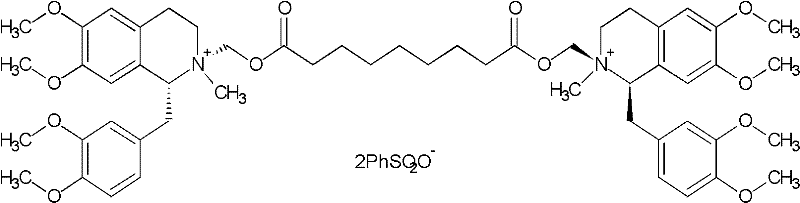
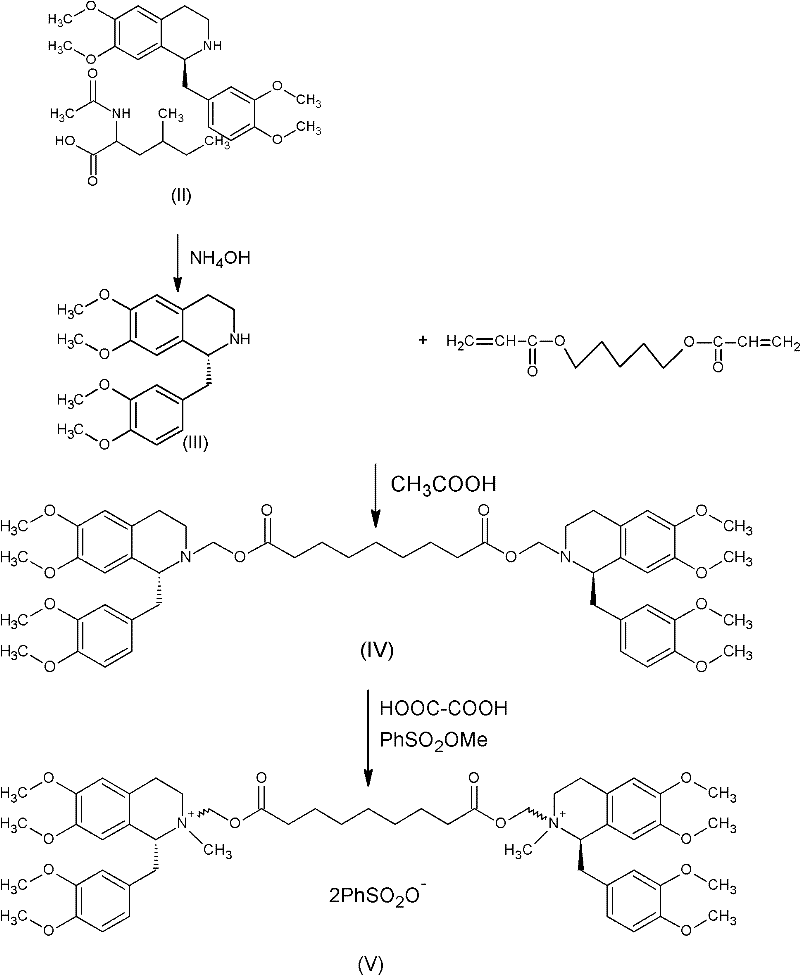
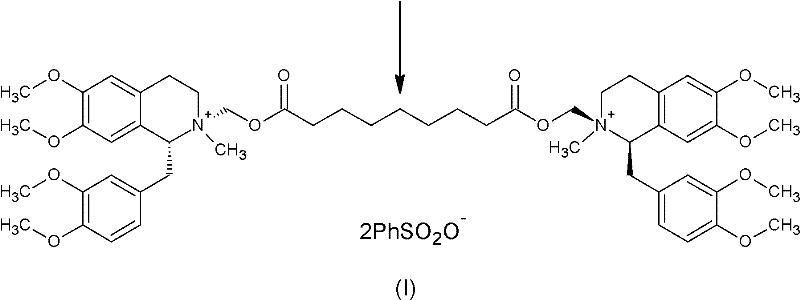
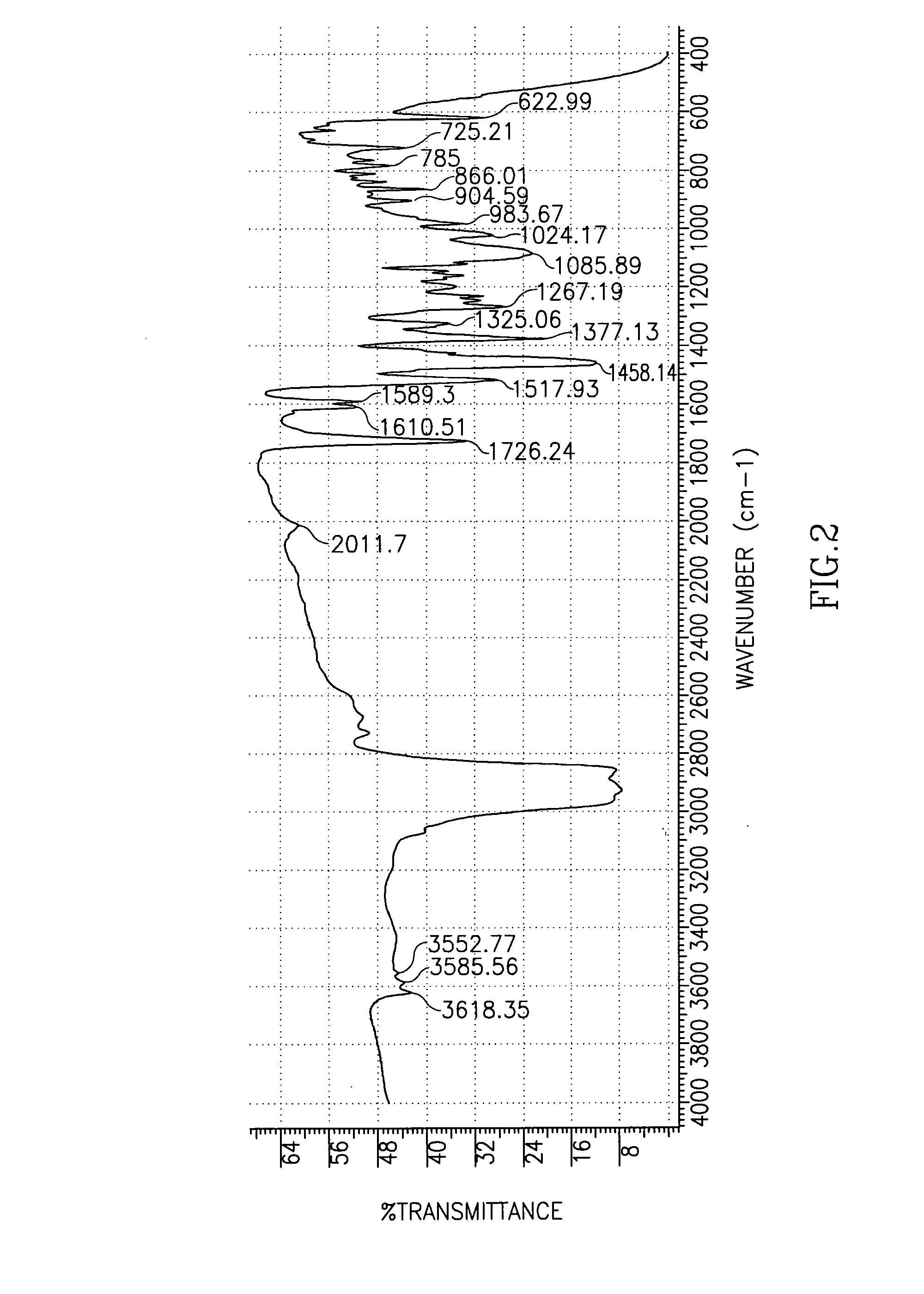
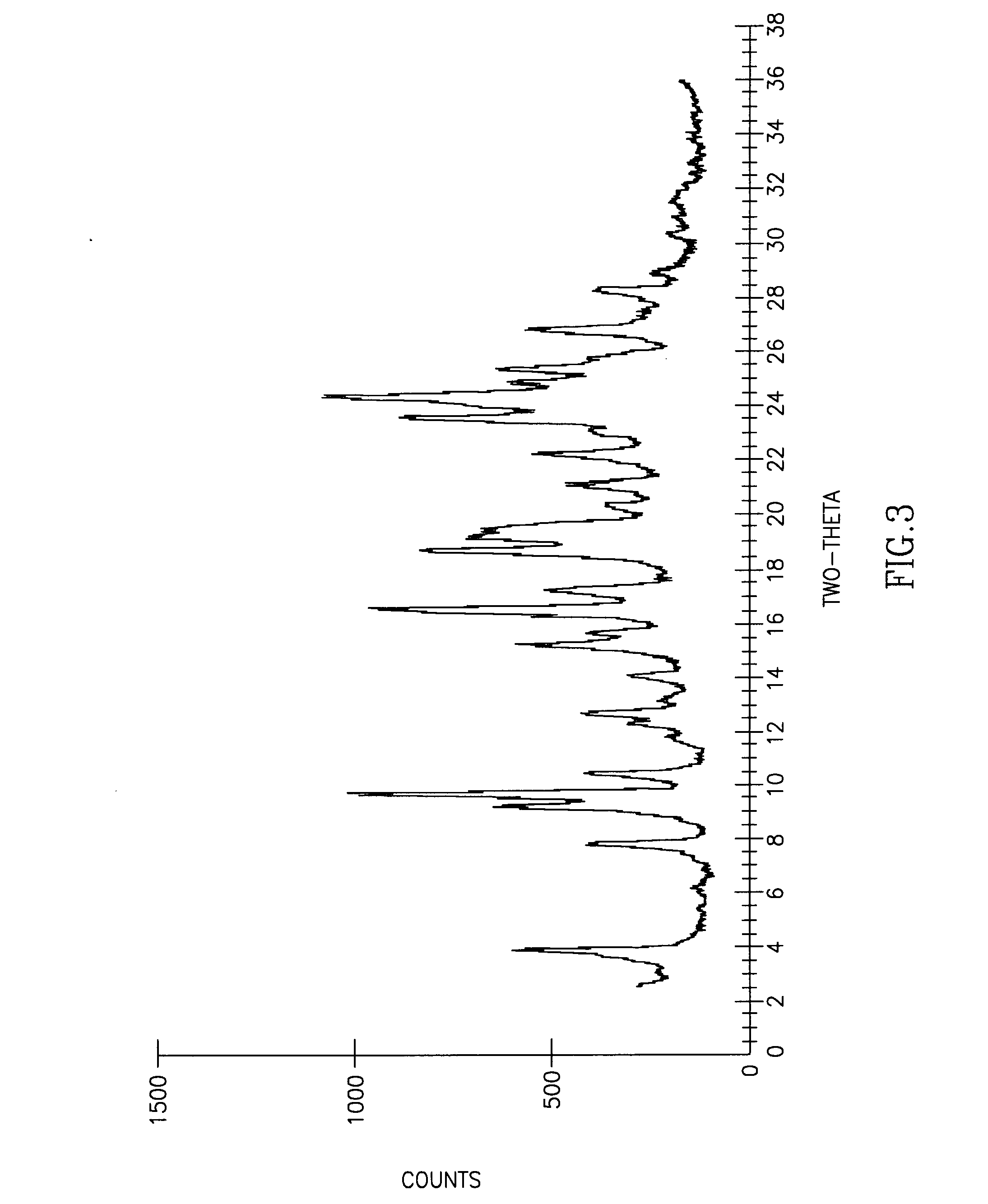
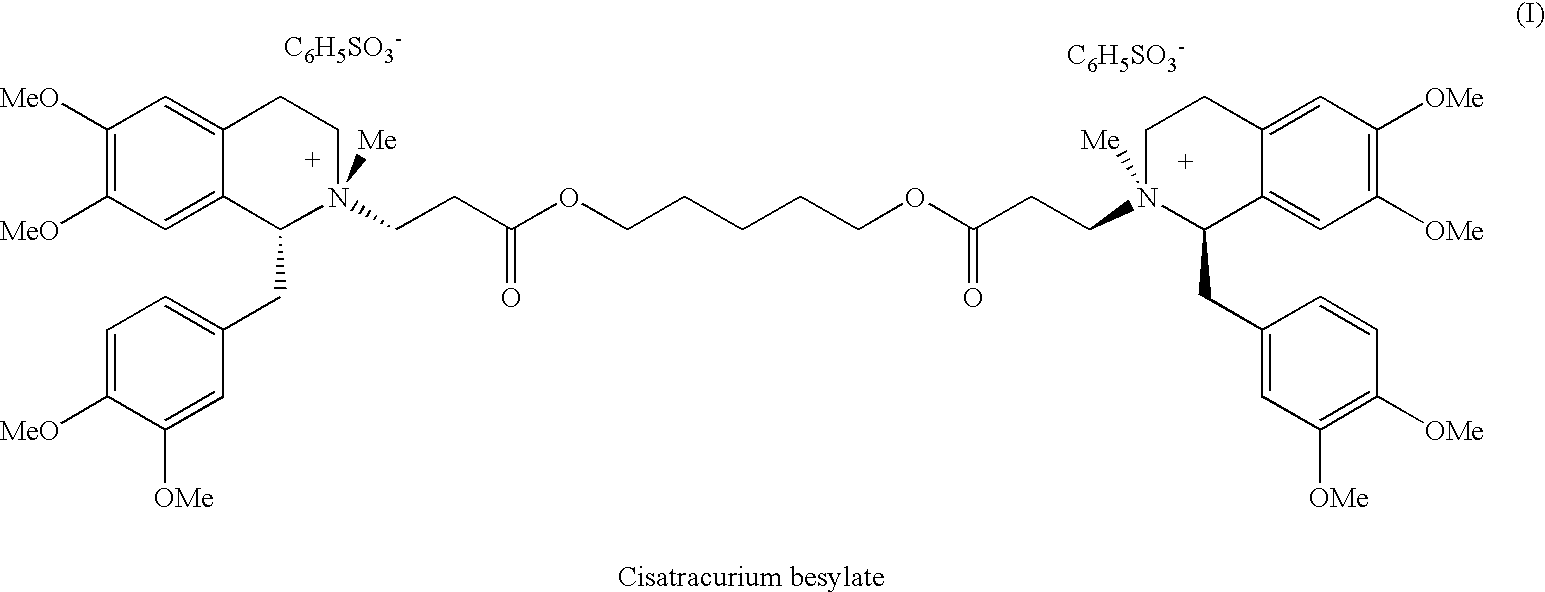
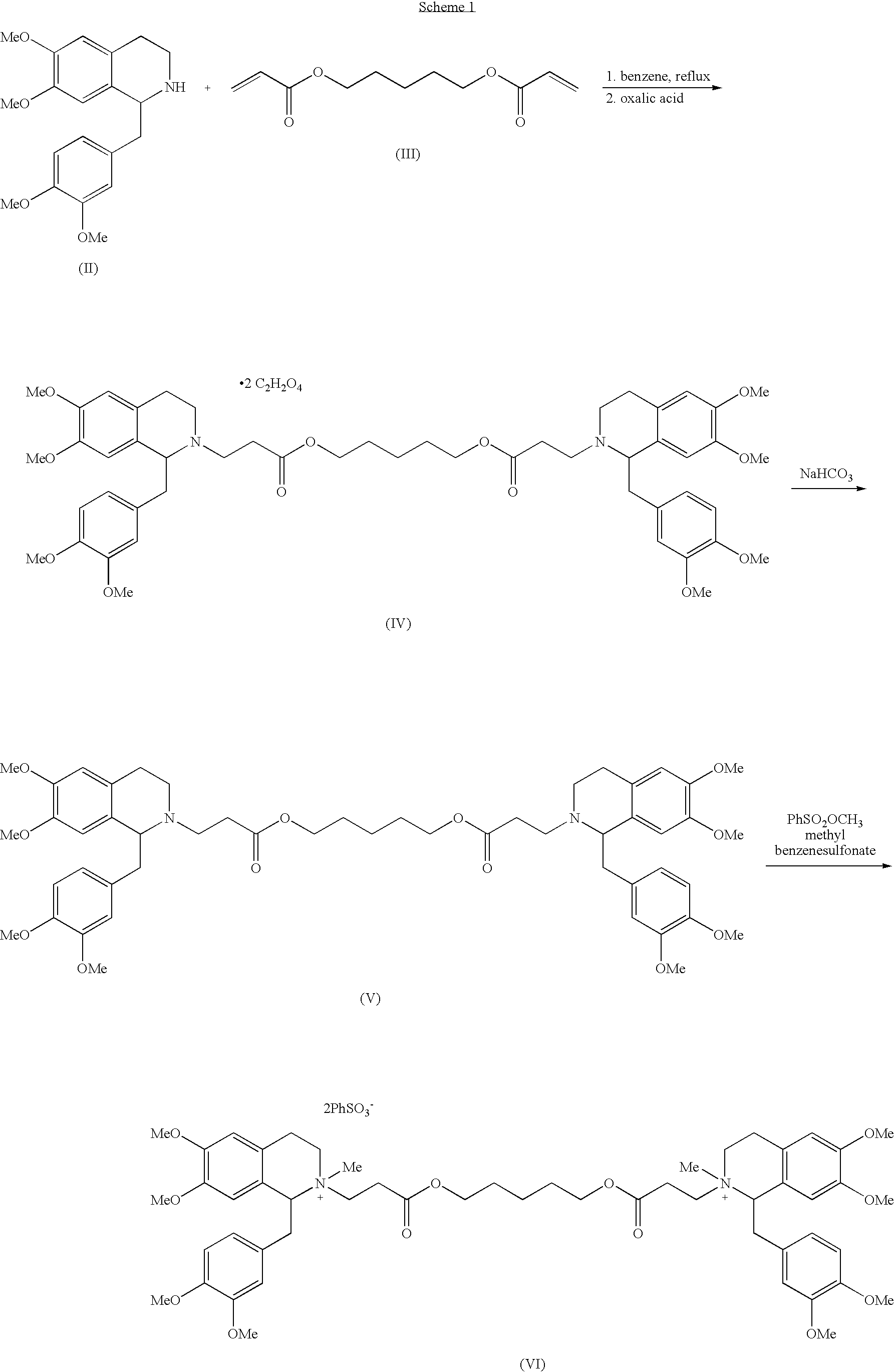
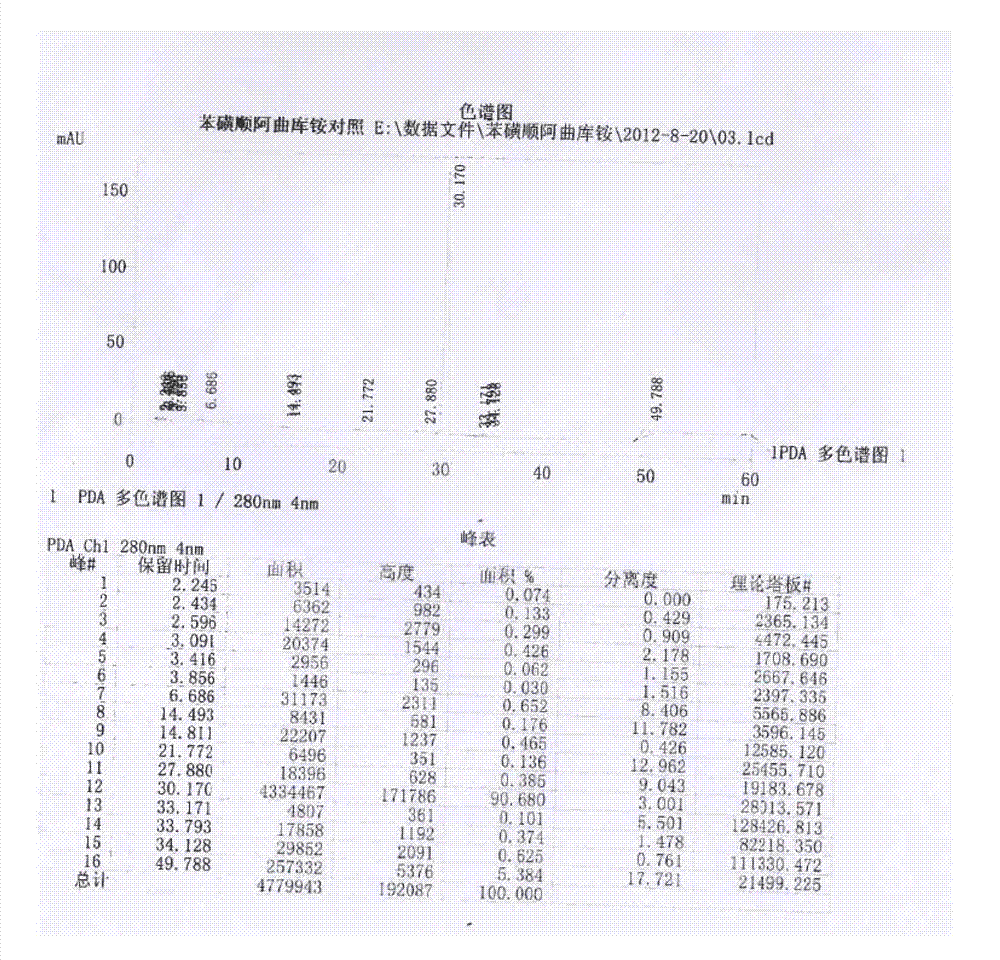
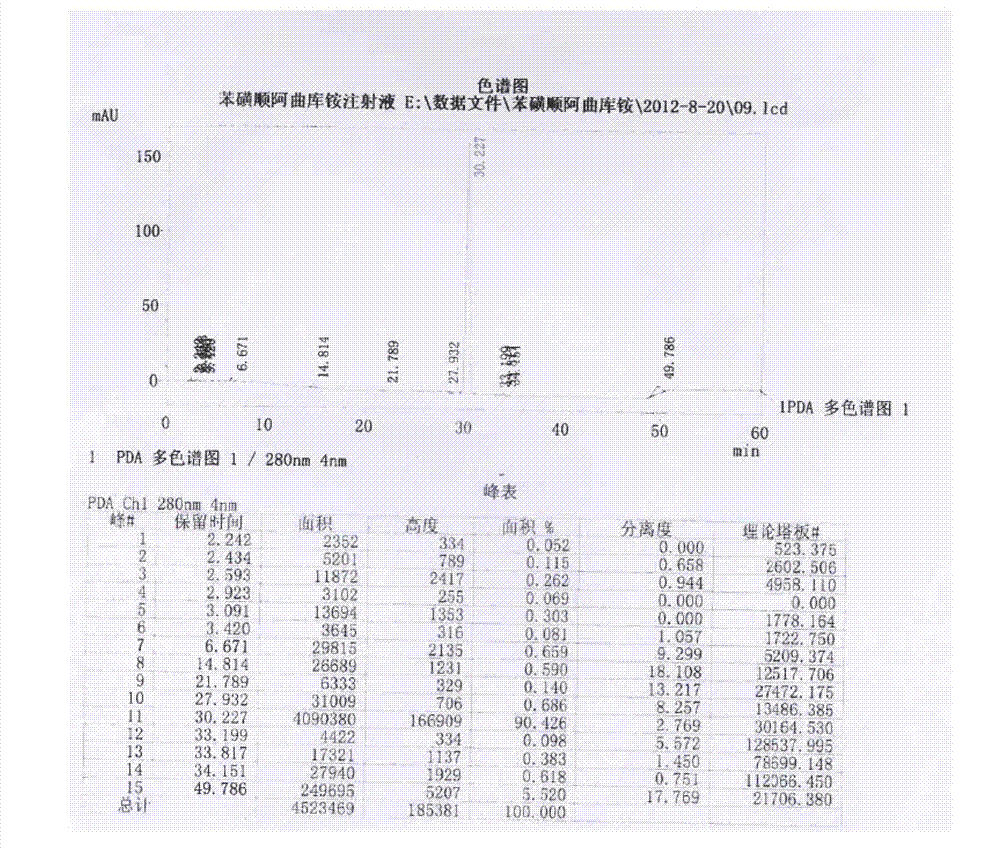
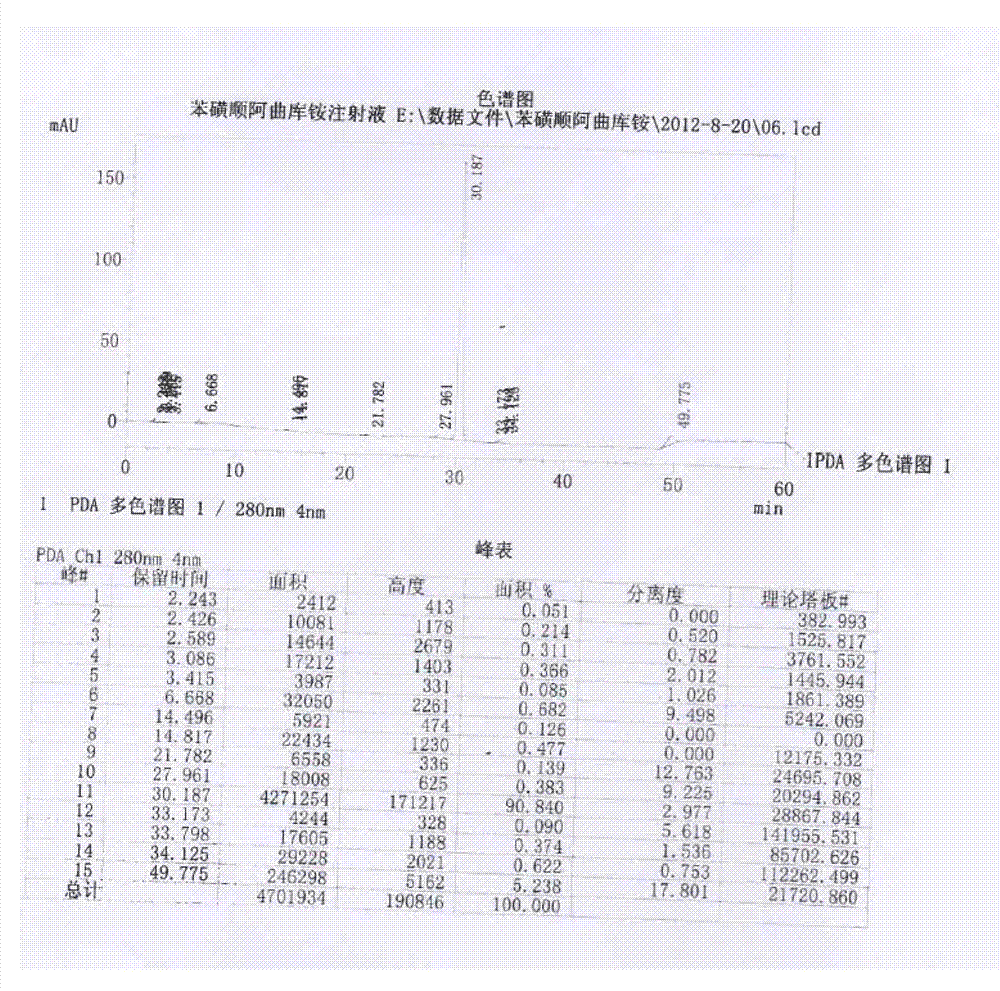
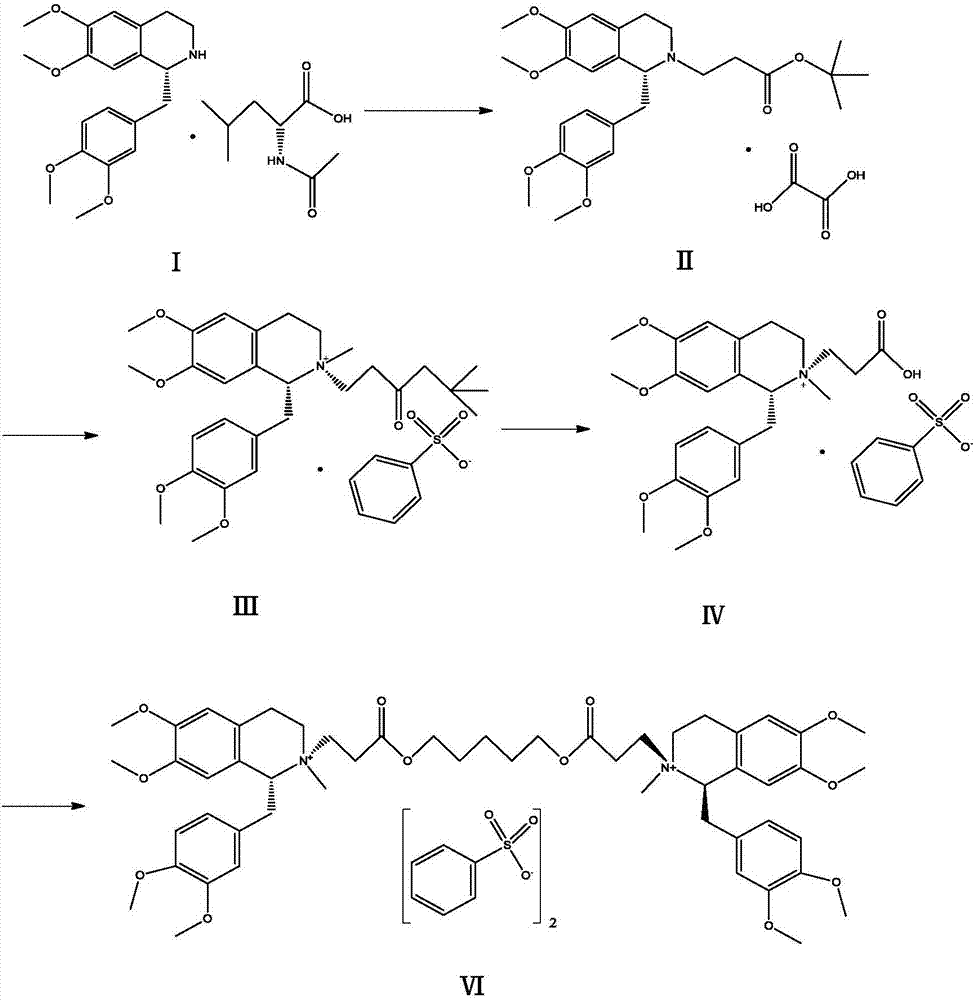
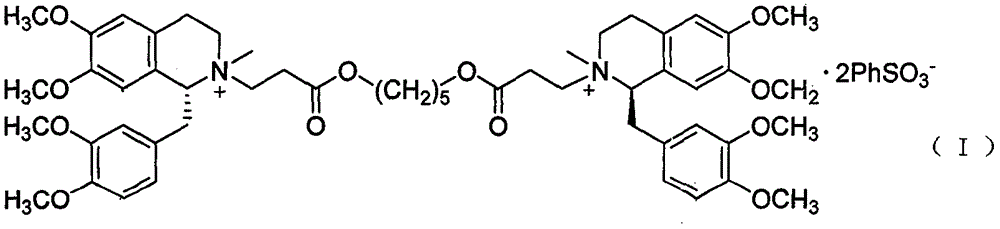
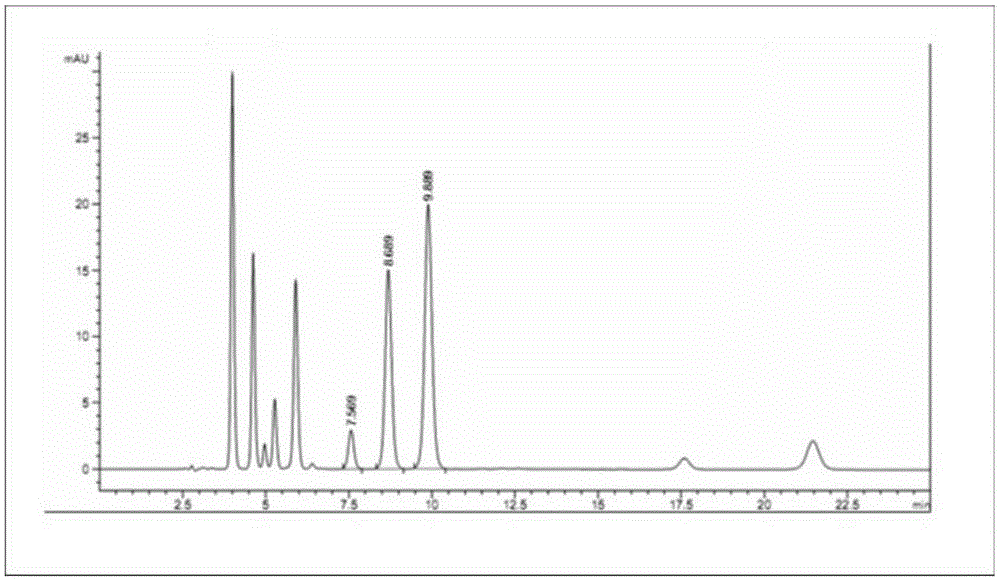
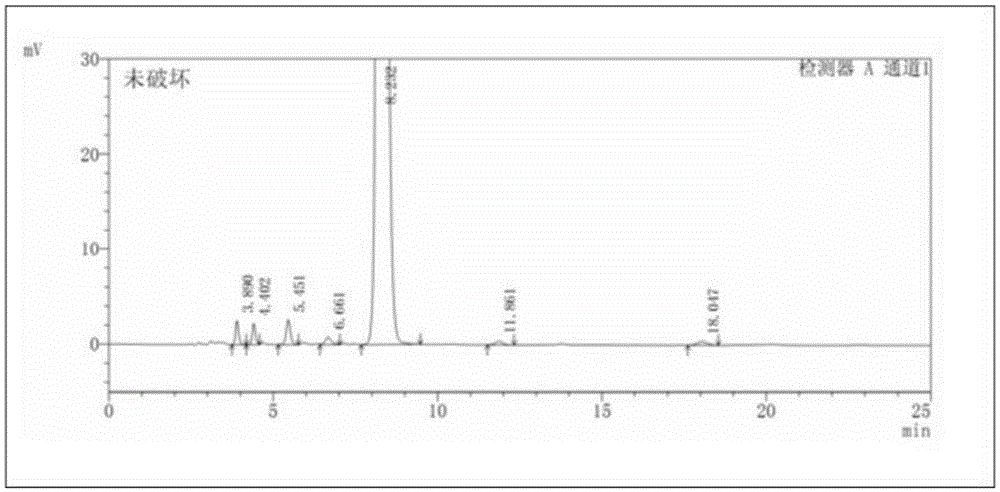
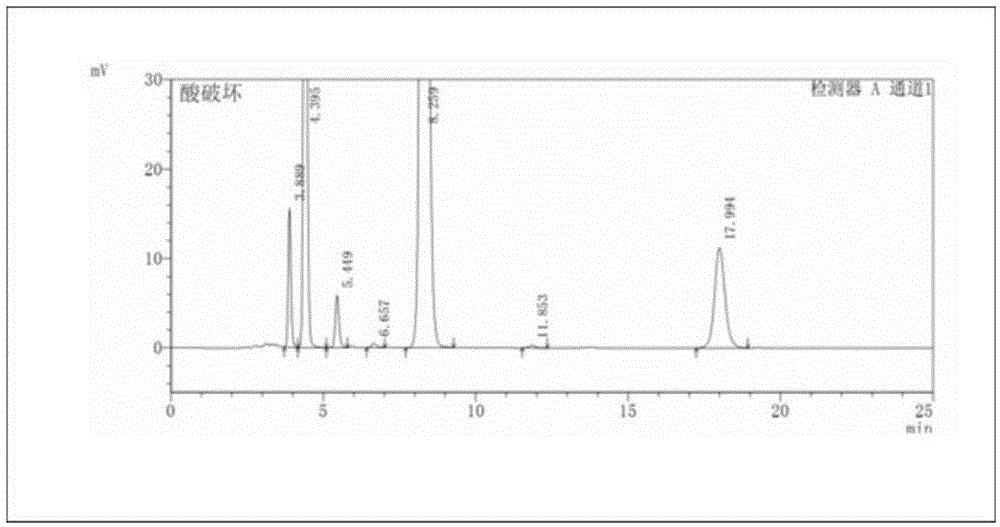
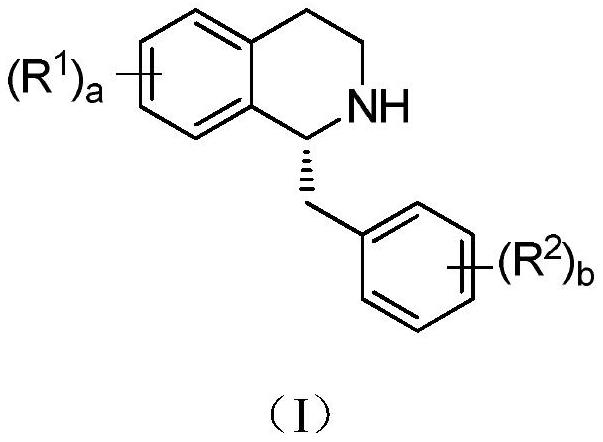
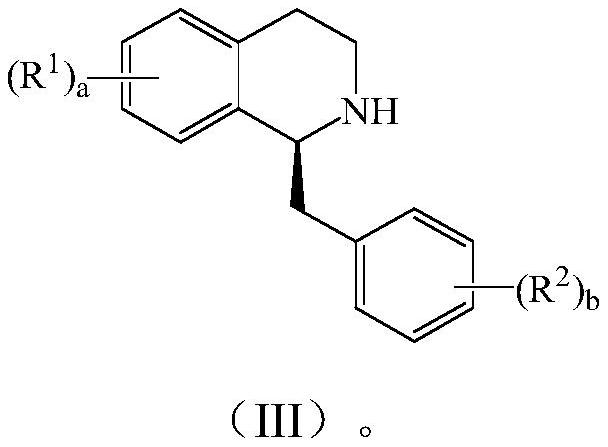
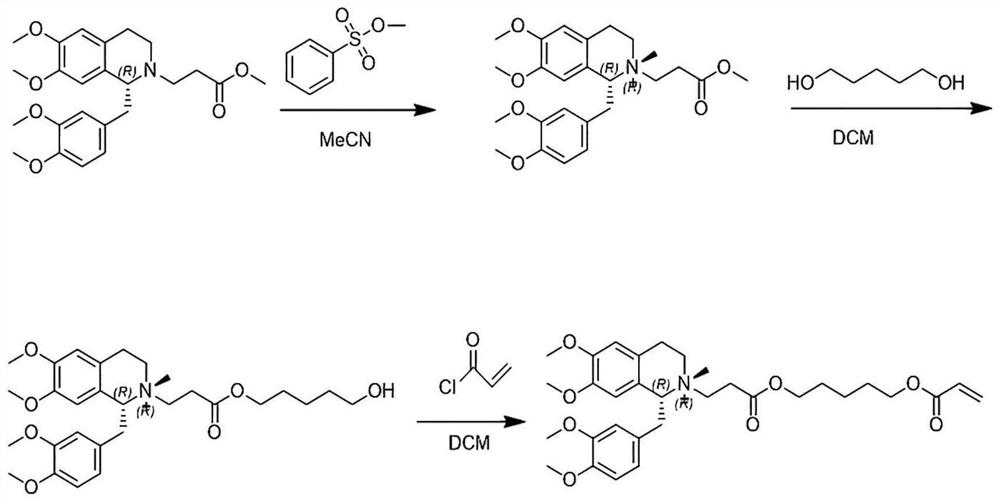
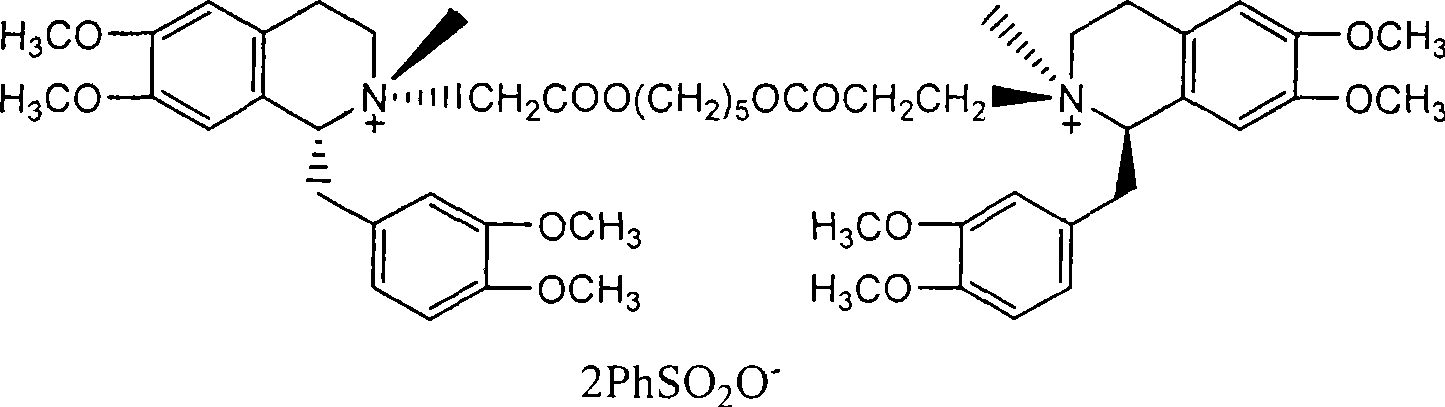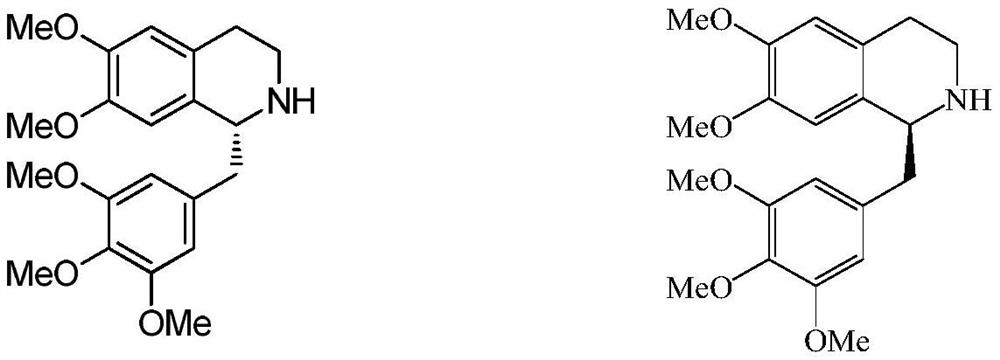Patents
Literature
30 results about "Cisatracurium Besylate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
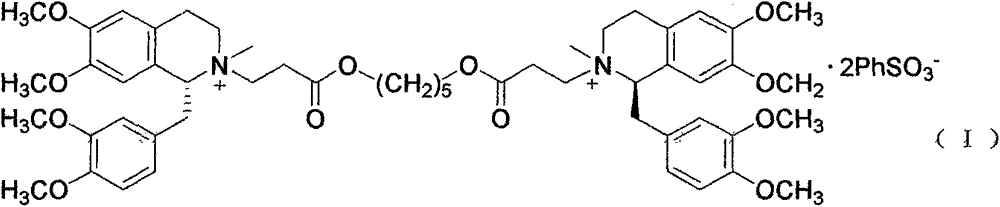
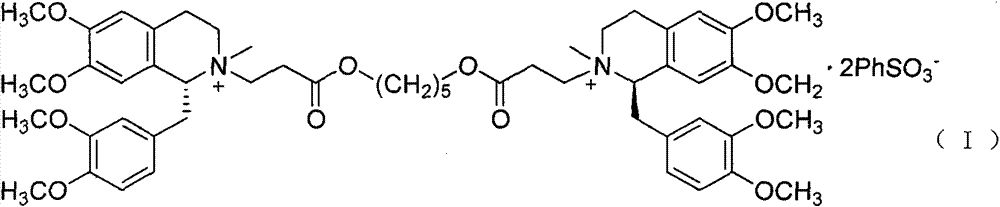
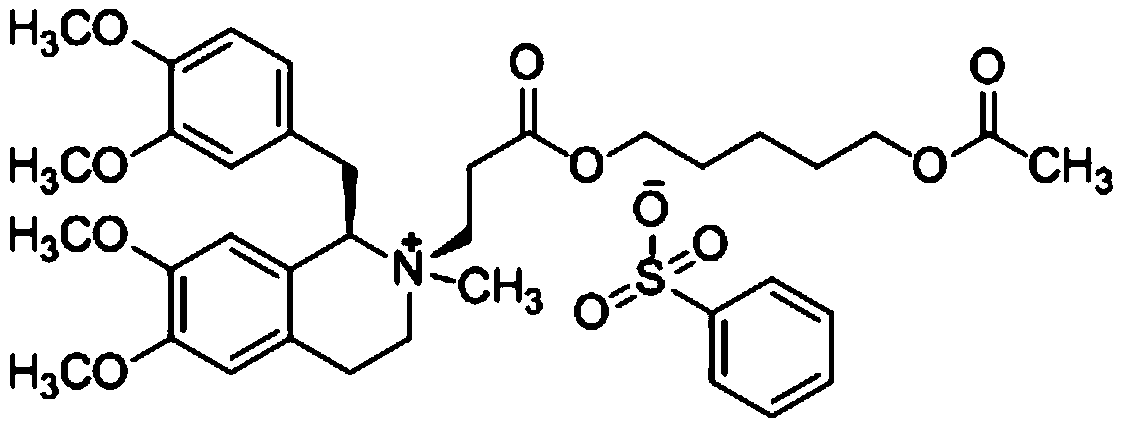
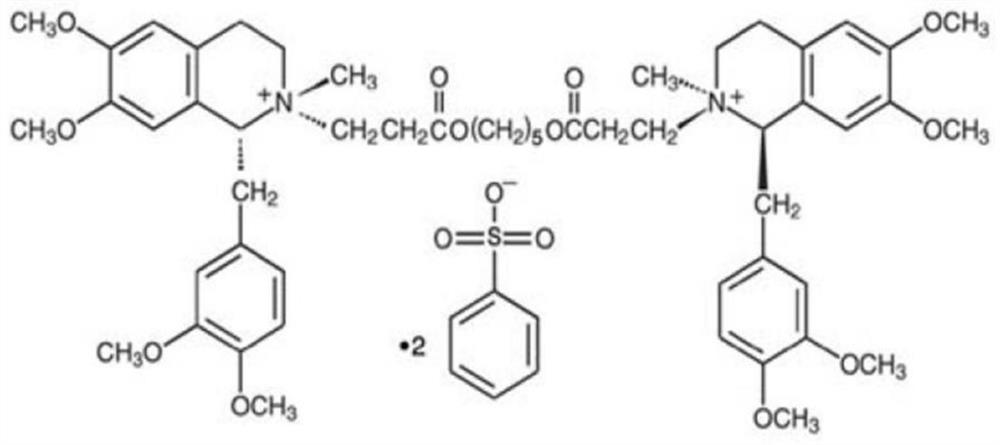
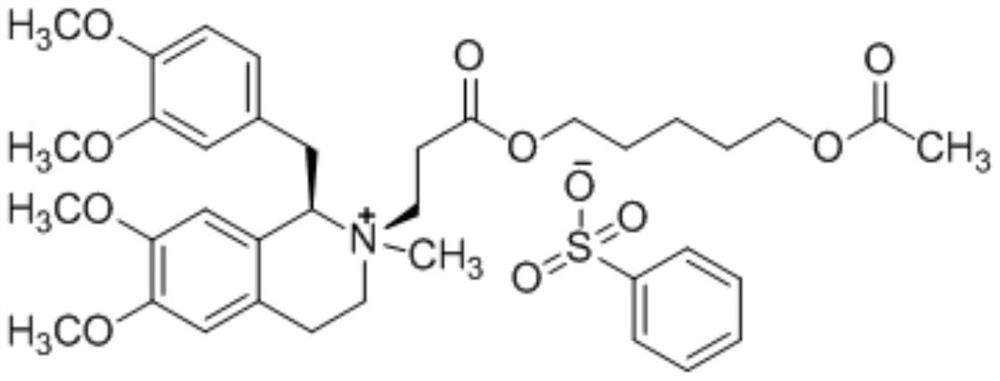
