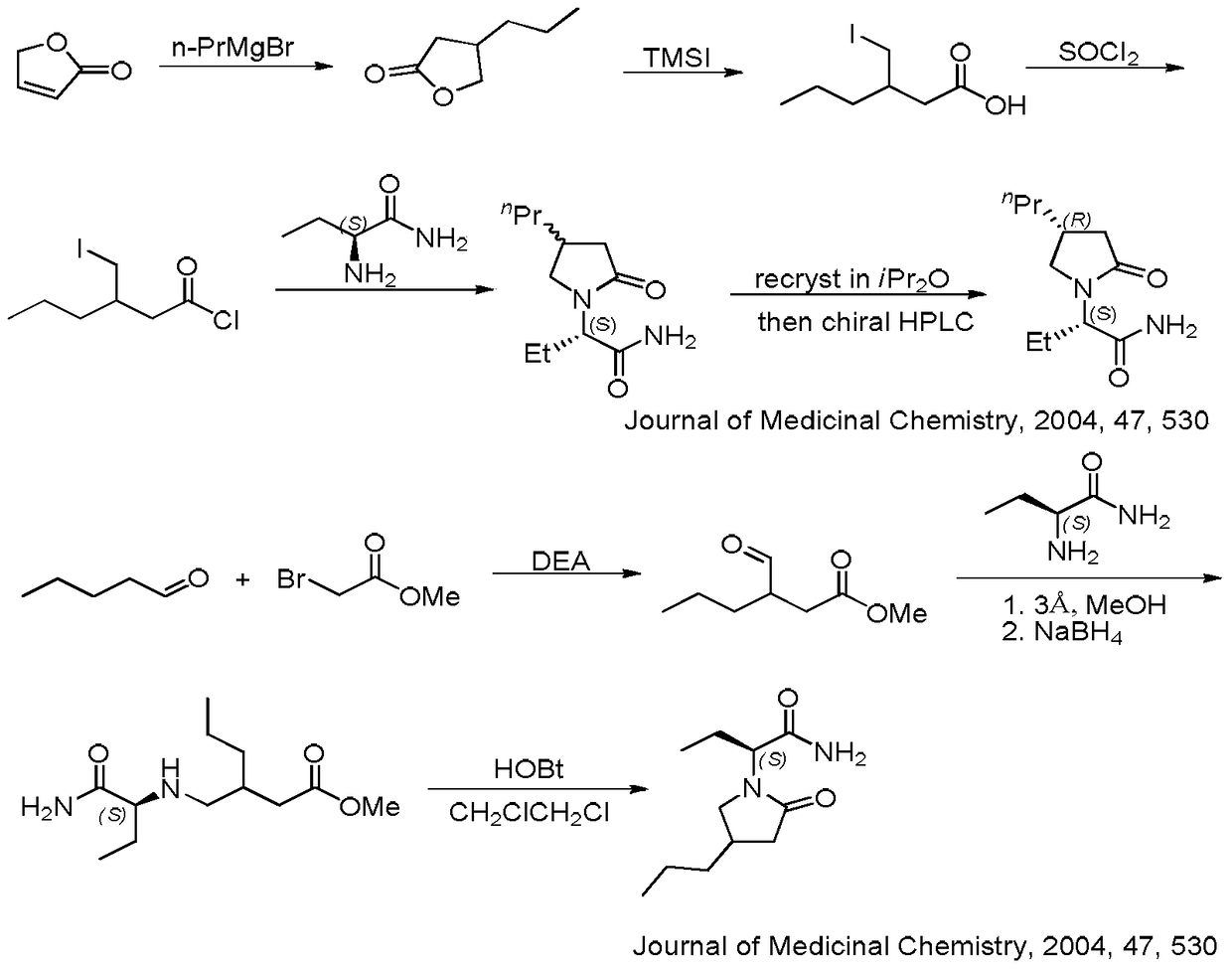
A compound, its preparation method and its use in the synthesis of Buvaracetam
A compound, the technology of cuprous iodide, applied in the field of compound and its preparation, and the synthesis of brivaracetam, can solve the problems of unconstructed butyrolactam and uneconomical use of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1 prepares compound 3
[0072] Sodium methoxide (2.05 g, 38 mmol) was added into 80 mL of absolute ethanol to dissolve completely. The reaction flask was placed in an ice-water bath, and diethyl malonate was added. Stirring at this temperature for 10 minutes, the system was raised to room temperature, and (R)-epichlorohydrin (ee 98%) (2.7 mL, 35 mmol) (purchased from Anaiji Chemicals) was slowly added to the reaction system, and the addition was completed. The system was reacted under reflux for 18 hours, the reaction was stopped, the system was cooled to room temperature, the solvent was spin-dried, 100 mL of water was added, and extracted 3 times with 100 mL of ethyl acetate. The organic phases were combined, dried over anhydrous sodium sulfate, filtered after drying, and the filtrate was spin-dried to obtain compound 3, which was distilled under reduced pressure to obtain a colorless liquid with a yield of 55%. Compound 3 chiral HPLC (ee 98%)
[0073] ...
Embodiment 2
[0074] Embodiment 2 prepares compound 4
[0075] Add CuI (9.5g, 50mol) into 100mL of dry THF, place the reaction flask in a low-temperature reaction bath at -30°C, add a THF solution of ethyl Grignard reagent (1.0M, 300mL, 300mmol) into the reaction flask and stir for 1 hour , and then the dry THF solution of compound 3 (20 g, 117 mmol) prepared by the method described in Example 1 was added dropwise to the reaction flask. After the dropwise addition was completed, after stirring at this temperature for 30 minutes, the temperature was slowly raised to -15°C. The reaction was quenched with saturated ammonium chloride, 1 L of water was added, extracted three times with 1 L of ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, after drying, filtered, and the filtrate was concentrated to obtain crude compound 4.
[0076] Purification by column chromatography (developing solvent polarity: petroleum ether / ethyl acetate=10 / 1) to obtain the nuclea...
Embodiment 3
[0078] Embodiment 3 prepares compound 5
[0079] The compound 4 crude product (in terms of 117mmol) prepared as described in Example 2 was added to DMSO / H 2 O (400 mL / 20 mL), LiCl (14.7 g, 350 mmol) was added to the reaction flask. After the system was reacted at 140°C for 18 hours, it was poured into 400 mL of water, extracted three times with 400 mL of ethyl acetate, the organic phases were combined, washed once with saturated NaCl solution, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated to obtain a crude product, and compound 5 was obtained by distillation under reduced pressure. , colorless liquid, together with embodiment 2 two-step total yield 50%
[0080] The NMR data of compound 5 are as follows: 1 H NMR (400MHz, CDCl 3 )δ4.42(1H,dd),3.92(1H,dd),2.52-2.65(2H,m),2.18(1H,dd),1.40-1.47(2H,m),1.40-1.47(2H,m) ,1.27-1.39(2H,m),0.94(3H,t).
[0081] The specific rotation of compound 5 is: [α] 23 D =+3.9 (C=10, CHCl 3 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com