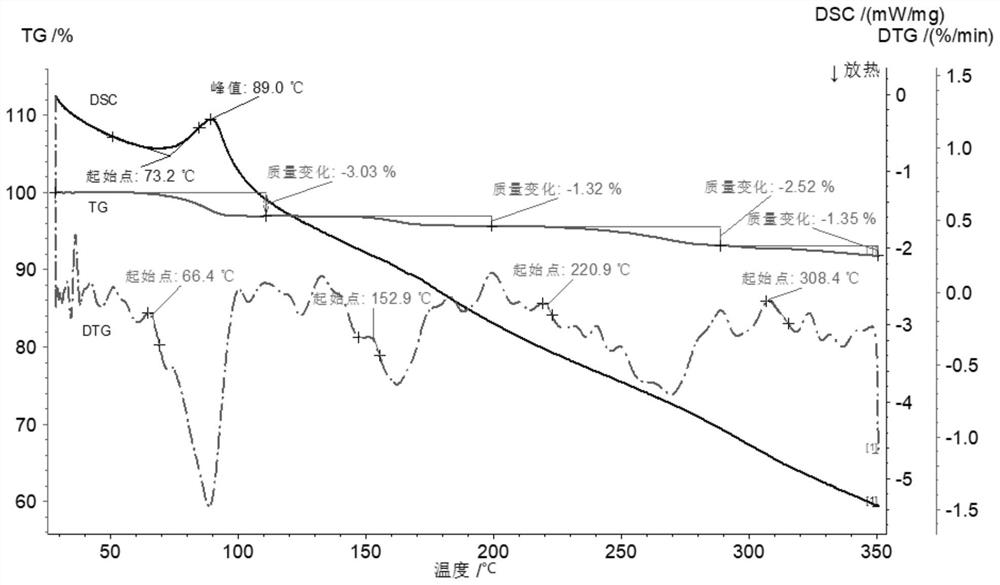
Novel crystal form cisatracurium besylate and crystallization method thereof
A technology of atracurium cis-benzenesulfonate and its crystal form, which is applied in the field of new crystal form of atracurium cis-benzenesulfonate and its crystallization, can solve problems such as difficult to reproduce, reduce usage and increase production Efficiency, good crystallization conditions and effect of purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] This embodiment provides a crystallization method of atracurium cis-benzenesulfonate and the obtained new crystal form of atracurium cis-benzenesulfonate, the operation is as follows:
[0057] (1) 10.02 g (purity 98.56%) of the amorphous atracurium cis-benzenesulfonate solid after precipitating was stirred and dissolved with 10.00 g of acetone at 22° C.;
[0058] (2) Add the acetone solution obtained in step (1) dropwise into 80 mL of ethyl acetate solvent at 25° C. for crystallization, and stir at a speed of 210 rpm while adding dropwise, and the dropwise addition is completed in 50 minutes;
[0059] (3) After the dropwise addition, continue to grow crystals at 20°C and 75rpm under stirring conditions for 2h, then filter with suction to obtain a filter cake, vacuum dry at 25°C for 48h, collect the materials, and obtain atracurium cis-benzenesulfonate crystals 9.80g, the purity is 99.89%, and the yield is 99.12%.
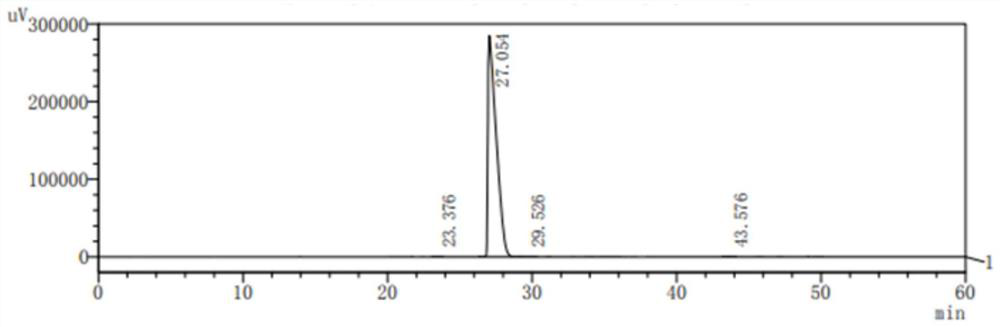
[0060] The product that obtains is carried out HPLC de...