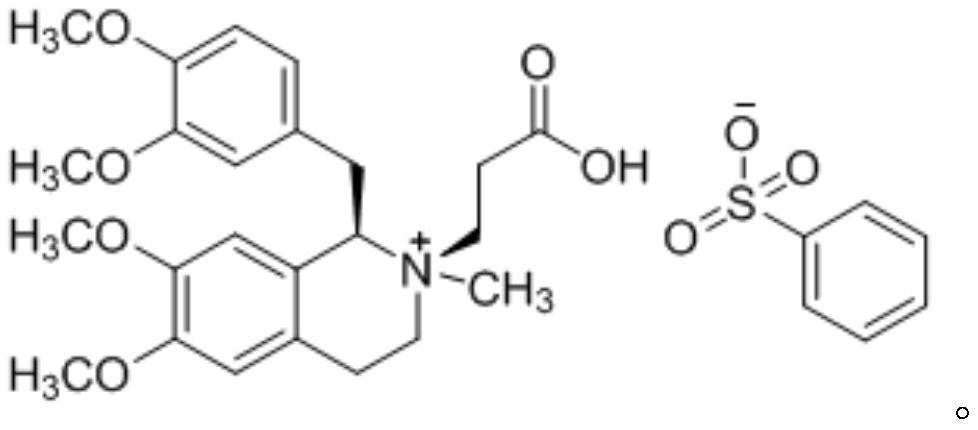
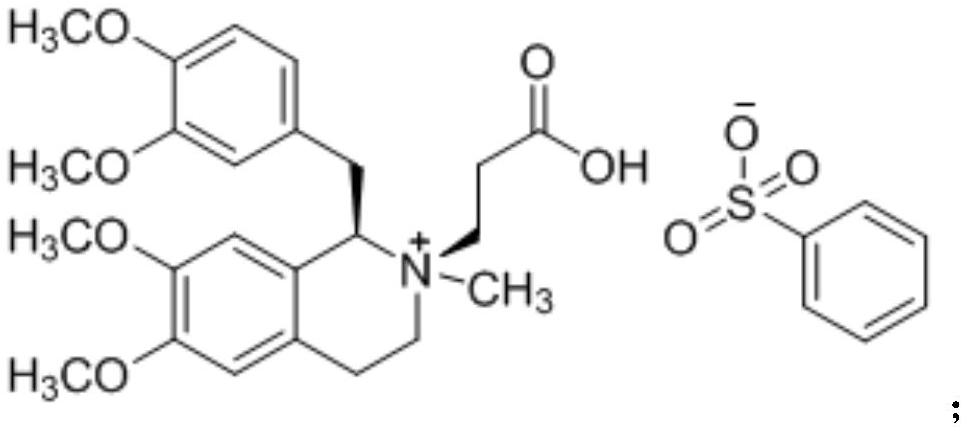
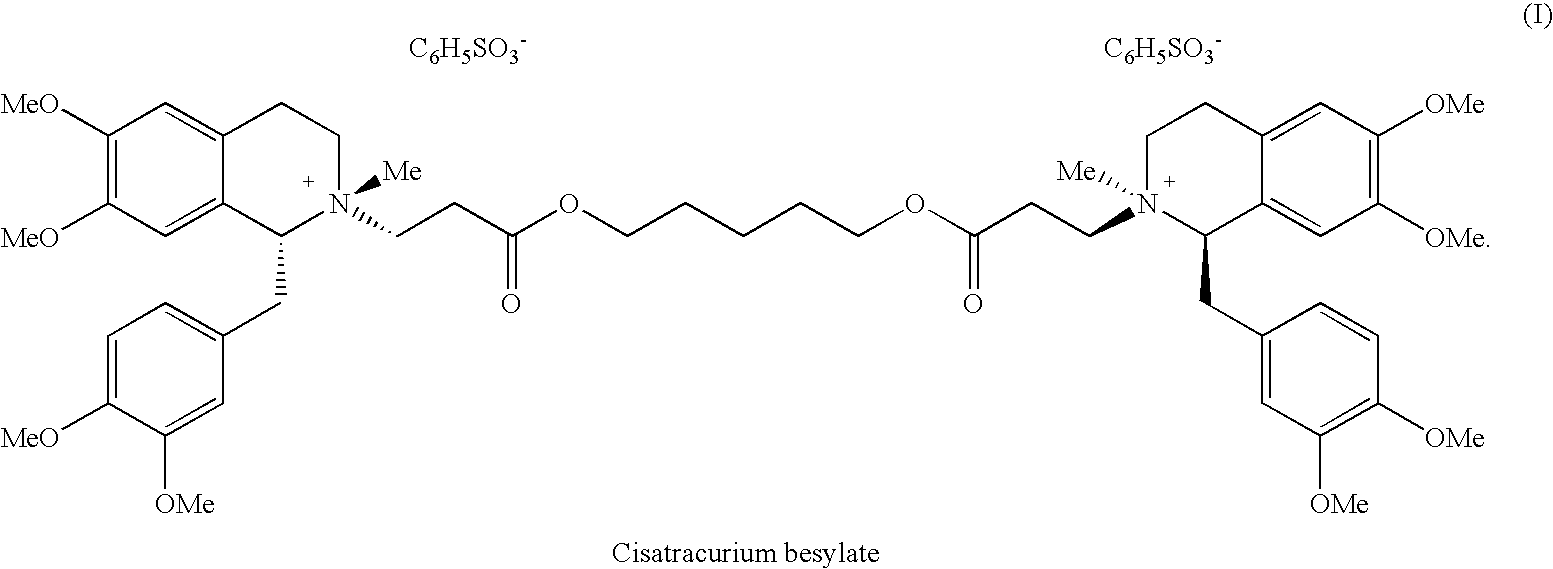
A non-depolarizing skeletal muscle relaxant of the benzylisoquinolinium class. Cisatracurium besylate acts as a competitive acetylcholine antagonist that binds to nicotinic receptors at the neuromuscular junction. Compared to other neuromuscular blocking agents, it is intermediate in its onset and duration of action. Cisatracurium besylate is used to maintain neuromuscular relaxation during major surgical procedures, primarily to facilitate endotracheal intubation. Cisatracurium besylate can cause bronchospasms, hypotension, and bradycardia.
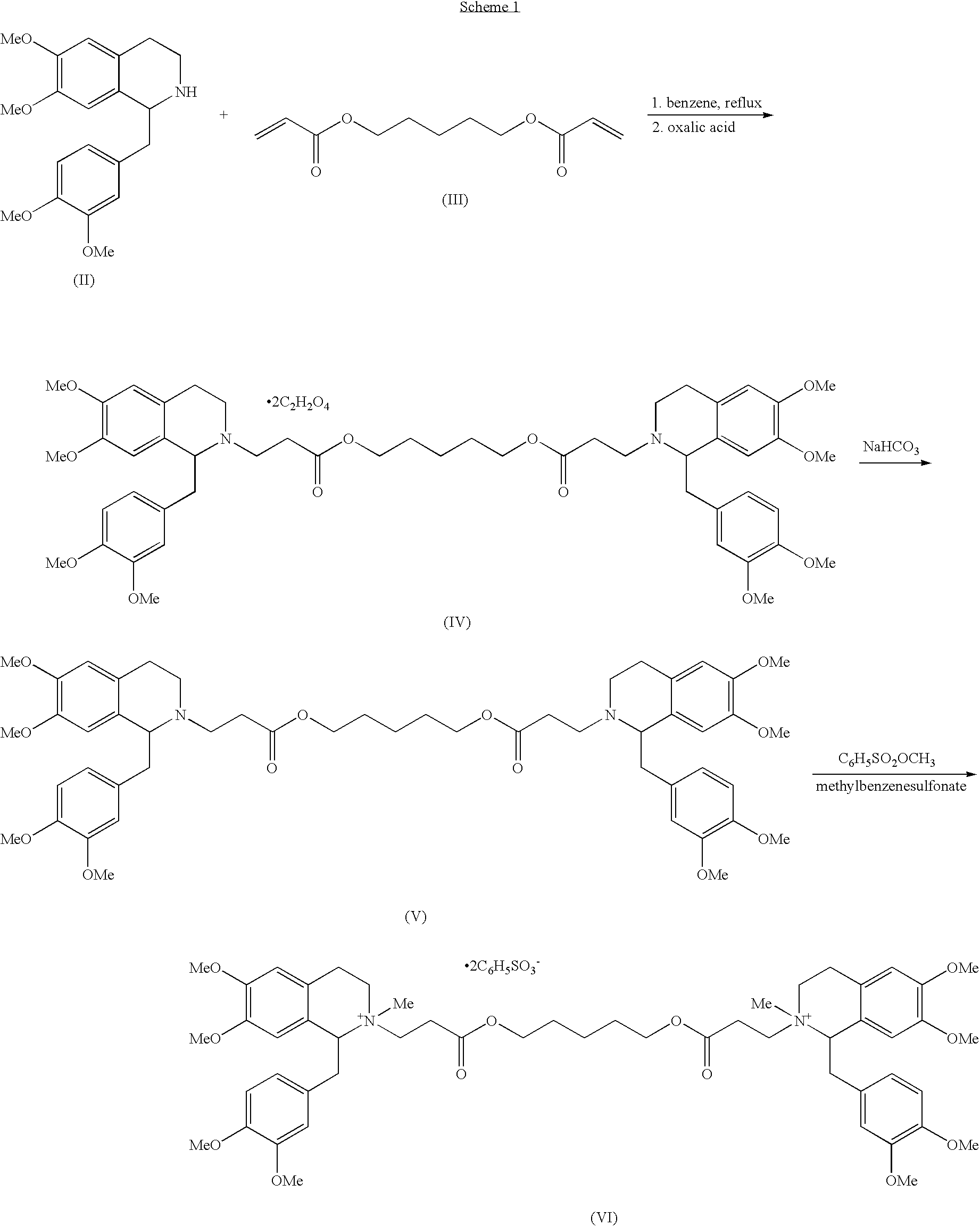
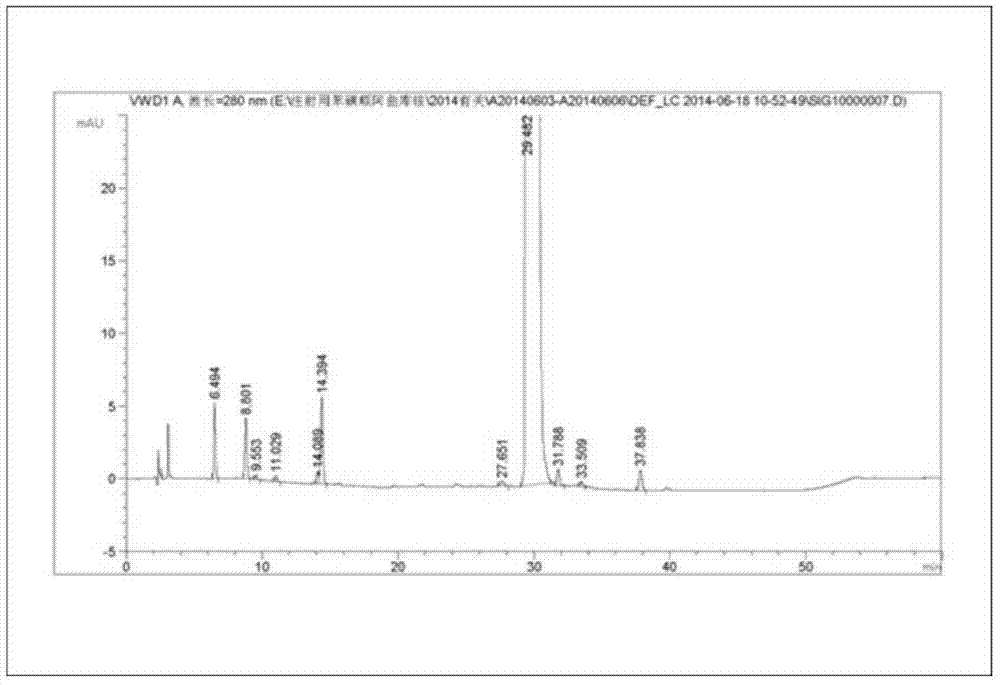
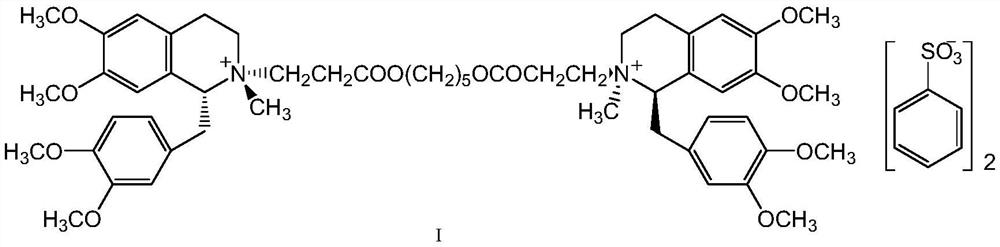
Method for separating and purifying cisatracurium besylate by preparative liquid chromatography
InactiveCN101475530AHigh purityHigh yieldIon-exchange process apparatusOrganic chemistryChromatographic separationSilica gel
The invention discloses a method for separating and purifying cis-benzene sulfonic acid atracurium through preparative liquid chromatography, which is to use a mobile phase to dissolve benzene sulfonic acid atracurium, load the benzene sulfonic acid atracurium on a preparative liquid-phase chromatographic silica gel column, perform normal-phase or opposite-phase liquid-phase chromatographic separation under the elution of the mobile phase, and reclaim the cis-benzene sulfonic acid atracuriums, wherein immobile-phase silica gel is modified bonded silica gel, and the particle diameter of the immobile-phase silica gel is between 1 and 200 micrometers; the load ratio of the immobile-phase silica gel to atracurium is not more than 1,000; and the pressure of the silica gel column is between 250 and 400 psi. The cis-benzene sulfonic acid atracurium obtained by the method has high purity, high yield and low cost; the amount of an organic solvent used is low; the pollution is light; and the maneuverability is strong. The treatment time of the method is shortened by approximately 10 times compared with the prior art, and simultaneously the method can feed samples continuously, realize automatic operation and the like.
