Preparation method of cisatracurium besilate impurity O
A technology of cisatracurium besylate and impurities, which is applied in the field of organic synthesis of medicines, can solve problems such as lack of and unfavorable research on impurities of cisatracurium besylate, and achieve simple preparation methods, cheap and easy-to-obtain reagents, and high yields. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
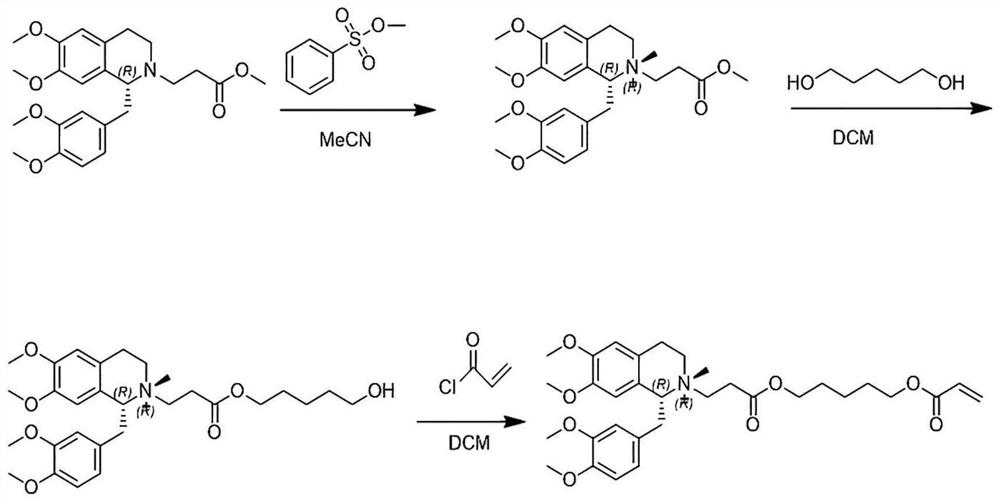
[0031] A kind of preparation method of cisatracurium besylate impurity O, it comprises the steps:
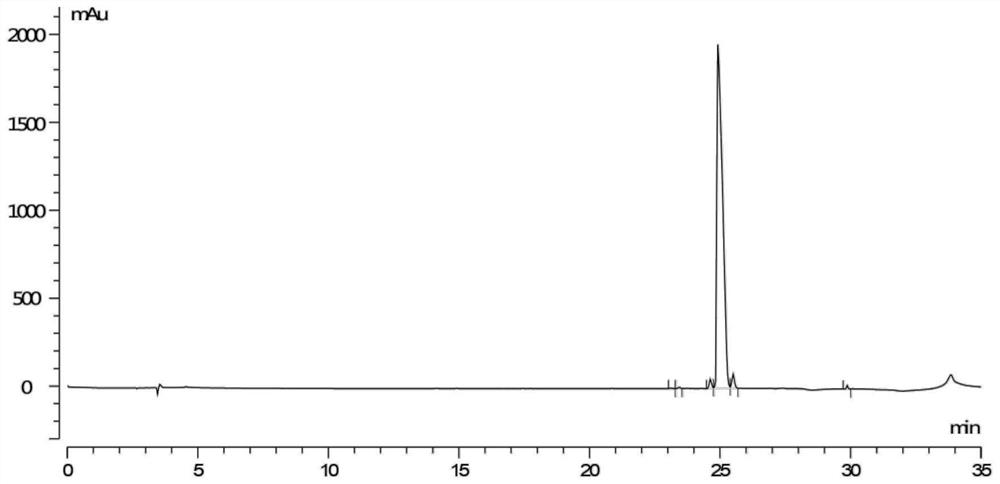
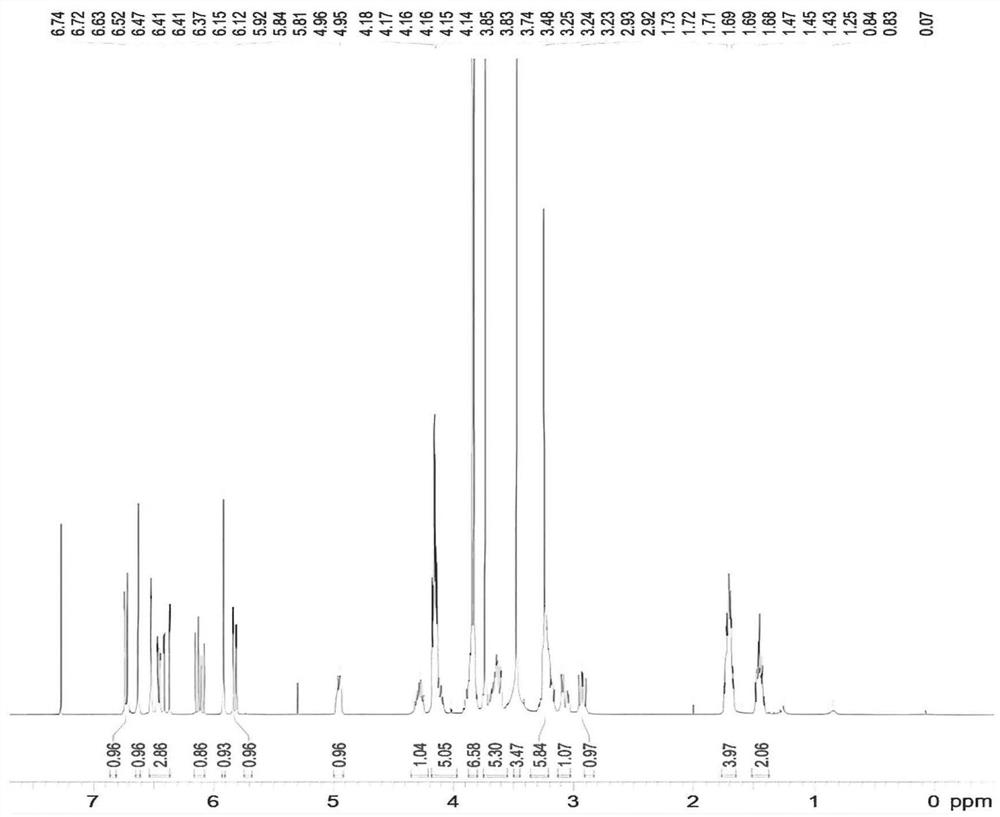
[0032] S1. Take (1R)-1-[(3,4-dimethoxyphenyl)-methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl Oxycarbonylethyl-isoquinoline 5.00g, methyl benzenesulfonate 4.00g, sodium bicarbonate 0.61g, acetonitrile 20mL in a reaction flask, react at 15-25°C for more than 48h, a large amount of white solids are precipitated during the reaction , the ratio of the peak areas of the two configurations in liquid phase detection is 3:1, suction filtration, and the filter cake is slurried with acetonitrile until the purity of impurity D is ≥98%, and finally 3.77g of impurity D (purity 98.21%) is obtained;