Embodiment 2
[0067] This embodiment provides a crystallization method of atracurium cis-benzenesulfonate and the obtained new crystal form of atracurium cis-benzenesulfonate, the operation is as follows:
[0068] (1) 10.03 g (purity 98.56%) of the amorphous atracurium cis-benzenesulfonate solid after precipitating was stirred and dissolved with 10.00 g of acetone at 25° C.;
[0069] (2) Add the acetone solution obtained in step (1) dropwise into 80 mL of ethyl acetate solvent at 25° C. for crystallization, and stir at a speed of 210 rpm while adding dropwise, and the dropwise addition is completed in 55 minutes;
[0070] (3) After the dropwise addition, continue to grow crystals at 25°C and 80rpm under stirring conditions for 2h, then filter with suction to obtain a filter cake, vacuum dry at 25°C for 48h, collect the materials, and obtain atracurium cis-benzenesulfonate crystals 9.86g, the purity is 99.85%, and the yield is 98.53%.
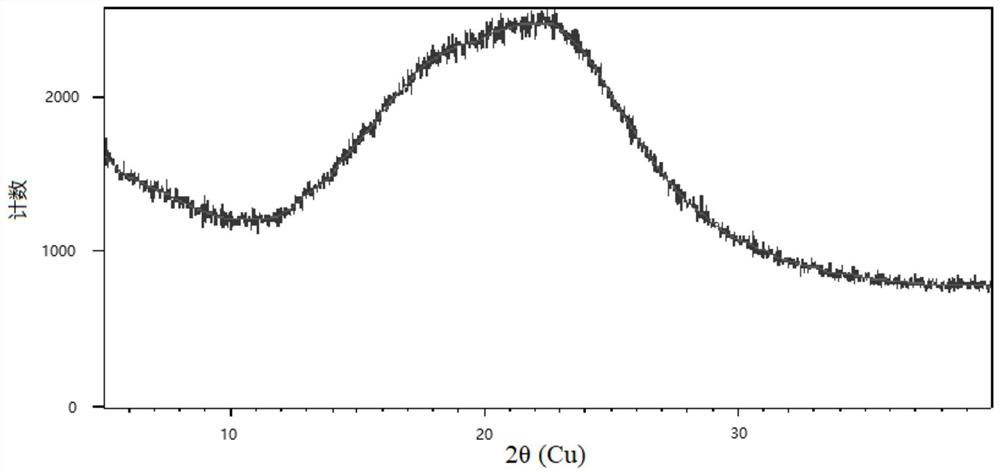
[0071] The obtained product was detected by XRD spectr...
Embodiment 3
[0073] This embodiment provides a crystallization method of atracurium cis-benzenesulfonate and the obtained new crystal form of atracurium cis-benzenesulfonate, the operation is as follows:
[0074] (1) 10.02 g (purity: 98.56%) of amorphous atracurium cis-benzenesulfonate solid after precipitating was stirred and dissolved at 18° C. with 10.00 g of acetone;
[0075] (2) Add the acetone solution obtained in step (1) dropwise into 100 mL of ethyl acetate solvent at 18° C. for crystallization, and stir at a speed of 220 rpm while adding dropwise, and the dropwise addition is completed in 58 minutes;
[0076] (3) After the dropwise addition, continue to grow crystals at 18°C and 75rpm under stirring conditions for 2.5h, then filter with suction to obtain a filter cake, vacuum dry at 30°C for 30h, collect the materials, and obtain atracurium cis-benzenesulfonate Crystal 9.91g, purity 99.60%, yield 99.08%.
[0077] Carry out XRD spectrogram detection to the product that obtains,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com