Owner:SHANGHAI PHARMA DONGYING JIANGSU PHARMA CO LTD
Novel isoquinolinium compounds useful in the preparation of cisatracurium and associated intermediates
InactiveUS20100168431A1Improve purification effectOrganic chemistryCisatracurium BesylateIsoquinoline
The present invention provides novel isoquinolinium compounds, methods of producing the isoquinolinium compounds, and methods for converting them into cisatracurium, e.g., cisatracurium besylate. The isoquinolinium compounds of the present invention can be obtained in the form of solids, which can be purified using simple techniques and can be used to afford pure cisatracurium besylate without HPLC purification
Owner:WAVELENGTH ENTERPRISES LTD
Method for preparing cisatracurium besylate
ActiveCN102249998AQuality improvementHigh purityOrganic chemistryCisatracurium BesylateDiacrylate ester
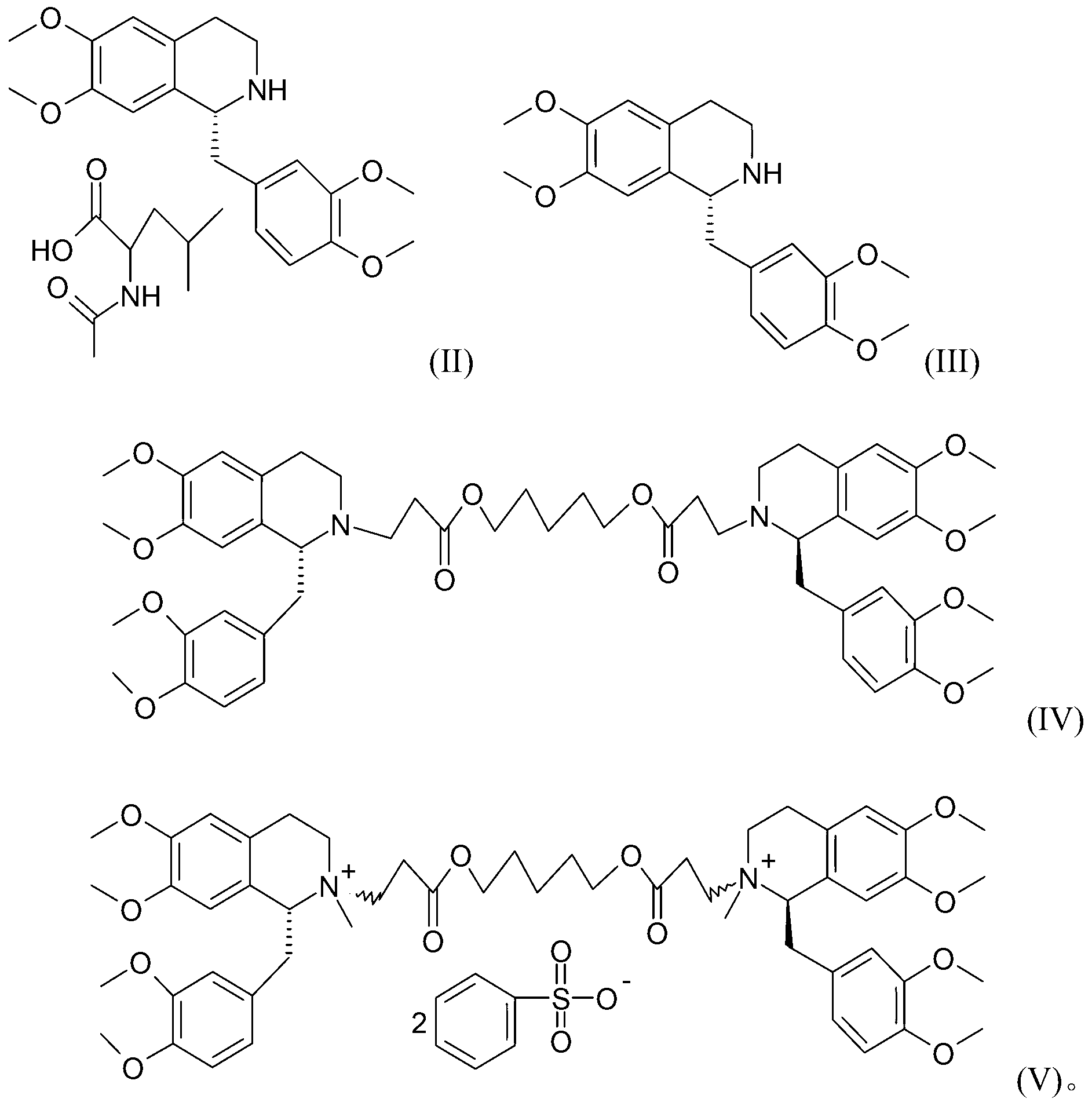
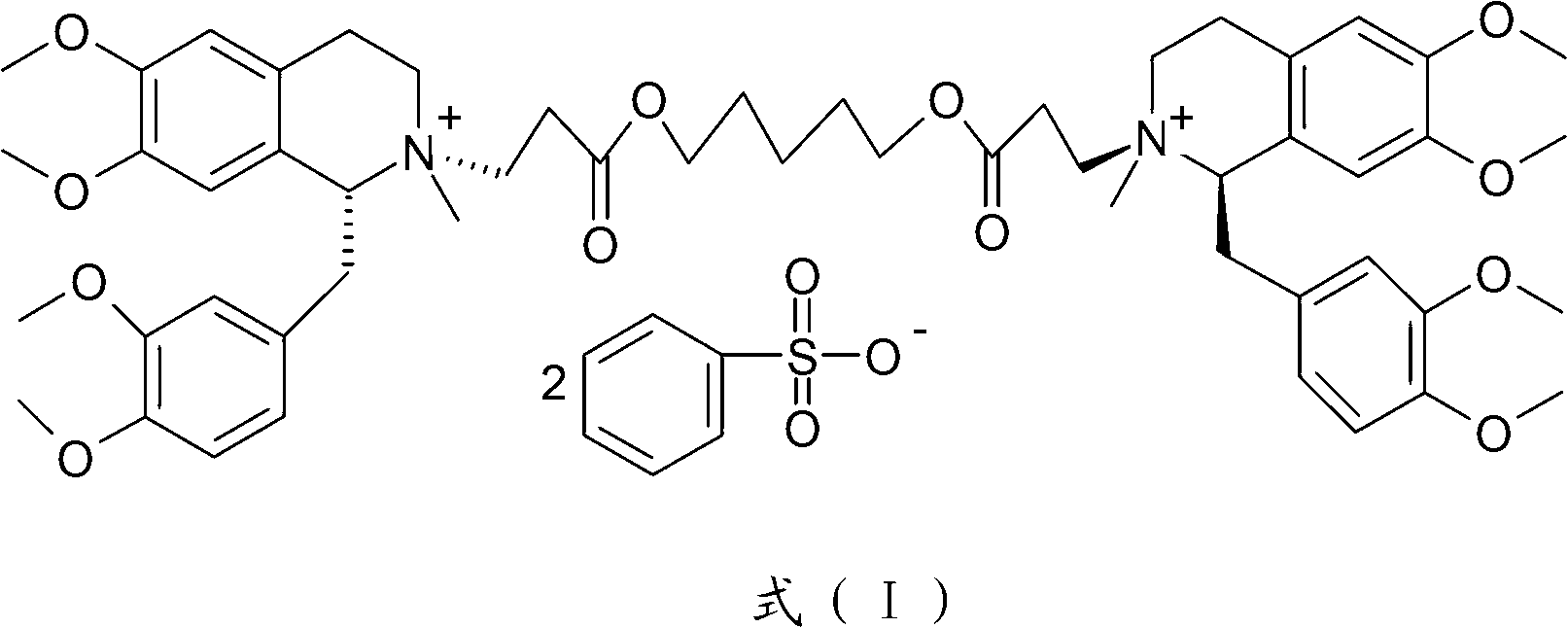
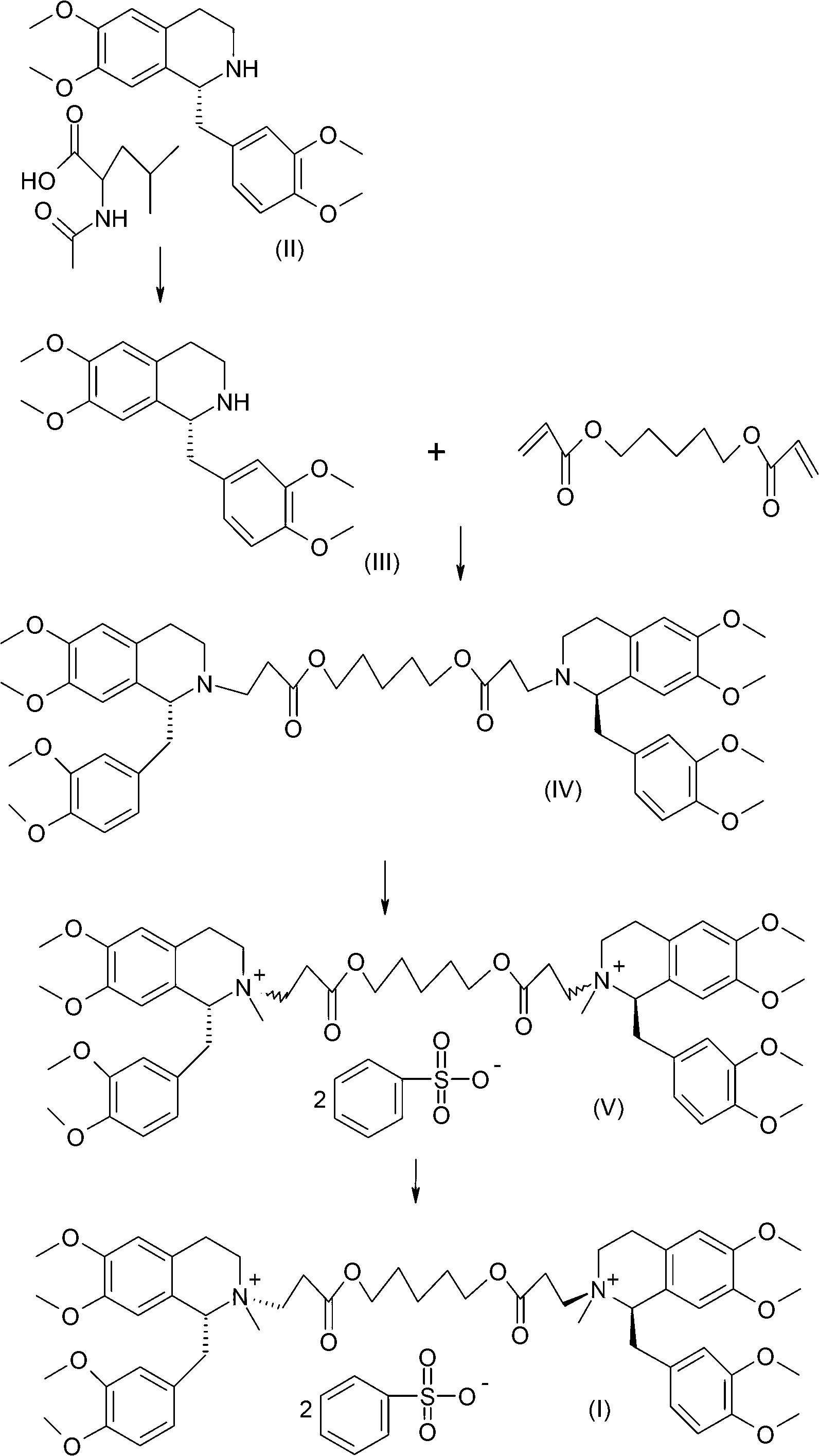
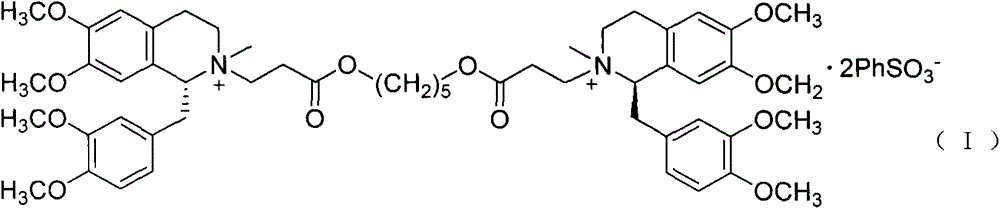
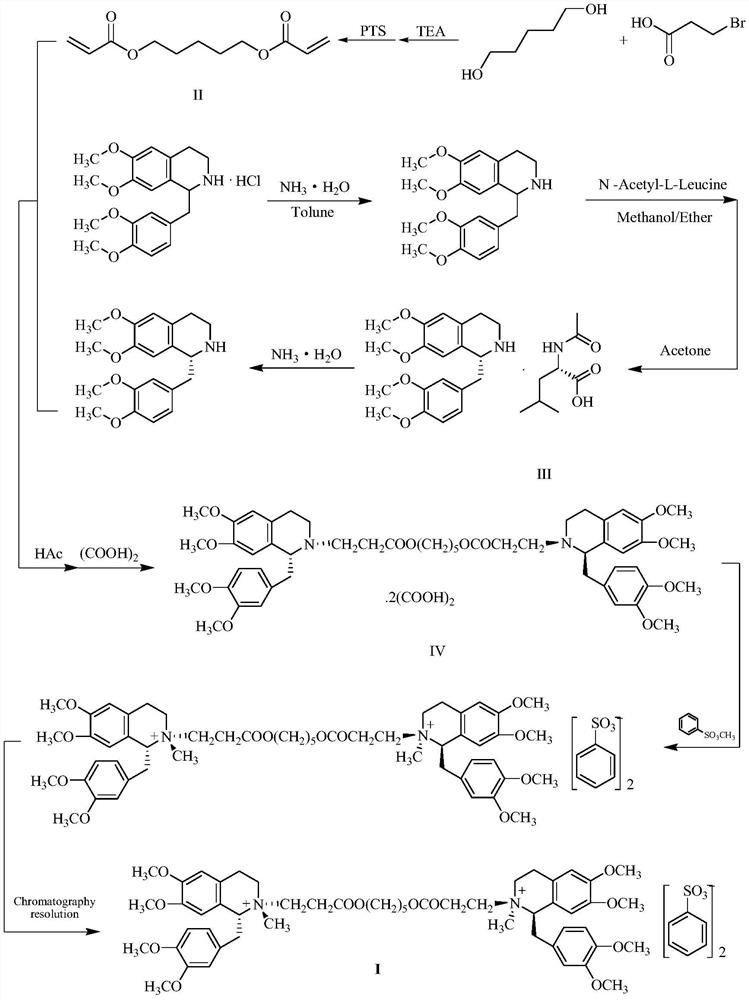
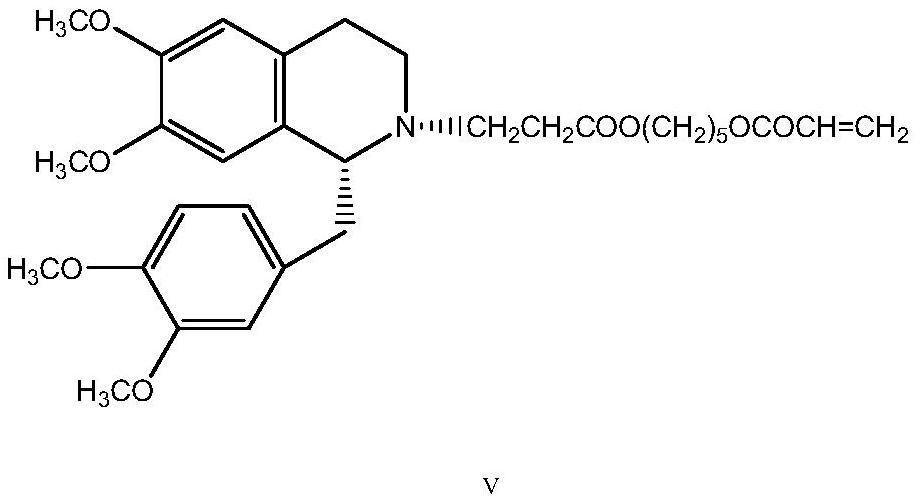
The invention discloses a preparation method of cisatracurium besylate. The method comprises the following steps: carrying out weal base dissociation on a R-tetrahydropapaverine-N-acetyl-L-leucine salt having a chiral purity of above 99.5% as a starting material so as to obtain R-tetrahydropapaverine; then carrying out a reaction on R-tetrahydropapaverine and 1,5-pentyl glycol diacrylate so as toobtain (1R,1'R)-2,2'-(3,11-dioxo-4,10-dioxa-1,13-subtridecyl) di[1,2,3,4-tetralin-6,7-dimethoxy-1-(3,4-dimethoxyl)benzyl]isoquinoline; after carrying out oxalate refinement and weal base dissociation, carrying out a reaction on (1R,1'R)-2,2'-(3,11-dioxo-4,10-dioxa-1,13-subtridecyl) di[1,2,3,4-tetralin-6,7-dimethoxy-1-(3,4-dimethoxyl)benzyl]isoquinoline and methyl benzenesulfonate so as to prepare1R,1'R-atracurium besilate; and finally, carrying out column chromatography separation on 1R,1'R-atracurium besilate so as to obtain cisatracurium besylate. By using the preparation method, the yieldof cisatracurium besylate can reach 30%, the purity of the product reaches above 98.6%, and single impurity content is below 0.3; and the process is brief, the yield is stable, and the product quality is good, thus the preparation method disclosed by the invention has an industrial application prospect.
Owner:ZHEJIANG XIANJU PHARMA
Novel r,r`-atracurium salts
The present invention provides R,R′-atracurium salts processes for producing and purifying such salts, and methods of using such salts to produce highly pure cisatracurium besylate.
Owner:WAVELENGTH ENTERPRISES LTD
Process for producing cisatracurium and associated intermediates
The present invention provides a process of producing cisatracurium compounds, e.g., cisatracurium besylate, from isoquinolinium salts of the structural formula (VIIA) wherein X− is an anion and R is H or a C1-C6 alkyl, or an activated form of the carboxylic acid with 1,5-pentanediol to form a cisatracurium salt, optionally via an intermediate compound (VIII). The cisatracurium compounds can be purified using simple techniques to afford pure cisatracurium besylate without the need for HPLC purification.
Owner:CHEMAGIS
Process for producing cisatracurium compounds and associated intermediates
The present invention provides processes for producing isoquinolinium compounds, and for converting them into cisatracurium salts, e.g., cisatracurium besylate
Owner:WAVELENGTH ENTERPRISES LTD
Cisatracurium besylate lyophilized preparation composition stable at room temperature and preparation method thereof
InactiveCN103070832AImprove stabilityEasy to storePowder deliveryOrganic active ingredientsBenzeneCisatracurium Besylate
The invention relates to a Cisatracurium besylate lyophilized preparation composition stable at room temperature and a preparation method thereof. The lyophilized preparation contains benzene sulfonic acid Cisatracurium besylate and a lyophilization excipient, a weight ratio of the lyophilization excipient to the benzene sulfonic acid Cisatracurium besylate (W / W) is 1:1-5:1. The lyophilized preparation overcomes the defect of cryopreservation of an existing lyophilized preparation, and is convenient for hospitals and patients for storage. The lyophilized preparation is used for muscle relaxation for operation patients.
