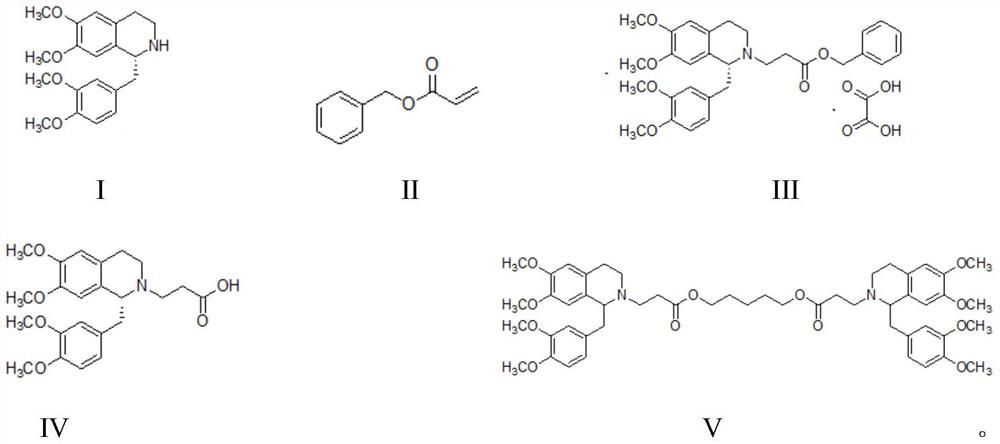
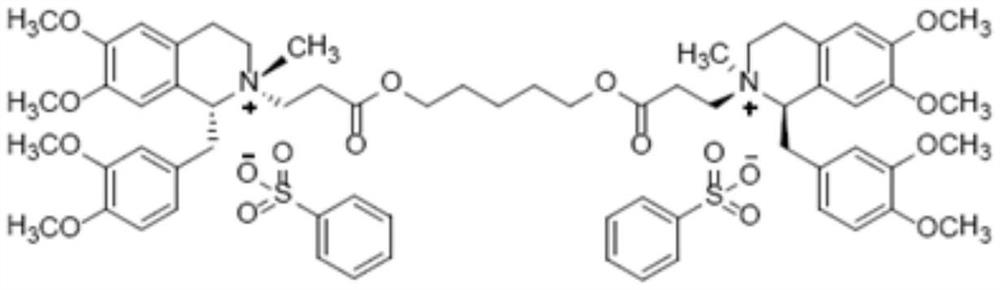
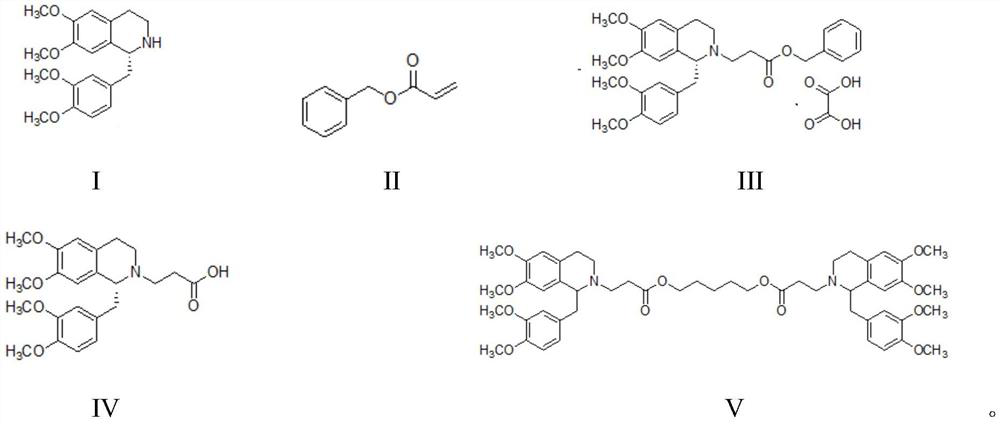
Method for synthesizing cisatracurium besilate
A technology for cisatracurium besylate and a compound, which is applied in the field of pharmaceutical synthesis, can solve the problems of complex steps, low product yield, low product purity, etc., to improve yield and purity, reduce synthesis cost, and ensure optical purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the synthesis of besylate cisatracurium
[0039] (1) Synthesis of compound T473-02:
[0040]
[0041] Anhydrous DMF (0.6 L) and acrylic acid (129.7 g, 1.8 mol) were added to a round bottom flask, potassium carbonate (273.6 g, 1.98 mol) was added in batches, and the temperature was controlled below 45°C. Then T473-02A (227.9 g, 1.8 mol) was added and after the addition was complete, the mixture was stirred at 100° C. for 3 hours. After the reaction was completed, the reaction solution was cooled to room temperature, and 1.0 L of pentane was added. The organic layer was washed with water (3×300 mL) and saturated brine (1×300 mL). The organic layer was collected, dried over anhydrous magnesium sulfate, filtered and distilled under reduced pressure to obtain 288.1 g of a colorless oily substance, namely T473-02, with a yield of 98.8%.
[0042] 1 H NMR (CDCl 3 , 300Hz): δ7.36-7.28 (m, 5H), 6.43 (dd, J 1=5.4Hz,J 2 = 10.8Hz, 1H), 6.13(dd, J 1 = 4.2Hz,J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com