[0033] S2. Take 3.00g of impurity D, 2.59g of 1,5-pentanediol, 2.37g of benzenesulfonic acid, and 15mL of dichloromethane in a reaction flask, heat to reflux for more than 8h, and HPLC detects that the raw materials have basically reacted, and the products are mainly Impurity F and a small amo...
Embodiment 2
[0040] A kind of preparation method of cisatracurium besylate impurity O, it comprises the steps:
[0041] S1. Take (1R)-1-[(3,4-dimethoxyphenyl)-methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl Oxycarbonylethyl-isoquinoline 5.00g, methyl benzenesulfonate 3.80g, sodium carbonate 0.77g, acetonitrile 20mL in a reaction flask, react at 15-25°C for more than 48h, a large amount of white solids are precipitated during the reaction, The ratio of the peak areas of the two configurations in liquid phase detection is 3:1, suction filtration, and the filter cake is slurried with acetonitrile until the purity of impurity D is ≥98%, and finally 3.56g of impurity D (purity 98.18%) is obtained;
[0042] S2. Take 3.00g of impurity D, 3.49g of 1,5-pentanediol, 2.87g of trifluoroacetic acid, and 15mL of dichloromethane in a reaction flask, heat to reflux for more than 8h, and HPLC detects that the raw materials have basically reacted, and the products are mainly Impurity F and a small amount...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com