Owner:CUREGEN JIANGSU PHARMA
Preparation method of high-purity cisatracurium besylate
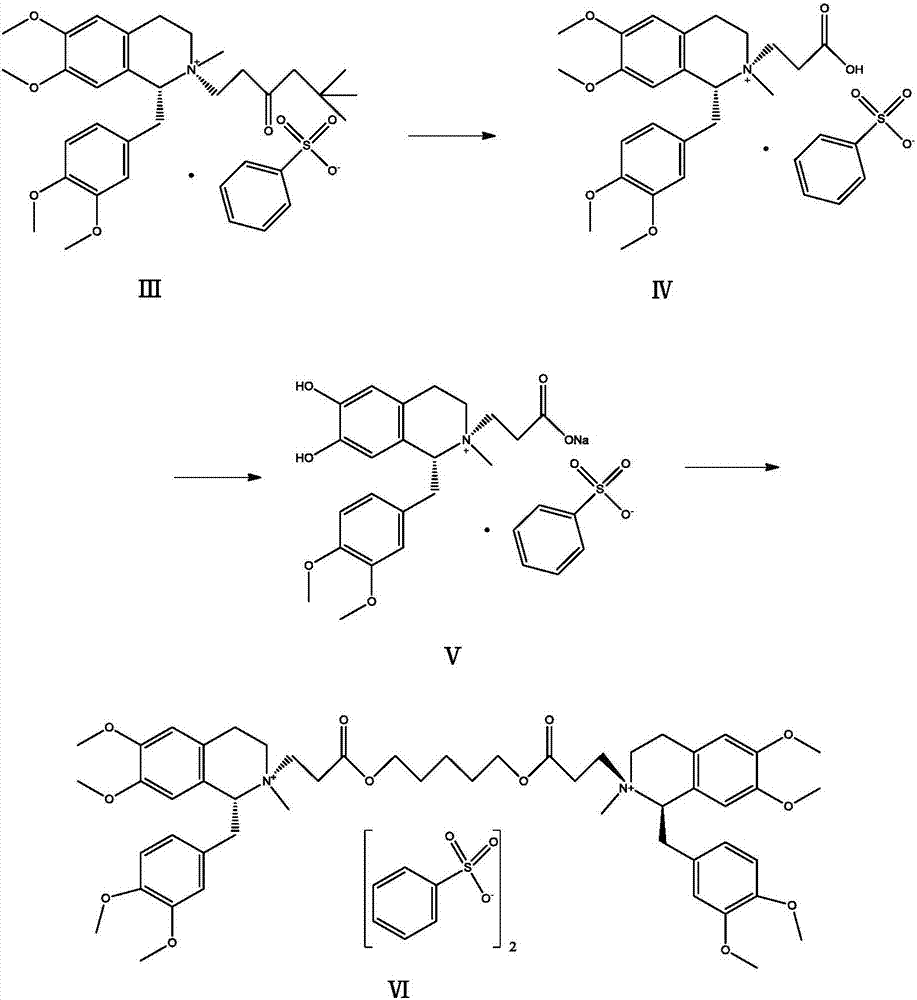
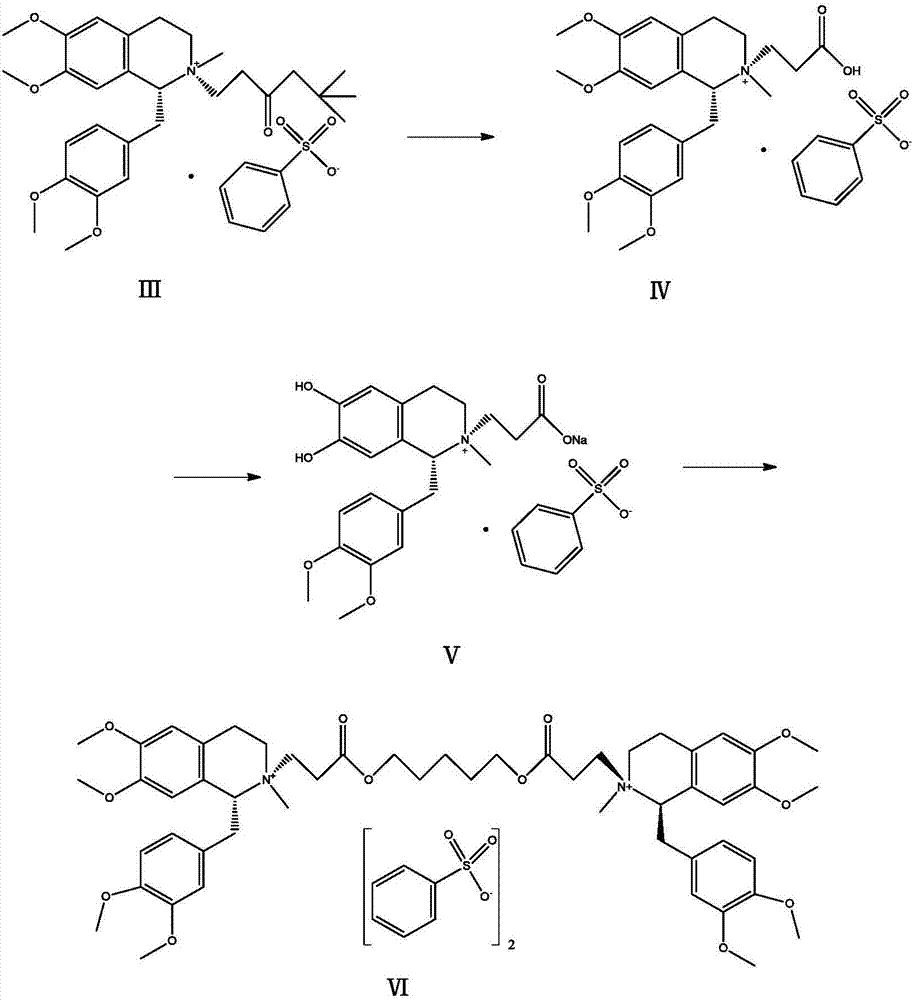
The invention relates to the drug synthesis field and particularly relates to a preparation method of high-purity cisatracurium besylate. The preparation method comprises the steps of reacting by virtue of a compound of a formula (III) and benzenesulfonic acid, carrying out substitution reaction on an obtained compound of a formula (IV) so as to generate a compound of a formula (V), and reacting by virtue of the compound of the formula (V) with 1,5-dichloropentane so as to generate cisatracurium besylate. An intermediate and sodium hydroxide or sodium hydrogen carbonate form sodium salt in a preparation process, namely that the intermediate is purified, so that reaction conditions of a laboratory and the production are easily met and controlled, the production efficiency is high, side reaction is little, the product purity is high, and sodium hydroxide or sodium hydrogen carbonate is easily available and relatively low in price.
Owner:LIANYUNGANG GUIKE PHARMA
Purification method for cisatracurium besylate
InactiveCN104892508ASimplify cumbersome operationsReduce consumptionOrganic chemistryCisatracurium BesylatePurification methods
The invention discloses an industrial purification and preparation method for cisatracurium besylate. According to the preparation method, the refining operation process is simplified, and firstly, under the circumstance of not changing a base, high-purity cisatracurium besylate is obtained in a non-column chromatography preparation mode. By adopting the preparation method disclosed by the invention, the production cycle is shortened greatly, productivity is improved, the yield reaches 40% or above, the content of cisatracurium besylate in a product is not less than 98%, the total content of impurities is less than or equal to 2%, the content of mono-quaternaries is less than or equal to 0.5%, the content of other single maximum impurity is less than or equal to 0.5%, and the content of all isomers is less than or equal to 0.1%.
Owner:TIANJIN ZHONGRUI PHARMA
Refinement method of cisatracurium besylate
ActiveCN104292161AReduce consumptionSimplify the refining processOrganic chemistryCisatracurium BesylateChromatographic column
The invention relates to an industrial preparation method of cisatracurium besylate. The preparation method simplifies the refinement operation process, and obtains the high-purity cisatracurium besylate in a non-chromatographic column chromatography preparation mode under the condition of not changing the salt base for the first time. The preparation method can greatly shorten the production cycle, enhance the productivity and increase the yield to 45% above. In the product, the content of cisatracurium besylate is not lower than 98%, the content of total impurities does not exceed 2%, the content of mono quaternary ammonium salts does not exceed 0.2%, the content of other maximum individual impurities does not exceed 0.3%, and the content of isomers does not exceed 0.1%.
Owner:大道隆达(北京)医药科技发展有限公司
Method for preparing cisatracurium besylate
The invention discloses a preparation method of cisatracurium besylate. The method comprises the following steps: carrying out weal base dissociation on a R-tetrahydropapaverine-N-acetyl-L-leucine salt having a chiral purity of above 99.5% as a starting material so as to obtain R-tetrahydropapaverine; then carrying out a reaction on R-tetrahydropapaverine and 1,5-pentyl glycol diacrylate so as toobtain (1R,1'R)-2,2'-(3,11-dioxo-4,10-dioxa-1,13-subtridecyl) di[1,2,3,4-tetralin-6,7-dimethoxy-1-(3,4-dimethoxyl)benzyl]isoquinoline; after carrying out oxalate refinement and weal base dissociation, carrying out a reaction on (1R,1'R)-2,2'-(3,11-dioxo-4,10-dioxa-1,13-subtridecyl) di[1,2,3,4-tetralin-6,7-dimethoxy-1-(3,4-dimethoxyl)benzyl]isoquinoline and methyl benzenesulfonate so as to prepare1R,1'R-atracurium besilate; and finally, carrying out column chromatography separation on 1R,1'R-atracurium besilate so as to obtain cisatracurium besylate. By using the preparation method, the yieldof cisatracurium besylate can reach 30%, the purity of the product reaches above 98.6%, and single impurity content is below 0.3; and the process is brief, the yield is stable, and the product quality is good, thus the preparation method disclosed by the invention has an industrial application prospect.
Owner:ZHEJIANG XIANJU PHARMA
A kind of refining method of atracurium besylate
ActiveCN104292161BReduce cleaning pressureQuality assuranceOrganic chemistryCisatracurium BesylateChromatographic column
The invention relates to an industrial preparation method of cisatracurium besylate. The preparation method simplifies the refinement operation process, and obtains the high-purity cisatracurium besylate in a non-chromatographic column chromatography preparation mode under the condition of not changing the salt base for the first time. The preparation method can greatly shorten the production cycle, enhance the productivity and increase the yield to 45% above. In the product, the content of cisatracurium besylate is not lower than 98%, the content of total impurities does not exceed 2%, the content of mono quaternary ammonium salts does not exceed 0.2%, the content of other maximum individual impurities does not exceed 0.3%, and the content of isomers does not exceed 0.1%.
Owner:大道隆达(北京)医药科技发展有限公司
Production process of injection cisatracurium besylate for storage and transportation at 25 DEG C
ActiveCN104983694AGood chemical stabilityReduce storage and transportation conditionsOrganic active ingredientsPowder deliveryCisatracurium BesylateFreeze-drying
The invention relates to injection cisatracurium besylate for storage and transportation at 25 DEG C and a preparation and freeze-drying production process of the injection cisatracurium besylate. The specific formula of the injection cisatracurium besylate includes 10 mg to 20 mg of cisatracurium besilate, 20 mg to 50 mg of dextran, 50 mg of mannitol and 2000 ml to 3000 ml of water for injection, wherein the PH of a solution containing 20% benzenesulfonic acid is properly adjusted. The freeze-drying production process includes pre-freezing, sublimation drying, re-drying, tamponade and cover rolling. By the adoption of the freeze-drying production process, the chemical stability of the medicine is improved, the cisatracurium besylate can be stored, transported and used at the temperature below 25 DEG C, product quality is guaranteed, storage and transportation conditions of a finished product are reduced, product quality is improved, and the cisatracurium besylate has the important significance in the medical application field.
Owner:SHANGHAI PHARMA DONGYING JIANGSU PHARMA CO LTD
Process for producing cisatracurium compounds and associated intermediates
Owner:WAVELENGTH ENTERPRISES LTD
Preparation method of impurity W in cisatracurium besylate
ActiveCN110256344AHigh purityEasy to prepareSulfonic acids salts preparationAcetic acidCisatracurium Besylate
The invention discloses a preparation method of an impurity W in cisatracurium besylate, and belongs to the technical field of synthesis of chemical substances. The preparation method includes the following steps: A, taking and reacting 1,5-pentanediol, glacial acetic acid and a catalyst to obtain a reaction solution; B, adding water and n-hexane to the reaction solution, performing stirring and liquid separation, and collecting the obtained aqueous layer solution; C, washing the aqueous layer solution with n-hexane, and collecting the washed aqueous layer solution; D, extracting the aqueous layer solution with dichloromethane, and collecting the dichloromethane layer solution; E, adding a drying agent to the dichloromethane layer solution, and filtering and concentrating the obtained solution to obtain 1,5-pentanediol monoacetate; and F, reacting the 1,5-pentanediol monoacetate with the impurity A in cisatracurium besylate, adding a solvent, stirring and filtering the obtained solution to obtain impurity W crystals, and drying the impurity W crystals to obtain the final product impurity W. The impurity of the prepared cisatracurium besylate is high, the preparation method is simple, and the prepared impurity W can be directly used in the quantitative detection process of the impurity W in the cisatracurium besylate as a standard.
Owner:江苏盈科生物制药有限公司
Cisatracurium besylate injection and preparation method thereof
InactiveCN112294756AImprove complianceImprove securityOrganic active ingredientsNervous disorderCisatracurium BesylateAlcohol ethyl
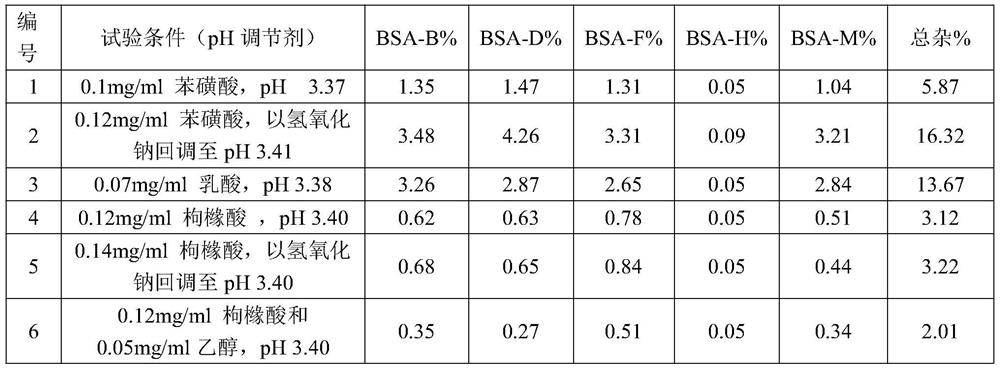
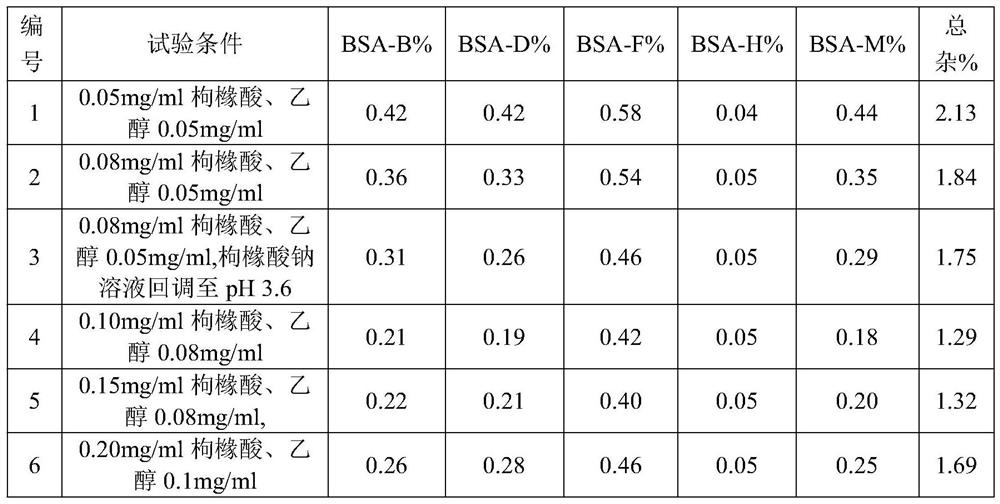
The invention discloses cisatracurium besylate injection and a preparation method thereof. The cisatracurium besylate injection can be subjected to constant-temptation fluid preparation and storage, also can be applied in a clinical compatibility way, is high in safety, is high in medicine compliance and provides a new choice for clinical application. The injection consists of cisatracurium besylate, citric acid and ethyl alcohol, is safe and stable, and has the advantages of a moderate fluid preparation technology and storage condition and good clinical compliance.
Owner:南京斯泰尔医药科技有限公司
Cisatracurium besylate composition for injection
ActiveCN107638391AImprove stabilityGood effectOrganic active ingredientsPowder deliveryCisatracurium BesylateVitamin C
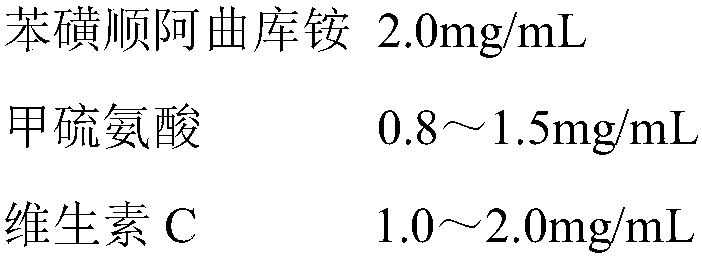
The present invention belongs to the technical field of medicine, and particularly relates to a cisatracurium besylate composition for injection, wherein the cisatracurium besylate composition comprises 2.0 mg / mL of cisatracurium besylate, 0.8-1.5 mg / mL of methionine, 1.0-2.0 mg / mL of vitamin C, an appropriate amount of a pH value adjusting agent, and 1000 mL of water for injection. According to the present invention, vitamin C provides the anti-oxidation effect, and methionine can increase the glutathione content in vivo so as to activate acetylcholinesterase, reduce the concentration of acetylcholine, and promote the combination between cisatracurium besylate and cholinergic receptors, such that the prepared pharmaceutical composition has advantages of good stability and excellent efficacy.
Owner:亿药通(河北)生物科技有限公司
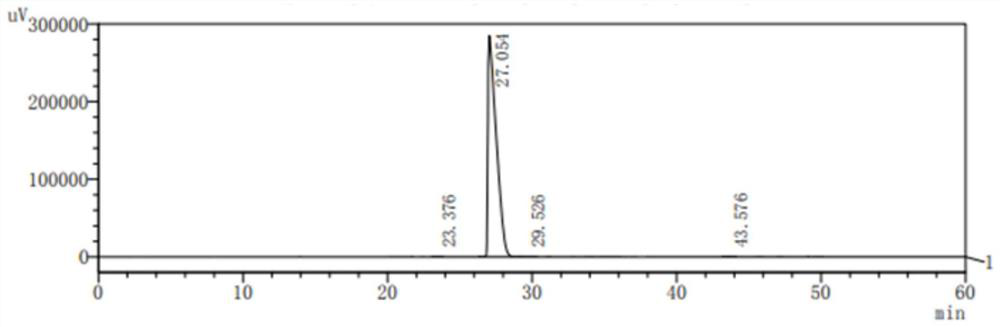
Method for determining content of cisatracurium besylate of raw materials by HPLC (High Performance Liquid Chromatography)
InactiveCN105277647AReduce consumptionExtend your lifeComponent separationCisatracurium BesylateIsocratic elution
The invention discloses a method for determining content of cisatracurium besylate of raw materials by an HPLC (High Performance Liquid Chromatography). New chromatographic conditions for determining the content of the cisatracurium besylate by the HPLC are established; an isocratic elution manner is adopted so that the preparation of a mobile phase is changed to one portion from two portions and the method is relatively convenient and saves time; and meanwhile, the isocratic elution is good for prolonging the service life of an instrument and a chromatographic column. According to the method, time consumed by single-needle sample feeding is 25 minutes and the checking efficiency is improved; and the consumption of the mobile phase is reduced so that the checking cost is reduced.
Owner:SHANGHAI PHARMA DONGYING JIANGSU PHARMA CO LTD
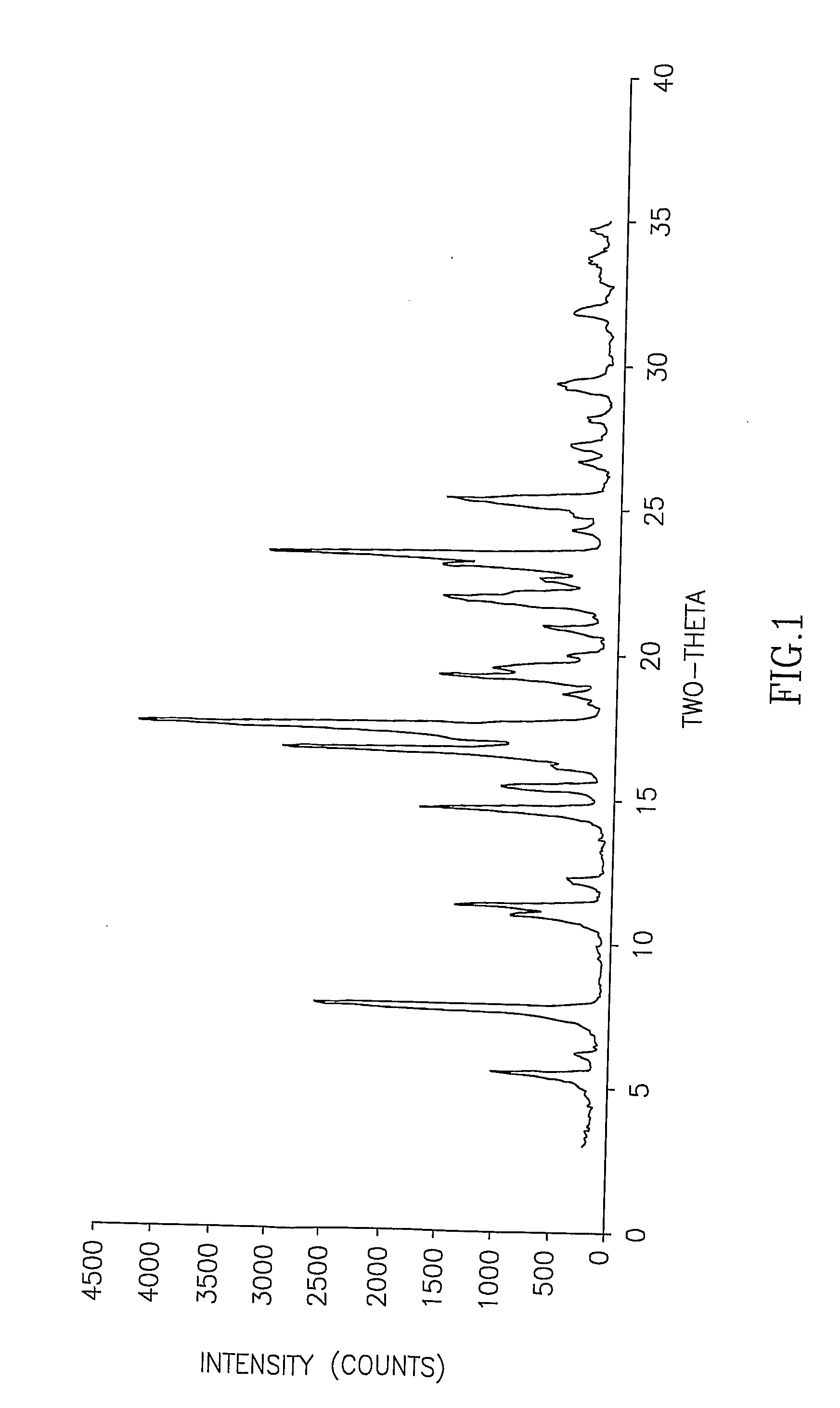
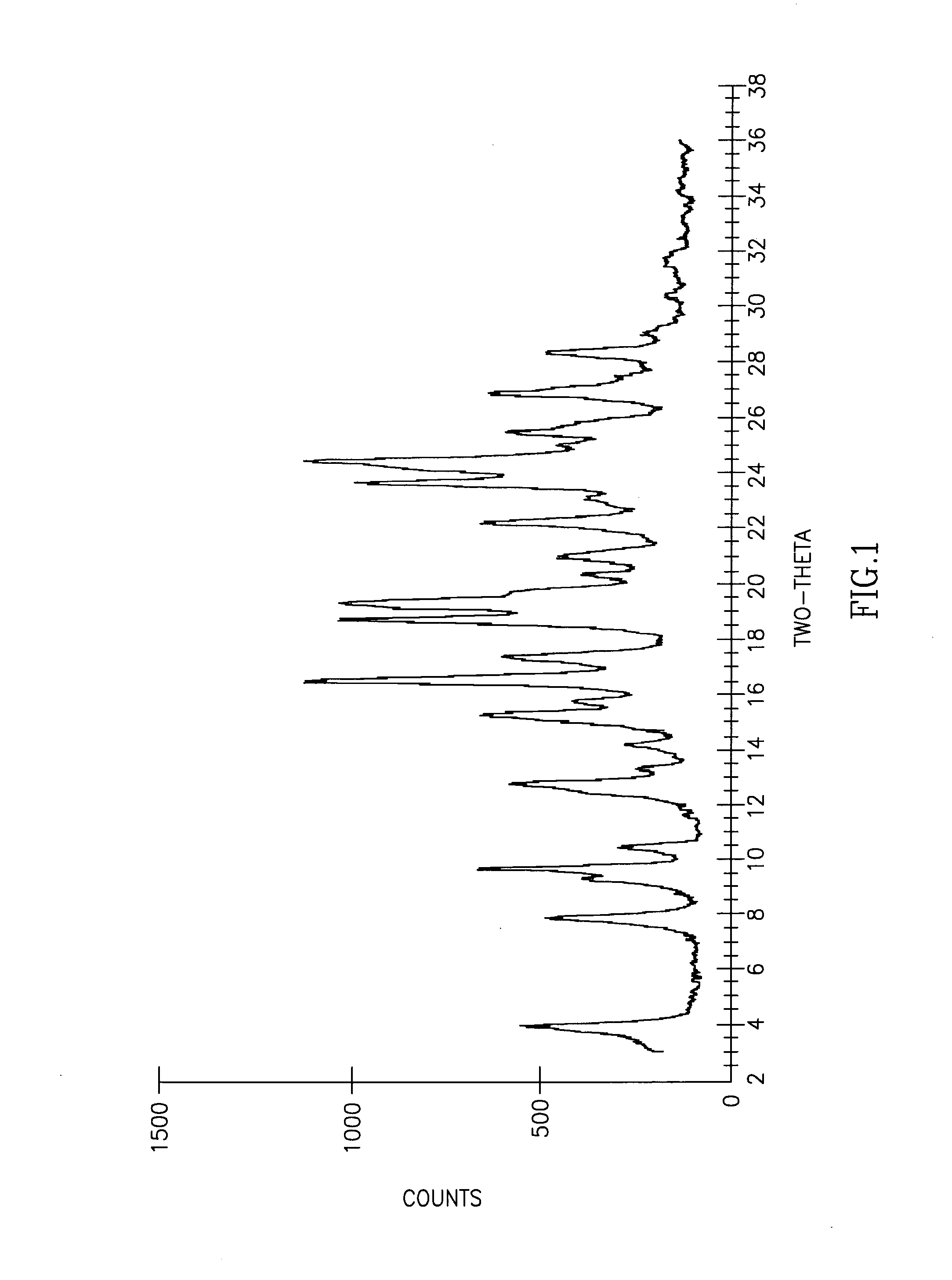
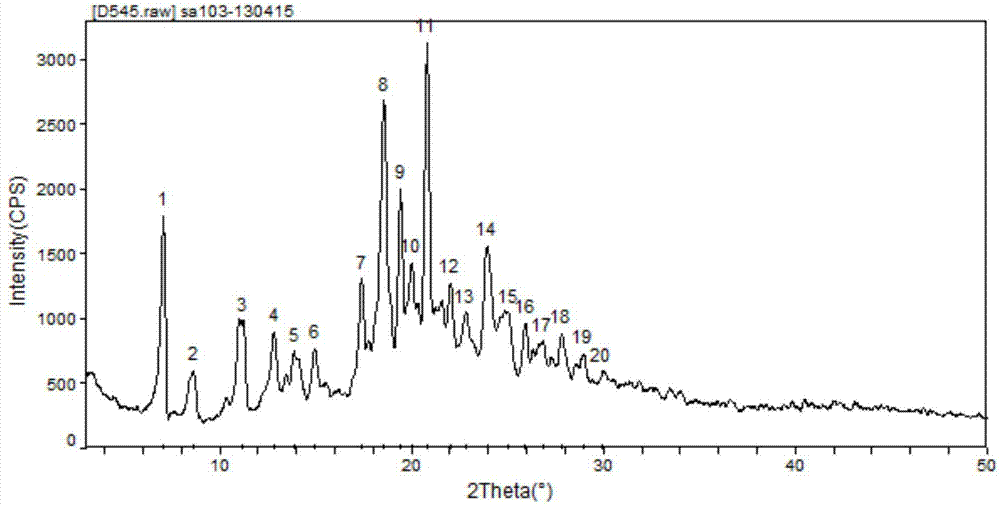
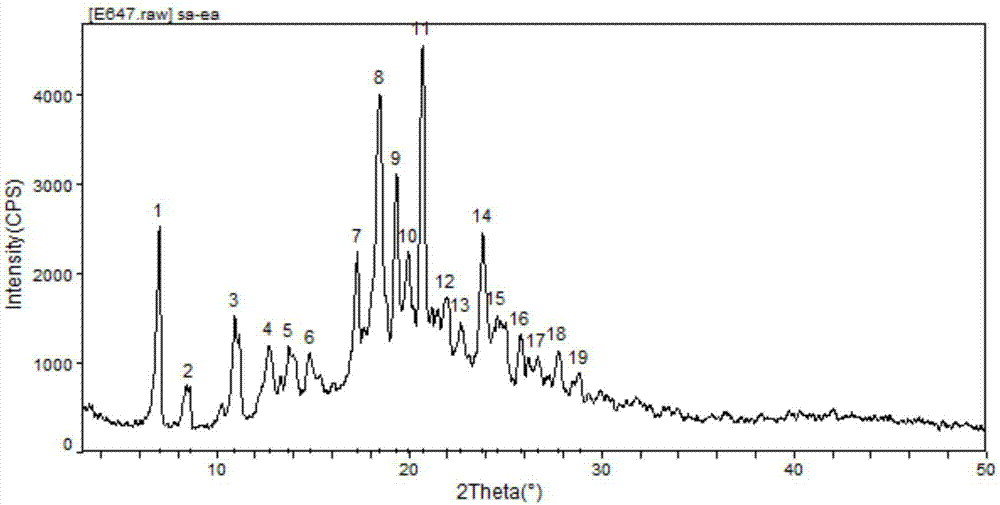
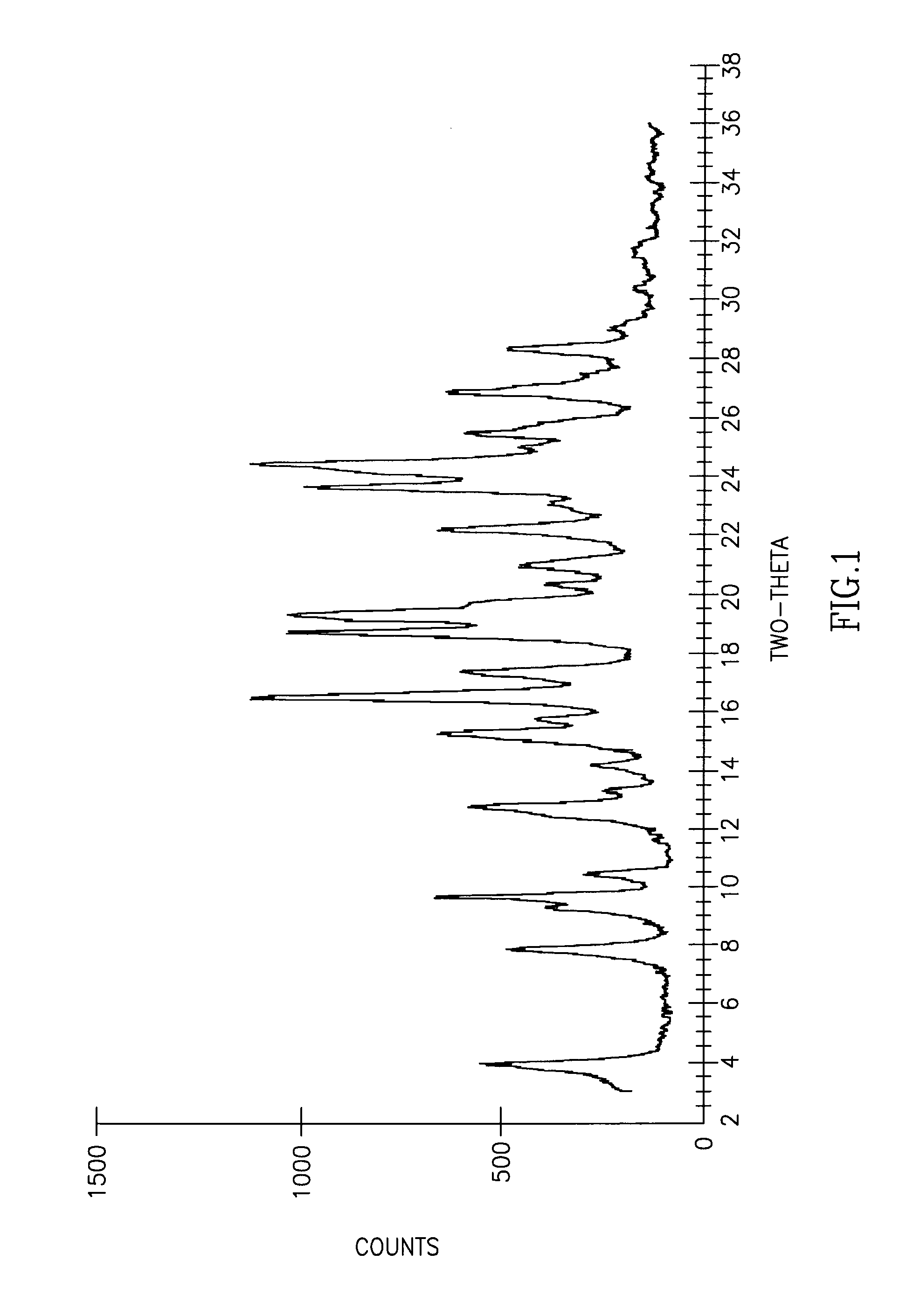
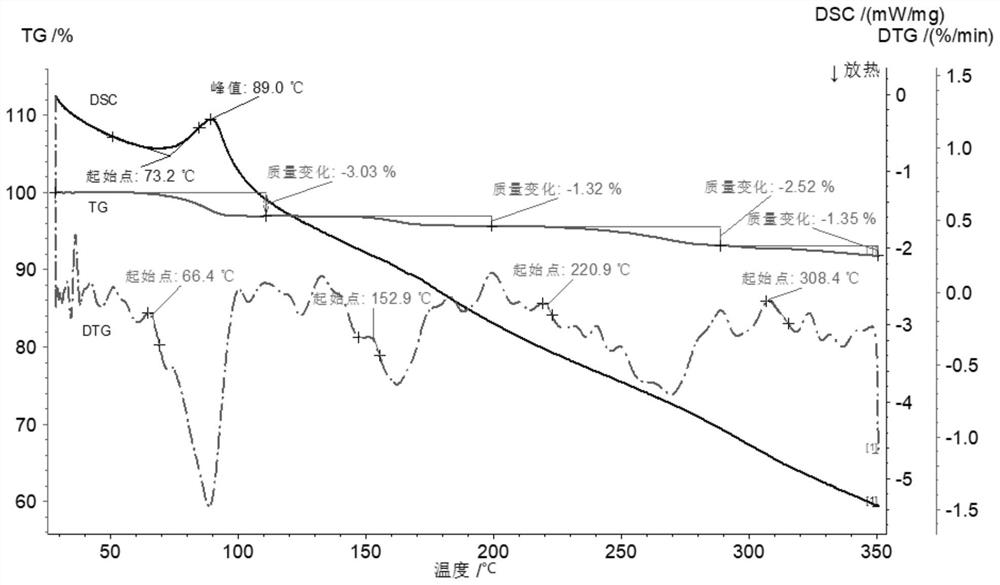
Cisatracurium besylate with new crystal form and purifying method thereof
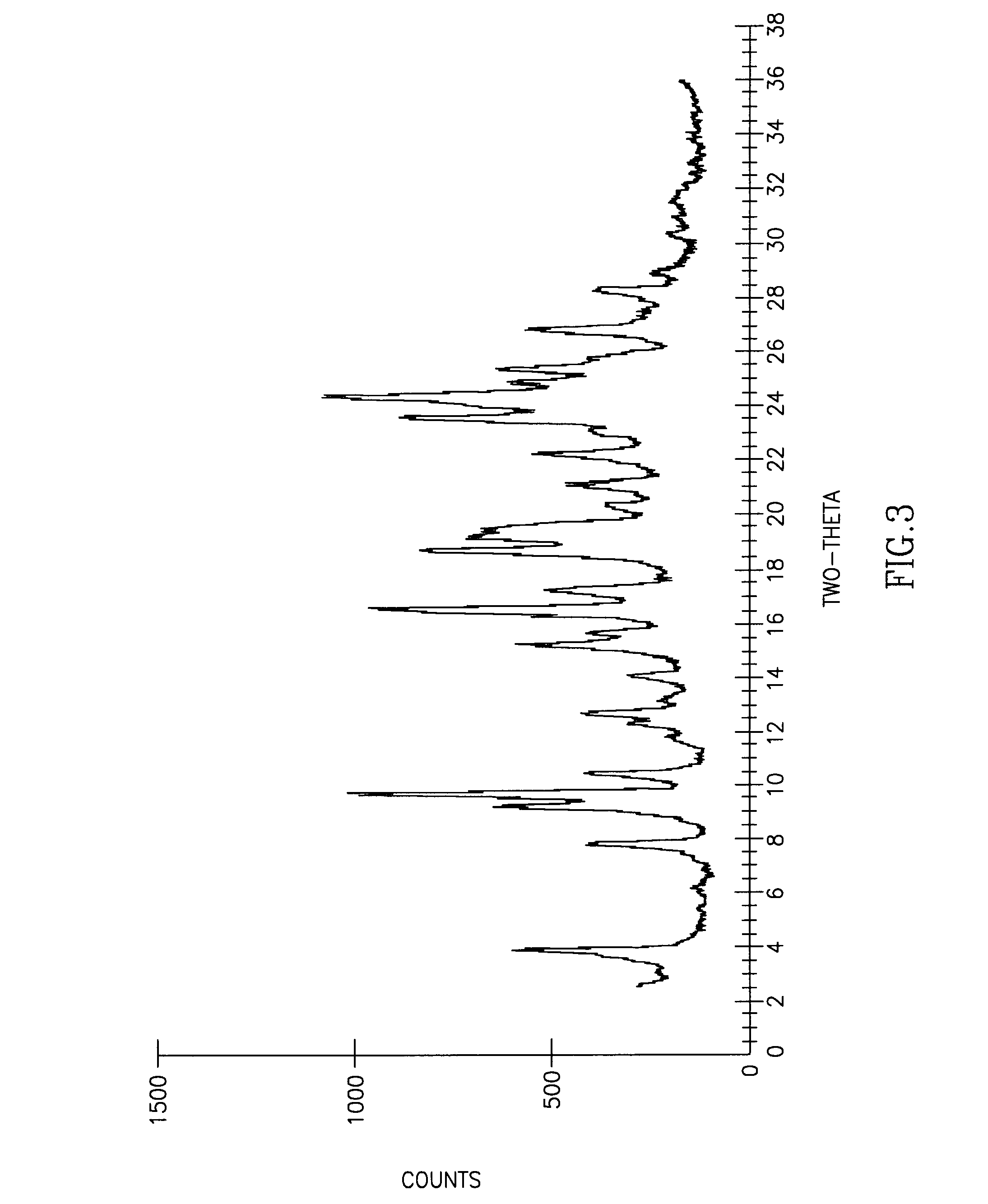
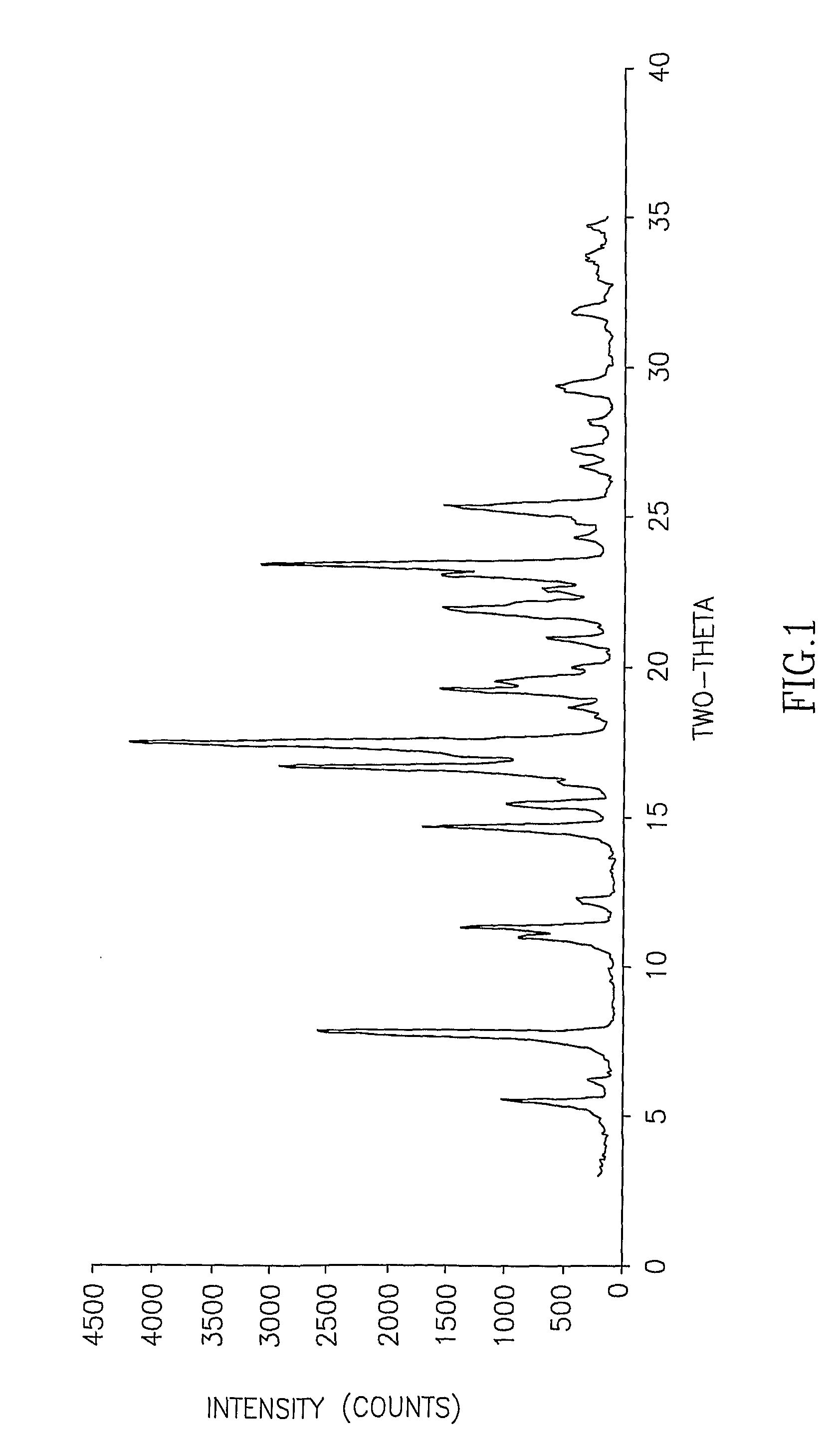
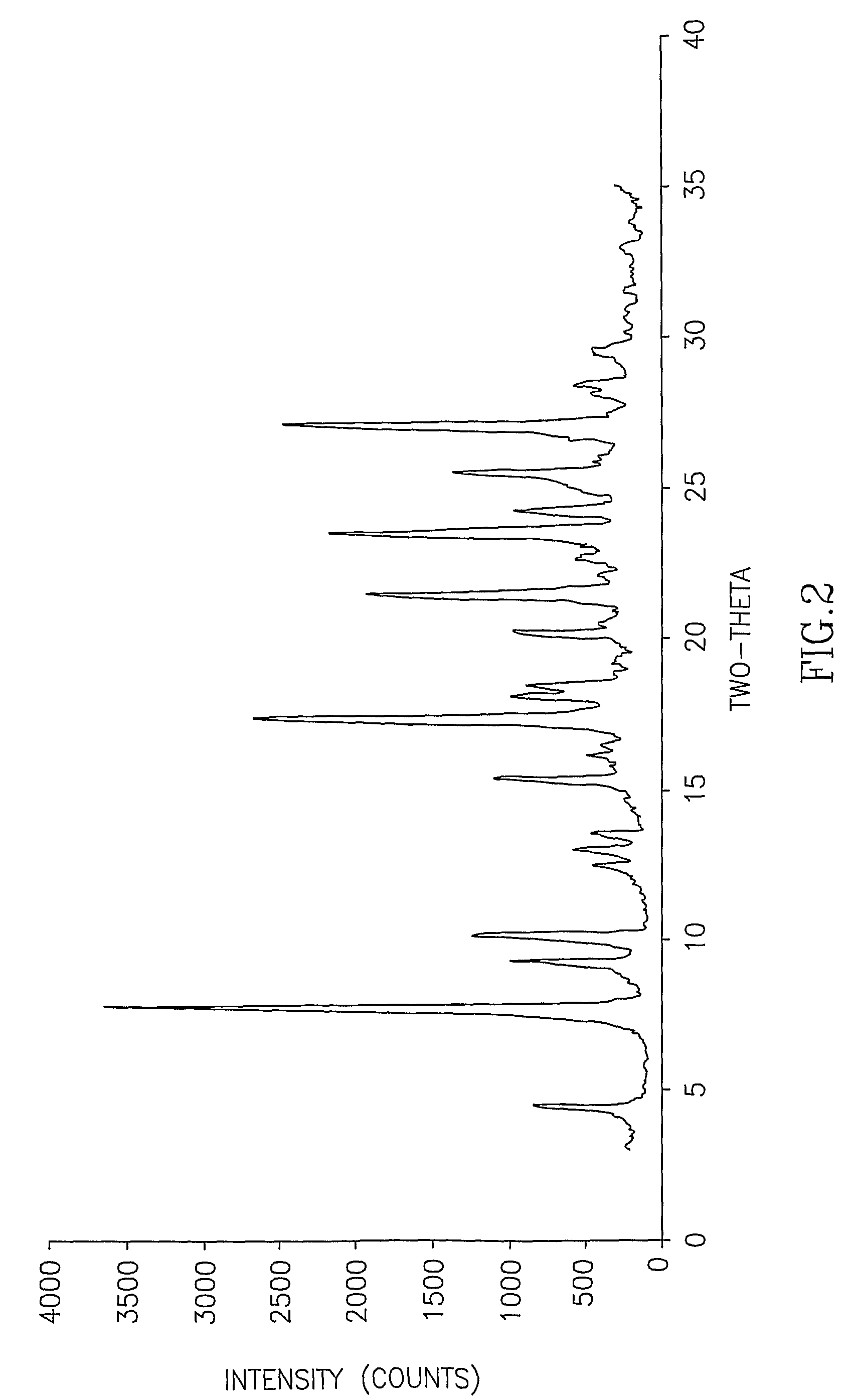
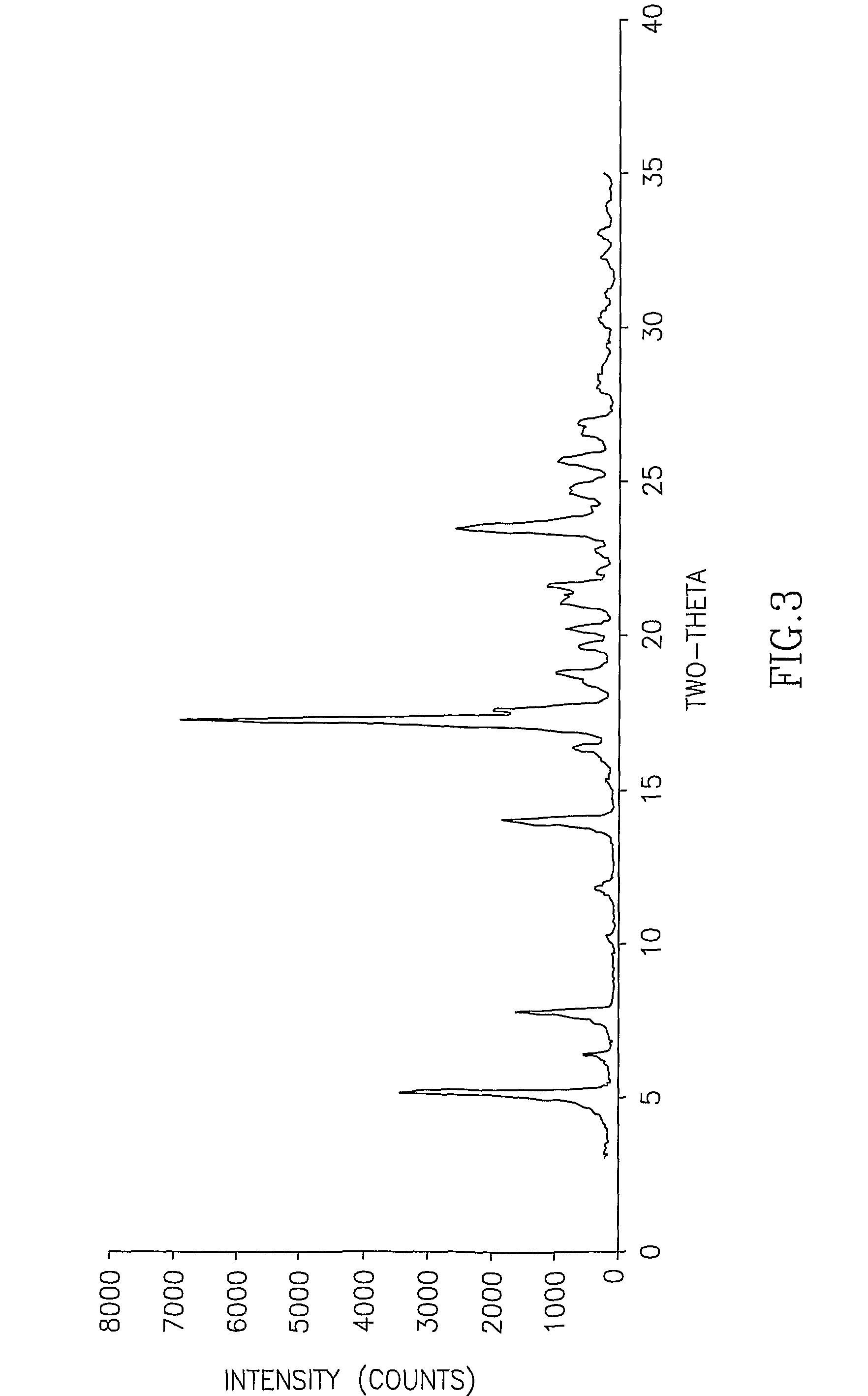
InactiveCN107353248AConvenient storage and transportationImprove stabilityOrganic chemistry methodsCisatracurium BesylateWhite powder
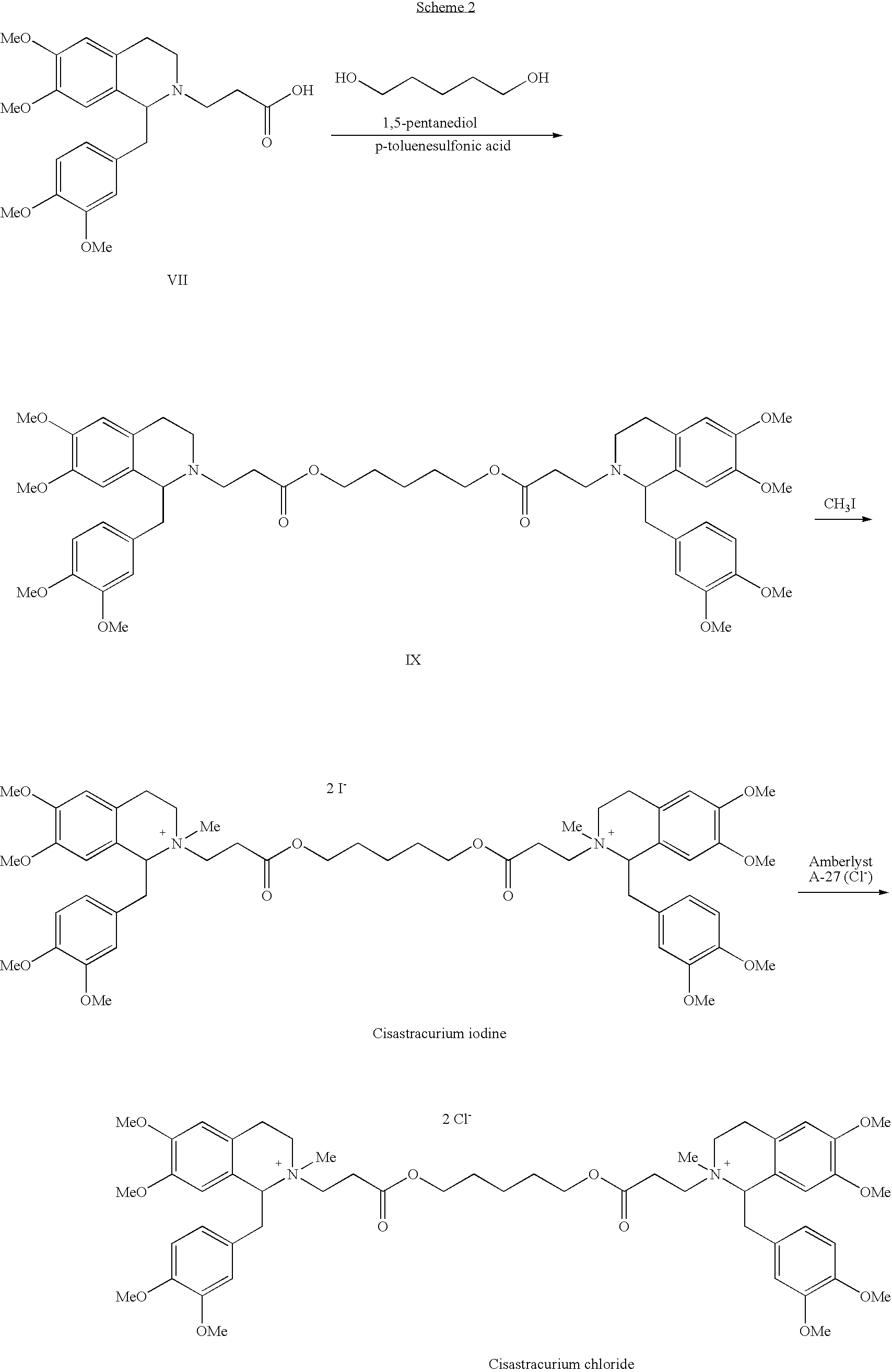
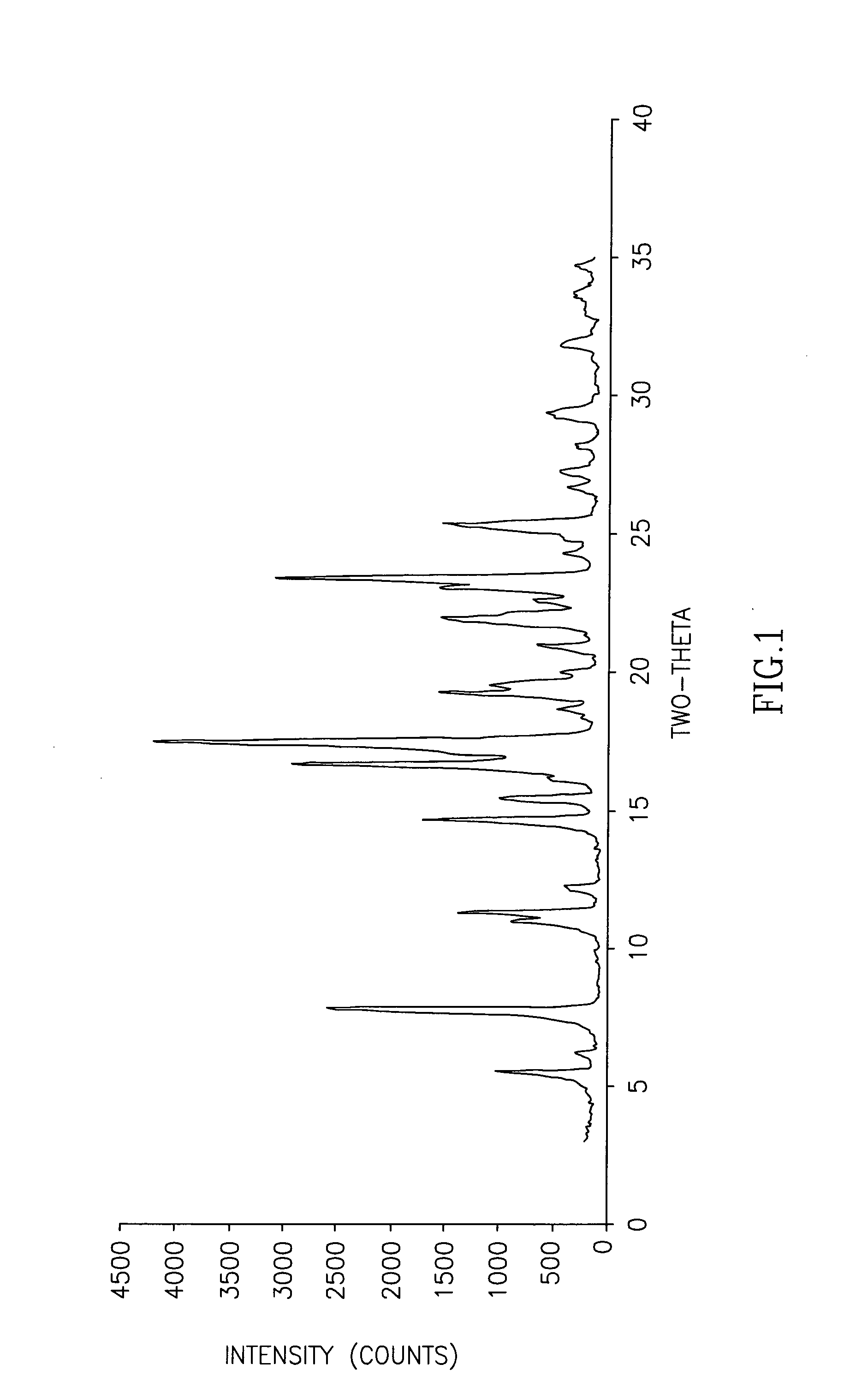
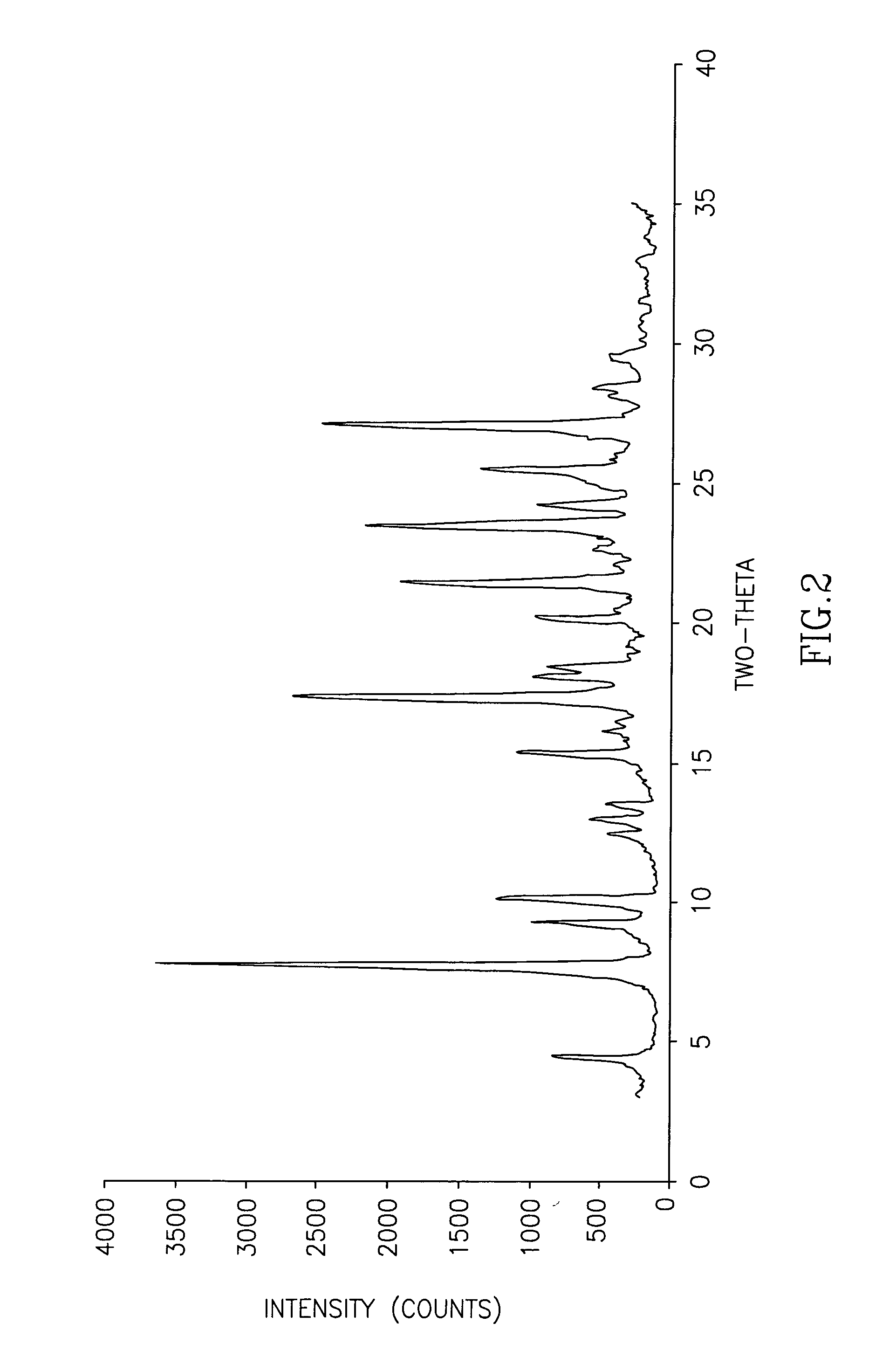
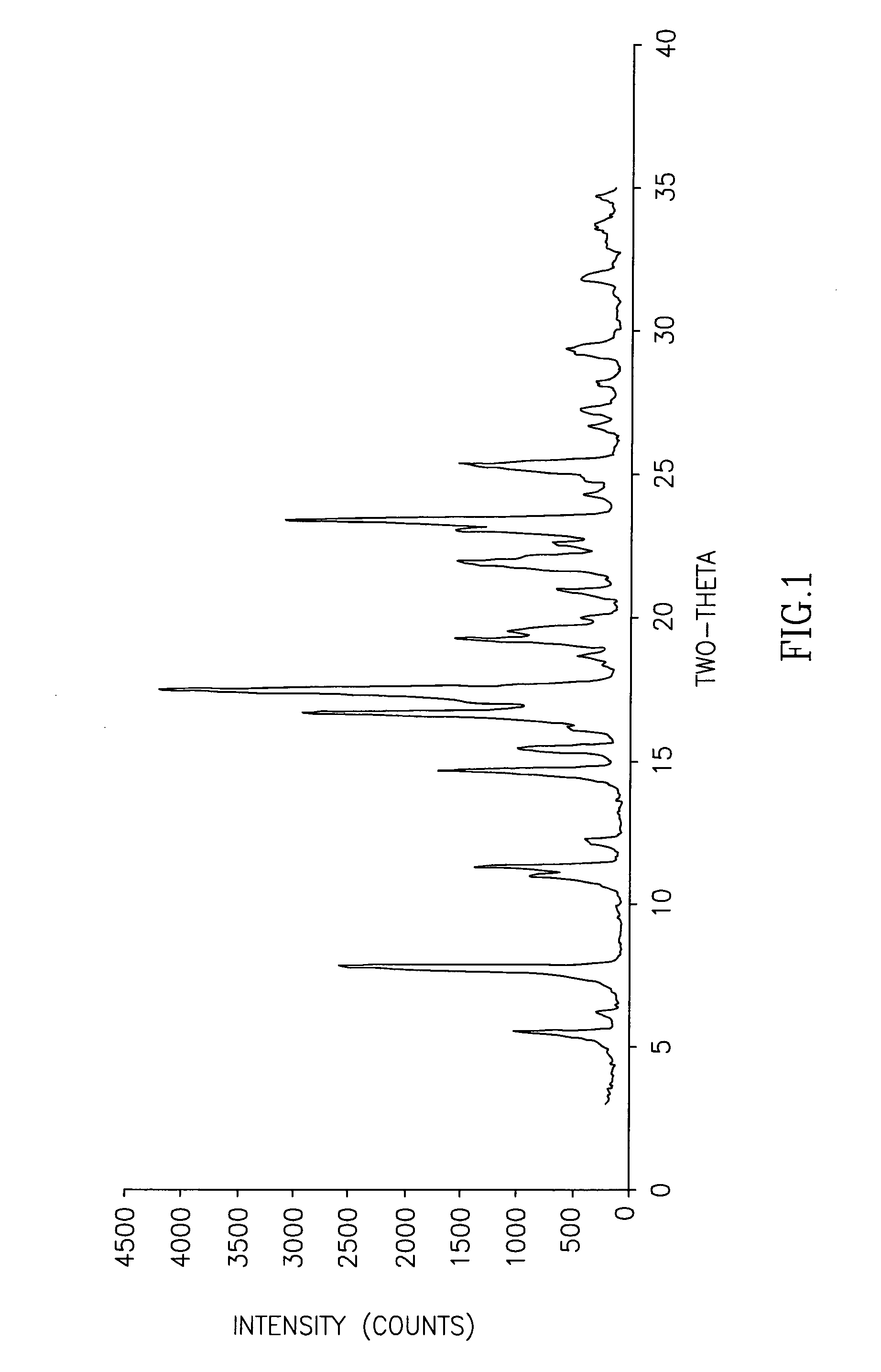
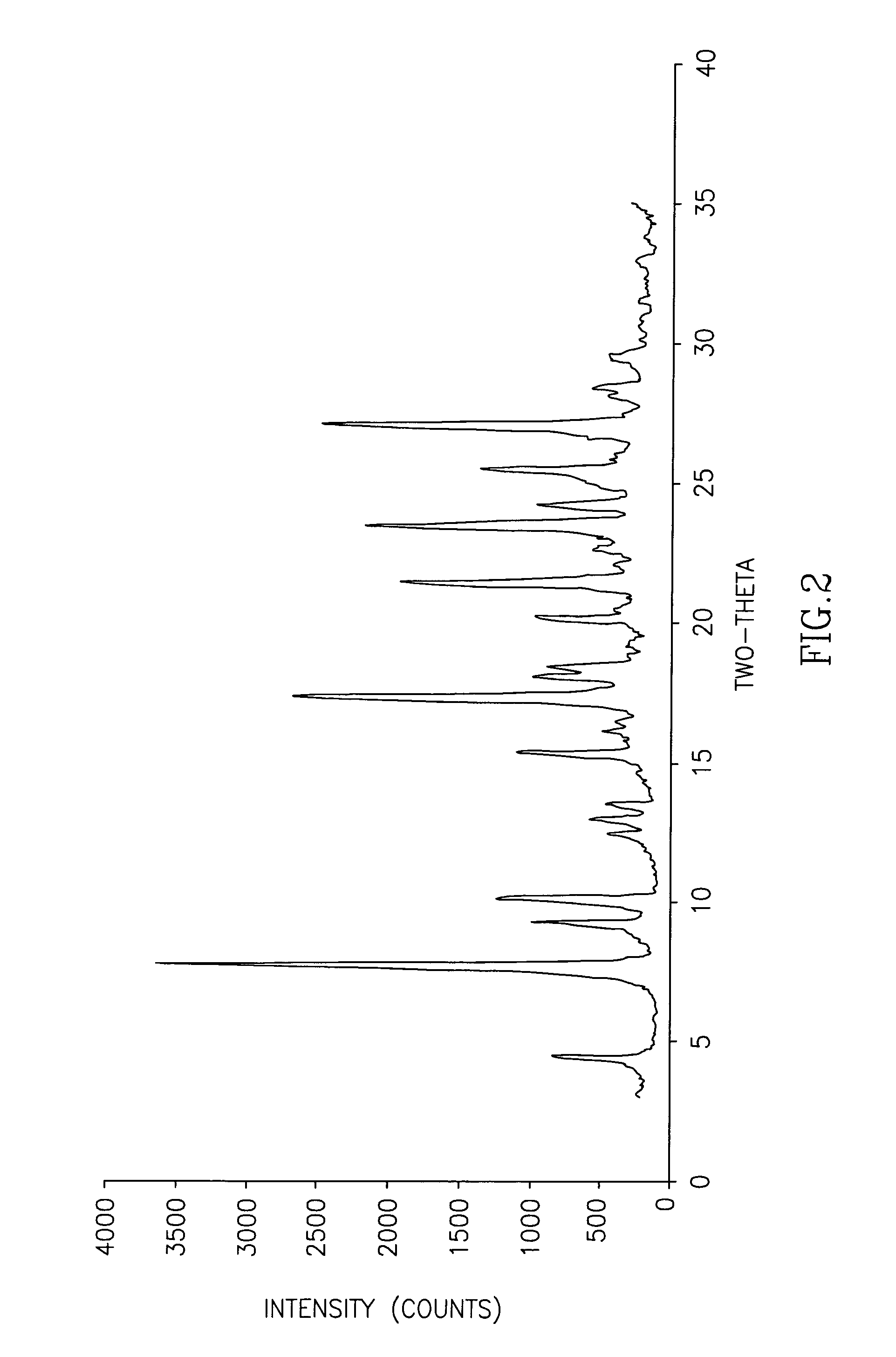
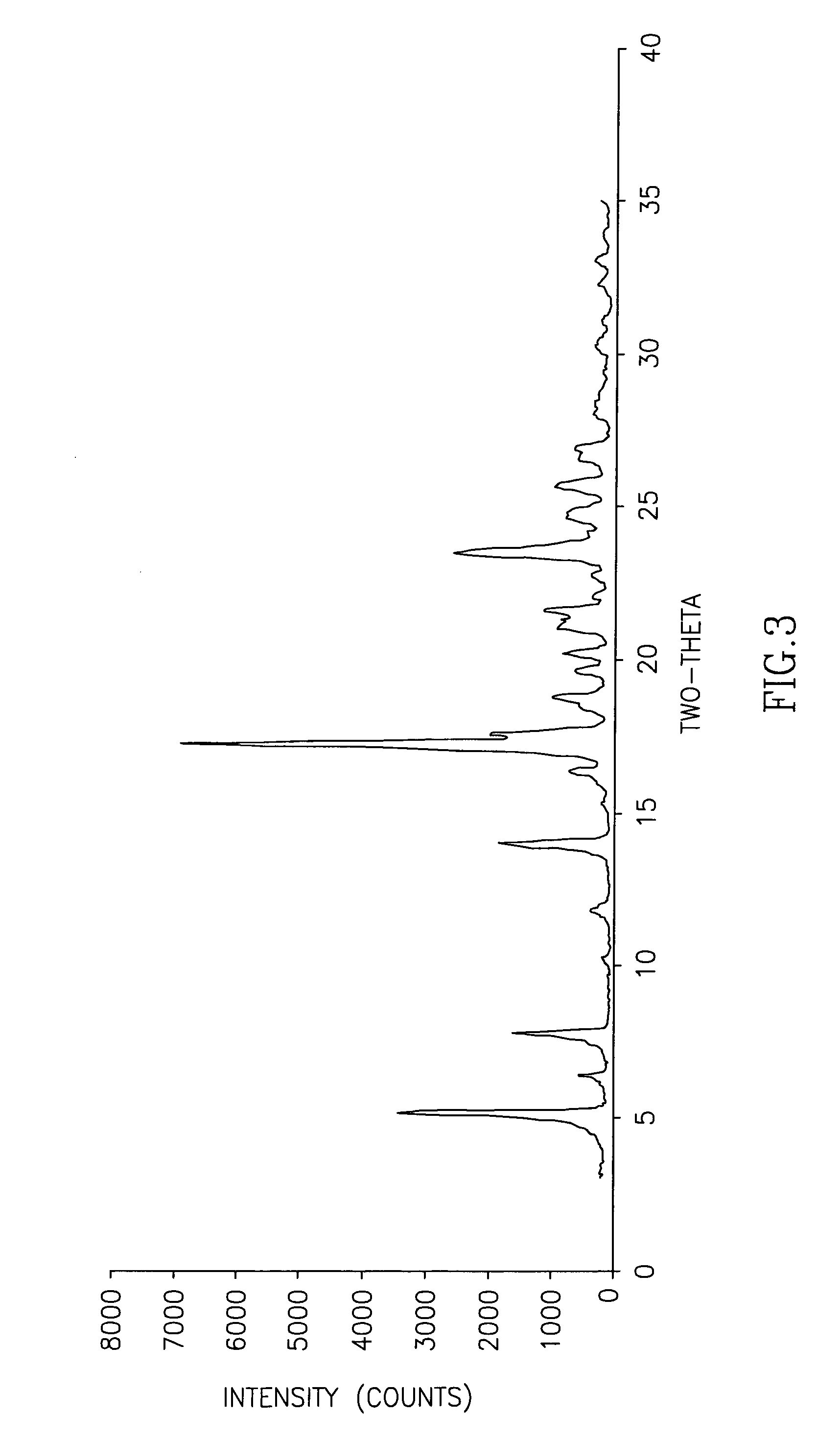
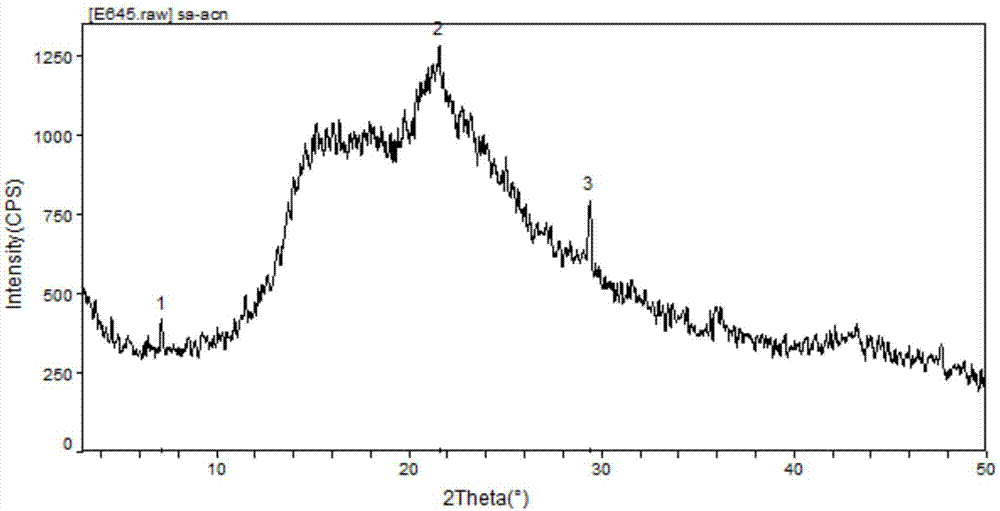
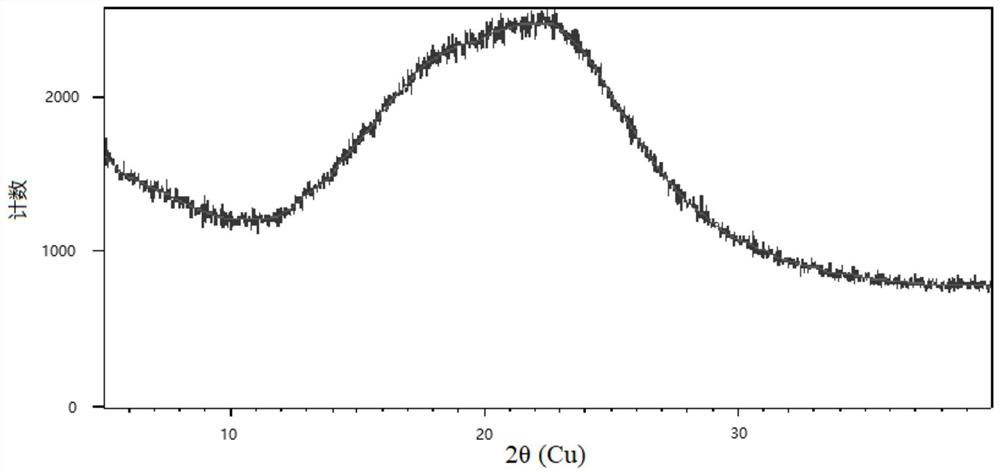
The invention discloses cisatracurium besylate with a new crystal form and a purifying method thereof. The purifying method comprises the following steps: (A) adding amorphous cisatracurium besylate into a polar solvent with the volume being 20-40 times of that of the amorphous cisatracurium besylate, and stirring till being completely dissolved; (B) filtering the obtained solution by a microporous filtering membrane, then putting filtrate at the temperature of 22+ / -5 DEG C, stirring for 5-30 hours; and (C) filtering to obtain filtering cakes, carrying out vacuum drying for 2-14 hours at the temperature of 30-40 DEG C to obtain white powder. The cisatracurium besylate and the purifying method disclosed by the invention have the advantages that 2theta in an X-ray diffraction pattern has main characteristic diffraction peaks at 7.0+ / -0.2, 8.6+ / -0.2, 11.2+ / -0.2, 12.8+ / -0.2, 17.4+ / -0.2, 18.5+ / -0.2, 19.4+ / -0.2, 20.8+ / -0.2 and 23.9+ / -0.2; the stability is better, and the storage and transportation of crude drugs are convenient; and the purifying method is simple and is suitable for industrial production.
Owner:江苏海悦康医药科技有限公司
R,R1-atracurium salts
The present invention provides R,R′-atracurium salts, processes for producing and purifying such salts, and methods of using such salts to produce highly pure cisatracurium besylate.
Owner:WAVELENGTH ENTERPRISES LTD
Isoquinolinium compounds useful in the preparation of cisatracurium and associated intermediates
The present invention provides novel isoquinolinium compounds, methods of producing the isoquinolinium compounds, and methods for converting them into cisatracurium, e.g., cisatracurium besylate. The isoquinolinium compounds of the present invention can be obtained in the form of solids, which can be purified using simple techniques and can be used to afford pure cisatracurium besylate without HPLC purification.
Owner:WAVELENGTH ENTERPRISES LTD
A kind of preparation method of impurity w in cisatracurium besylate
ActiveCN110256344BHigh purityEasy to prepareSulfonic acids salts preparationCisatracurium BesylateAcetic acid
The invention discloses a method for preparing impurity W in cisatracurium besylate, belonging to the technical field of chemical substance synthesis, including A. Take 1,5-pentanediol, glacial acetic acid, and a catalyst to react to obtain a reaction solution; B. Add water and n-hexane to the reaction solution, stir and separate the liquid, and collect the aqueous layer solution; C. Washing the aqueous layer solution with n-hexane, collecting the washed aqueous layer solution; D. Extract the aqueous layer solution with dichloromethane, and collect the dichloromethane layer solution; E. Add a desiccant to the dichloromethane layer solution, filter, and concentrate to obtain 1,5-pentanediol acetate monoester; F. 1,5-pentanediol acetic acid monoester reacts with impurity A in cisatracurium besylate, adds a solvent, stirs, and filters to obtain impurity W crystallization, and dry to obtain final product impurity W. The cisatracurium besylate impurity W prepared by the present invention has high purity, and the preparation method is simple, and the prepared impurity W can be directly used as a standard for the quantitative detection process of the impurity W in the cisatracurium besylate.
Owner:江苏盈科生物制药有限公司
A kind of cisatracurium besylate composition for injection
ActiveCN107638391BImprove stabilityGood effectPowder deliveryOrganic active ingredientsVitamin CReceptor
The present invention belongs to the technical field of medicine, and particularly relates to a cisatracurium besylate composition for injection, wherein the cisatracurium besylate composition comprises 2.0 mg / mL of cisatracurium besylate, 0.8-1.5 mg / mL of methionine, 1.0-2.0 mg / mL of vitamin C, an appropriate amount of a pH value adjusting agent, and 1000 mL of water for injection. According to the present invention, vitamin C provides the anti-oxidation effect, and methionine can increase the glutathione content in vivo so as to activate acetylcholinesterase, reduce the concentration of acetylcholine, and promote the combination between cisatracurium besylate and cholinergic receptors, such that the prepared pharmaceutical composition has advantages of good stability and excellent efficacy.
Owner:亿药通(河北)生物科技有限公司
(1R,1′R)-atracurium salts separation process
InactiveUS8357805B2Easy to applyHighly effectiveBiocideAnimal repellantsChromatographic separationCisatracurium Besylate
Owner:WAVELENGTH ENTERPRISES LTD
Preparation method and application of cisatracurium besilate
ActiveCN112778200AReduce typesImproved production safetyOrganic chemistryMuscular disorderCisatracurium BesylatePropanoic acid
The invention provides a preparation method and application of cisatracurium besylate, and the preparation method comprises the following steps: (1) dissolving R-tetrahydropapaverine-N-acetyl-L-leucine salt, adjusting the pH value with an alkaline solution, and then mixing with methyl 3-bromopropionate and alkali for reaction to obtain an intermediate A; (2) mixing and reacting the intermediate A obtained in the step (1) with alkali to obtain an intermediate B; (3) mixing and reacting the intermediate B obtained in the step (2) with a methylation reagent to obtain an intermediate C; and (4) mixing and reacting the intermediate C obtained in the step (3), a catalyst and a dehydrating agent to obtain cisatracurium besylate. The preparation method provided by the invention is safe in production process, high in yield, few in isomer and low in maximum single impurity.
Owner:JIANGSU CHENGXIN PHARMA
Preparation method of tetrahydrobenzyl isoquinoline compound
PendingCN114716375AHigh yieldHigh chemical purityOrganic chemistry methodsCisatracurium BesylateOrganic acid
The invention relates to a preparation method of a tetrahydrobenzyl isoquinoline compound, which is characterized in that the method comprises the step of adding a specific chiral organic acid into a mixture containing a compound shown as a formula (I) to form a salt, and the chemical purity and optical purity of the prepared product of the compound shown as the formula (I) are greatly improved while the high yield is ensured. The method is favorable for improving the total yield and controlling the product quality in the synthesis process of the mivacurium chloride or cisatracurium besylate finished product, provides a powerful guarantee for improving the safety and effectiveness of the finished product medicine, and is suitable for industrial production.
Owner:SICHUAN CREDIT PHARMA
Preparation method of cisatracurium besilate impurity O
PendingCN114539146AHigh purityEasy to prepareOrganic chemistryBulk chemical productionMeth-Ptru catalyst
The invention relates to a preparation method of a cisatracurium besylate impurity O. The preparation method comprises the following steps: S1, taking (1R)-1-[(3, 4-dimethoxyphenyl)-methyl]-1, 2, 3, 4-tetrahydro-6, 7-dimethoxy-2-methoxycarbonyl ethyl-isoquinoline, methyl benzenesulfonate, acetonitrile and a catalyst to react, so as to obtain a cisatracurium besylate impurity D; s2, taking the impurity D, 1, 5-pentanediol, dichloromethane and a catalyst to react to obtain an impurity F; s3, taking the impurity F, acryloyl chloride, dichloromethane and a catalyst to react to obtain an impurity O crude product; and S4, preparing a purified impurity O pure product through a liquid phase. According to the method, the defect that an effective method for preparing the cisatracurium besylate impurity O is lacked in the prior art is overcome, the purity of the prepared impurity O is high, the preparation method is simple, the used reagent is low in price and easy to obtain, the yield is high, and the impurities D and F can be prepared in the synthesis route.
Owner:武汉绿合医药科技有限公司
Production process of cisatracurium besylate for injection for storage and transportation at 25°C
ActiveCN104983694BGood chemical stabilityReduce storage and transportation conditionsOrganic active ingredientsPowder deliveryCisatracurium BesylatePolymer science
Owner:SHANGHAI PHARMA DONGYING JIANGSU PHARMA CO LTD
A kind of preparation method of besylate cisatracurium intermediate
ActiveCN110724100BHigh purityAvoid introducingOrganic chemistryOXALIC ACID DIHYDRATECisatracurium Besylate
The invention discloses a preparation method of a cisatracurium besylate intermediate. The method is as follows: compound III is freed by adding alkali, reacts with tert-butyl acrylate under the catalysis of acetic acid, and then adds oxalic acid to form a salt to obtain the compound of formula VI; compound of formula VI is freed by adding alkali, and then firstly becomes salt, and then hydrolyze the tert-butyl ester in it into carboxylic acid under heating to obtain the compound of formula VII, and then carry out esterification reaction with 1,5-pentanediol in situ to generate an esterified product, after washing the excess compound of formula VII with water, add Alkali alkalization, and then salify with oxalic acid to obtain the intermediate of formula IV. The product of the present invention has high yield and high purity, can be directly put into the cisatracurium besylate for the last step reaction without refining to finally obtain the cisatracurium besylate, and has simple aftertreatment and is easy for industrialized production.
Owner:SHANDONG BOYUAN PHARM CO LTD
Novel crystal form cisatracurium besylate and crystallization method thereof
PendingCN114014806AReduce usageReduce dosageOrganic compound preparationOrganic chemistry methodsCisatracurium BesylateOrganic solvent
The invention relates to novel crystal form cisatracurium besylate and a crystallization method thereof. The crystallization method comprises the following steps of: dissolving amorphous cisatracurium besylate with acetone, adding the obtained solution into an ethyl acetate solvent, and crystallizing to obtain a cisatracurium besylate crystal. The crystallization method only relates to two organic solvents, namely acetone and ethyl acetate, the dosage is relatively small, the crystallization time is short, and the crystallization method is more suitable for industrial production from the angle of amplification. Meanwhile, the prepared novel crystal form cisatracurium besylate is very high in quality, the purity can reach 99.89%, the yield can reach 99.12%, and a very good crystallization condition and purification method for cisatracurium besylate are provided.
Owner:JIANGSU CHENGXIN PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com